
Change in Clinico-Epidemiological Pattern of Leptospirosis in Andaman & Nicobar Islands: A Retrospective Analysis of the Past Decade (2010-2020)
*Corresponding Author(s):
Paluru VijayachariICMR- Regional Medical Research Centre, Port Blair, Post Bag No. 13, Dollygunj, Port Blair 744101, Andaman & Nicobar Islands, India
Email:fatemaambreen@gmail.com
Abstract
Context: Leptospirosis, a zoonotic disease, is re-emerging with numerous outbreaks in the Andaman and Nicobar Islands of the Indian subcontinent.
Methods and Materials: Retrospective data analysis of patient records from 2010 to 2020 (41,910) maintained at ICMR-Regional Medical Research Centre, Port Blair, was carried out.
Settings and Design: Suspected cases of leptospirosis who reported to PHCs, CHCs and district hospitals. Statistical analysis used: Chi-square test, prevalence rate, incidence rate, and positivity percentage to analyse study variables.
Results: The annual incidence of leptospirosis varied from 4.47/100,000/year to 207.05/100,000/year, much higher than in other parts of the globe. The proportion of cases was high among males (χ2 = 117.2, p < 0.0001) and reported more from rural areas (χ2 = 290.2, p < 0.0001), with the mean age of the affected population being 34.75 years. 41,910 patients were suspected, and the yearly positivity rate varied from 0.90 % to 9.49 % and showed a declining trend. The most typical symptom reported was fever (100 %), followed by headache (63.91 %).
Conclusion: Annual incidence rate in these islands is high compared to other regions, where the predominantly male and rural populations are affected.
Keywords
Endemic; Incidence; Positivity rate; Rural
Introduction
Leptospirosis is a zoonotic disease caused by a spirochete from the genus Leptospira [1]. The clinical presentations are diverse and overlapping symptoms with other infections. Even though the clinical picture of severe leptospirosis is well-defined and easily recognised by experienced doctors in endemic locations, rare and unexpected clinical presentations may confuse diagnosis [2].
Leptospirosis is re-emerging all over the world [3]. Recently, leptospirosis epidemics have erupted in many countries, mainly South America and India. Natural calamities such as cyclones and floods were to blame for some of these [4]. Each year, an estimated 1.03 million cases and 58,900 deaths are reported worldwide. According to a recent estimate [5], in tropical climates, the annual incidence rate exceeds 10 cases per 100 000 people, but in temperate climates, the rate is significantly lower (0.1-1 per 100 000). In high-exposure risk groups, the disease incidence can ascend to 100 per 100,000 [6].
Andaman and Nicobar Islands are a group of islands situated around 1300 km from the mainland of India in the Bay of Bengal. Leptospirosis continues to be an endemic disease in these Islands [7]. Previous studies report that 52 %-55 % of the general population is seropositive to Leptospira and 62 % among agricultural workers [8]. Any mammal is a potent animal reservoir for the organism, and human infections are often from rodents [1]. Transmission can be directly or indirectly to humans [9]. Leptospirosis causes a wide range of clinical symptoms affecting various organs. Jaundice, bleeding, and renal failure are symptoms of the most severe form of leptospirosis. The distant hospitals where these patients have been treated need more intensive care units and hemodialysis facilities, making it impossible to give the best care [7].
It is the first study of the clinical-epidemiological pattern of leptospirosis in Andaman and Nicobar Islands for such a prolonged duration; hence the surveillance data of the last decade covering the samples from five primary health centers, one community health center, and one referral hospital in South Andaman and Nicobar Islands analyzed. This study will provide a better epidemiological understanding of this infectious illness which is necessary to develop effective prevention and control initiatives based on a "One Health" approach [4]. The analysis includes comparing clinically diverse presentations of the disease, which will aid in assessing the shift in the clinical spectrum and better precision in diagnosing the illness.
Materials and Methods
Study Area
Over 500 islands make up the archipelago of the Andaman and Nicobar Islands, which is located in the Bay of Bengal and lies between 92° and 94° East and 6° and 14° North. There are 38 inhabited islands. The population is 379,944 [10].
Study type
Retrospective analysis of clinically suspected patients of leptospirosis reported to hospitals and samples received at the ICMR-RMRC Port Blair (RMRCPB).
Sample collection
Confirmed (1990) and suspected cases (41,910) of leptospirosis were included from GB pant hospital and primary health facilities. Even general practitioners and consulting clinicians sent clinical specimens from the suspected cases of leptospirosis to the RMRCPB for laboratory evaluation.
GB Pant is the only tertiary care hospital in the Andaman and Nicobar Islands. The severe cases from North and Middle Andaman were also referred to the GB Pant hospital. The samples of the suspected cases of leptospirosis were referred to RMRCPB for diagnosis and confirmation of the disease in the period mentioned (2010-2020).
About 2 ml of blood was collected by vein puncture and used to separate the serum.
Criteria for clinical suspicion of leptospirosis
The criteria used for suspecting leptospirosis in any patient were those who reported febrile illness of sudden onset, headache, and body aches, related to any of the following symptoms: (i) calf muscle tenderness, (ii) bleeding tendencies, including sub-conjunctival haemorrhage, (iii) cough, hemoptysis, and breathlessness, (iv) jaundice and (v) oliguria.
Criteria for laboratory confirmation of leptospirosis
Clinical signs and symptoms are consistent with leptospirosis and any one of the following for the evaluation of samples: (i) a fourfold increase in MAT titer in acute and convalescent serum samples (ii) MAT titer ≥ 1 in 400 in single or paired serum samples (iii) RT - PCR; (iv) IgM antibodies [11].
MAT for detecting anti-leptospiral antibodies in the serum samples was performed per the protocol [12]. We utilised a panel of 11 references Leptospira strains and a local isolate spanning nine serogroups. viz., Australis (Ballico), Autumnalis (Bangkinang 1), Canicola (Utrecht IV), Grippotyphosa (Moskva V), Grippotyphosa (CH 31) Icterohaemorrhagiae (RGA), Pomona (Pomona), Sejroe (Hardjoprajitno), Hebdomadis (Hebdomadis), Pyrogenes (Salinem), Semaranga (Patoc 1), and the local isolate, Icterohaemorrhagiae. (AF 61), MAT was performed on doubling serum dilutions, starting from an initial dilution of 1 in 20. Positive samples were titrated up to the endpoint (40,960).
Results
Socio-demographic characteristics: The confirmed cases' ages ranged from 3 to 85 years. Among 1990 patients, 1316 (66.13%) were males, and 674 (33.86 %) were females. The mean age was 34.75 years). Females (mean age: 35.19 years, Standard deviation (σ) 14.63) males (mean age 34.31 years, σ 15.48). From 2010 to 2020, 41,910 tests were carried out on suspicion of leptospirosis, and 1,990 (4.74%) were laboratory confirmed. As mentioned in Table 1, the proportion of leptospirosis was high among the male population, and this difference between the male and female populations was statistically significant at p < .05 (χ2 = 117.2, p < 0.0001).
|
Characteristics |
Laboratory confirmed cases |
Suspects |
95% Confidence Interval (Lower, Upper) |
χ2 Value |
P-Value |
|
Male |
1316 |
22528 |
5.236, 5.815
|
117.3
|
<0.0001 |
|
Female |
674 |
19382 |
3.12, 3.619
|
Table 1: Gender-wise comparison of cases infected with leptospirosis.
As given in Table 2, the proportion of leptospirosis among people from rural areas was higher than in urban areas and was statistically significant at p < .05 (χ2 = 290.2, p < 0.0001). Total rural suspects constitute 21,491, of which confirmed cases were 1409 (6.55 %), rural female suspects were 9,465 in which 460 (4.86 %) were positive, the number of rural male suspects was 12,026, and 949 (7.89 %) were confirmed. The overall urban suspects were low at 20,419, and 581 (2.84 %) were positive. There was a male preponderance with 368 (3.50 % positive of total male suspects) and 213 female (2.13% positive of total female suspects).
|
Characteristics |
Laboratory confirmed cases |
Suspects |
95% Confidence Interval (Lower, Upper) |
χ2 Value |
P-Value |
|
Rural |
1409 |
21491
|
5.849, 6.472 |
|
<0.0001 |
|
Urban
|
581 |
20419 |
2.553, 2.997 |
Table 2: Comparison of cases infected with leptospirosis by place of residence.
The positivity rate by health facilities reporting leptospirosis cases (2010-2020) is given in Table 3. Maximum suspects reported were from GB Pant district hospital and Garacharma CHC, which lies in the urban part of the island, in which out of 20,419 suspects, 581 (2.84%) were confirmed. Tusnabad is the rural area with the highest number of confirmed patients, 668 out of 9,551 suspects (6.99%), followed by Manglutan and Ferrergunj. The higher positivity percentage of interisland patients is due to sampling referral from the severe cases [13] of leptospirosis after initial screening at PHC/ CHC level by the interisland clinicians.
|
Area |
Total patients tested |
Confirmed patients |
Positivity rate |
|
GB Pant District hospital + Garacharma CHC (Urban) |
20,419 |
581 |
2.84 |
|
Tusnabad PHC (Rural) |
9,551 |
668 |
6.99 |
|
Manglutan PHC (Rural) |
5,868 |
357 |
6.08 |
|
Ferrargunj PHC (Rural) |
2,991 |
225 |
7.52 |
|
Wimberly Gunj PHC (Rural) |
1,620 |
68 |
4.19 |
|
Chouldari PHC (Rural) |
1,436 |
68 |
4.73 |
|
Patients from interisland PHC's* (Ruralx) |
25
|
23 |
88.46 |
|
Total |
41,910 |
1990 |
4.74 |
Table 3: Positivity rate of leptospirosis cases reported to different health facilities (2010-2020).
*(Baratang, kadamtala, Rangat and Neil, and Havelock Island PHCs), Interisland patients were initially screened at the PHC level, and samples were sent to RMRC, Port Blair for laboratory diagnosis.
Figure 1 shows the distribution of confirmed cases of leptospirosis by age group and gender-wise. The lowest age group infected was 0-5 years, with only 3 (0.22 positivity rate) cases. The highest positivity rate in the age group of 26-30 years, in which total positive cases were 364 (7.92%) out of 4594 tested, but the maximum number of tests were done for the age group 21-25 years is 4680 confirmed 202(4.32%). Among females, the positivity rate was highest at 26-30 years (7.80 %), followed by 31-35 years (6.15%). The positivity rate in males was higher than in females. Overall, it was found that the age group of 26-30 is the most affected in both genders.
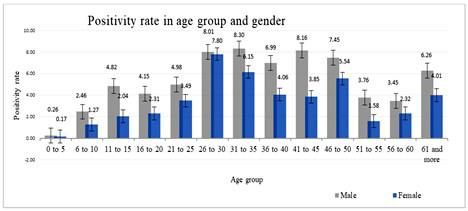 Figure 1: Age and gender-wise positivity rate distribution for a decade (2010-2020).
Figure 1: Age and gender-wise positivity rate distribution for a decade (2010-2020).
The estimated population of the A & N islands in 2021 is calculated based on the decal growth rate of the population. The overall decadal growth rate of the Andaman and Nicobar islands is 6.68 %, where the male has 4.85 %, and the female has an 8.85% decadal growth rate [10]. The trend of leptospirosis incidence is shown in Figure 2. The incidence varied between 4.47/100,000/year in 2010 to 207.05/100,000/year in 2013, where 2013 showed the highest incidence rates. The maximum number of samples, 7354, was tested in 2013, in which 698 were positive, indicating a 9.49 positivity rate, and in 2018 the lowest was with a 0.90 positivity rate. The trend of leptospirosis positivity rates and their association with the average rainfall is shown in Figure 3, indicating a decline in the disease positivity without any significant rainfall association [14].
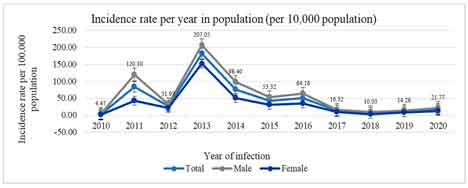 Figure 2: Incidence rates of leptospirosis in Andaman and Nicobar Islands.
Figure 2: Incidence rates of leptospirosis in Andaman and Nicobar Islands.
*Incidence of leptospirosis in Andaman and Nicobar Islands from (2010-2020). The light blue line indicates the total incidence rate per 100,000. The blue line indicates the incidence rate among female cases. (Number of cases in female/100,000 female population). The lighter black line indicates the incidence rate among male cases (Number of cases in male/100,000 male population). The total population of Andaman and Nicobar Islands (3,77,406 in 2010), (3,79,944 in 2011), (3,82,482 in 2012), (3,85,036 in 2013), (3,87,608 in 2014), (3,90,197 in 2015), (3,92,803 in 2016), (3,95,426 in 2017), (3,98,067 in 2018), (4,00,726 in 2019) and (4,03,402 in 2020).
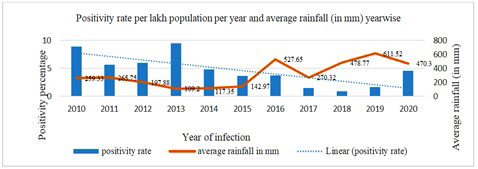 Figure 3: Association of average rainfall (in mm) and positivity rate distribution per year of leptospirosis in Andaman and Nicobar Islands (2010-2020).
Figure 3: Association of average rainfall (in mm) and positivity rate distribution per year of leptospirosis in Andaman and Nicobar Islands (2010-2020).
The most typical symptom reported was fever (100 %), followed by headache (63.91 %), body ache (61.35 %), and chills (45.42 %), respectively. Breathlessness was reported by 13.16 %, possibly due to lung involvement, resulting in difficulty breathing. Other less common symptoms which are not summarised in Table 4 are Eye irritation (2.61 %), muscle pain (2.46 %), burning micturition (2.3 1%), icterus (0.75 %), and oliguria (0.45 %) are among less reported symptoms. Central nervous system involvement indicated by Neck stiffness was observed in 15 patients (0.75%). Bleeding tendencies such as hemoptysis reported was 1.80 %, and hematuria, conjunctival haemorrhage, hematemesis, and epistaxis consist of 1.00 %, 0.85 %, 0.70 %, and 0.55 %, respectively.
|
Signs and Symptoms |
Frequency |
Percentage (%) |
|
Fever |
1990 |
100 |
|
Headache |
1272 |
63.91 |
|
Body ache |
1221 |
61.35 |
|
Chills |
904 |
45.42 |
|
Cough |
895 |
44.97 |
|
Arthritis |
397 |
19.94 |
|
Cold |
379 |
19.04 |
|
Giddiness |
358 |
17.98 |
|
Rigor |
323 |
16.23 |
|
Vomiting |
323 |
16.23 |
|
Abdominal pain |
312 |
15.67 |
|
Breathlessness |
262 |
13.16 |
|
Chest pain |
247 |
12.41 |
|
Yellow urine |
231 |
11.6 |
|
Arthralgia |
213 |
10.7 |
|
Nausea |
210 |
10.55 |
|
Weakness |
121 |
6.08 |
|
Back pain |
113 |
5.67 |
|
Diarrhoea |
104 |
5.22 |
|
Muscle tender |
77 |
3.86 |
|
Loss of appetite |
72 |
3.61 |
|
Runny nose |
63 |
3.16 |
|
Throat pain |
58 |
2.91 |
Table 4: Signs and symptoms of leptospirosis.
Discussion
Scientific data on the clinical-epidemiological aspect of leptospirosis is limited in Andaman and Nicobar Islands [15]. The current research shed light on the clinical-epidemiological status of Leptospirosis in the Andaman and Nicobar Islands. As agriculture is the primary occupation in the Andaman Islands' rural areas, males are predominantly involved due to conventional agricultural techniques, employing cattle or buffalo for dry and wet farming [8,16]. Hence these occupational groups are at high risk of leptospiral infection [17,18]. However, a study from Thailand by Narkkul U et al. found no gender difference when evaluating risk factors of leptospirosis [19]. However, our analysis found that the laboratory-confirmed male population of combined urban and rural areas are at higher risk of getting infected, while the. At the same time, the confirmation percentage was relatively low.
The prevalence in rural areas (95 % CI 5.849, 6.472) and in urban (95 % CI 2.553, 2.997) (χ2 = 290.2, p <0.0001), our study is in agreement with the previous research.[16] Farming activities, wet rice farmers and labourers working without protective equipment like shoes and gloves, and cattle used for field ploughing are all significant causes of rural leptospirosis.[20] As mentioned in Table 3, the highest positivity rate of leptospirosis was from Ferrargunj PHC rural area (R) 7.52 %, followed by Tusnabad (R) 6.99 % and Manglutan (R) 6.08 %, respectively. G.B. Pant hospital and Garacharma CHC, which fall in an urban area combined [10], had a positivity rate of 2.84 % from 20,419 tests that yielded 581 confirmed cases. This suggests that increased awareness among clinicians and people about the disease and ease of access to hospitals have resulted in more suspects in urban areas, resulting in increased testing over the years and, in turn, decreasing the positivity rate. However, there is still a need for more awareness in rural and urban areas of the islands for early diagnosis and control of the disease progression.
A recent study from Ecuador suggests an annual incidence ranging from 0.27 to 2.45 cases per 100,000 residents and a fatality rate of 3.06% from 2020 to 2020. The highest affected age group is adult males [21].
In our analysis of Figure 1, the middle-aged populations are predominantly affected. The primary age group affected was between 25 to 30 years, and where male cases were (10.85 %) high compared with female cases (7.33 %), which is higher than any other age group [16,22]. A possible reason for this could be their occupational exposure to the culprit organism. The annual incidence of leptospirosis varied from 4.47/100,000/year to 207.05/100,000/year, where 2013 showed the highest incidence rates. Our findings agree with the previous data regarding tropical zone incidence rates [6].
A total of 41,910 patients were suspected of leptospirosis in the last decade, and the yearly positivity rate varied from 0.90 % to 9.49 %. The positivity rate in recent years showed a declining trend, and it had no significant association with the average rainfall of the respective year. Better quality of surveillance and awareness programs resulted in a decline in the positivity rate when more samples were tested, and the proportion of confirmation of disease was decreased compared to previous years. In 2020 (4.59 %), due to the COVID-19 pandemic, fewer suspected leptospirosis patients were reported to health facilities. Improved surveillance and quality of case identification and diagnosis have helped control the disease to a much greater extent.
The signs and symptoms of the disease are summarised in Table 4 from the acute phase of the disease and complications reported by patients apart from the common signs and symptoms like fever, cough and cold etc. Severe eye irritation reported was from cases consisting of 2.61 %, while some patients reported burning micturition which makes the total percentage of 2.31 %. The potential underlying case could be renal involvement. Bleeding tendencies were rare [23], hemoptysis was reported in 1.80 %, hematuria, hematemesis, and epistaxis consisted of 1.00 %, 0.70 % and 0.55 %, respectively, observed in severe leptospirosis 15, Many patients also complained of giddiness, consisting of 17.98 %. Breathlessness, as reported by many patients related to pulmonary involvement (13.16 %) [24]. One study in a similar geographical area [15] reported headache at 93.1 %, diarrhoea at 24.1 %, and neck stiffness at 12.1 %, which were much higher than our findings where headache at 63.91 %, diarrhoea at 5.22 %, and only 0.75 % of patients reported neck stiffness. These differences in the values may be due to a shift in the clinical presentation of the disease over the period. Our study estimates that average annual rainfall is not significantly related to a higher incidence of confirmed cases.
Limitations
The samples were referred from healthcare centres on suspicion of leptospirosis. In our study, the positivity rate of interisland samples was high, which could result from only severe case samples being referred for testing to RMRCPB. Another limitation of our analysis is the cases from North and Middle Andaman. Cases mild in severity might have been treated at the local hospital but could have been missed.
Conclusion
Leptospirosis continues to be an endemic disease in Andaman and Nicobar Islands. Predominantly male and rural populations were affected. The incidence of cases and positivity rate among suspected cases show a declining trend. We conclude that an effective surveillance system and awareness programs leading to early reporting and the diagnosis of the cases decreased the positivity rate among the islands’ urban and rural populations. The knowledge of control measures and timely diagnosis and treatment has reduced the incidence of leptospirosis in the islands to a much larger extent.
Statistical analysis
The Statistical Package for Social Sciences (SPSS) was used to evaluate the data, version 28.0 (SPSS Inc., Chicago, IL, USA). Graphs were prepared in MS Office 19.
Acknowledgements
The authors acknowledge the help of Dr Manjunatha R for his efficient and prompt guidance in the analysis of statistical methods, proofreading of the manuscript, and overall inputs throughout the process Dr M. G. Madanan, Dr I. P. Sunish, and Dr Rehnuma Parvez for their guidance in preparing the manuscript.
Consent
Informed consent was obtained from all the subjects before sample collection.
Conflicts of interest
None to declare.
Ethical approval
The study was approved by the institutional ethics committee.
References
- Faine S (1994) Leptospira and leptospirosis. Crc Press Inc 353.
- WHO HL (2003) Guidance for Diagnosis, Surveillance and Control. World Heal. Organ. MaltA.
- Hartskeerl RA, Collares-Pereira M, Ellis WA (2011) Emergence, control and re-emerging leptospirosis: Dynamics of infection in the changing world. Clin Microbiol Infect 17: 494-501.
- Goarant C (2016) Leptospirosis: Risk factors and management challenges in developing countries. Res Rep Trop Med 7: 49-62.
- Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, et al. (2015) Global Morbidity and Mortality of Leptospirosis?: A Systematic Review. A Syst Rev Plos Neglected Trop Dis 1371: 1.
- http://www.leptonet.net/
- Vijayachari P, Sugunan AP, Shriram AN (2008) Leptospirosis: An emerging global public health problem. J Biosci Springer 33: 557-569.
- Sharma S, Vijayachari P, Sugunan A, Natarajaseenivasan K, Sehgal SC (2006) Seroprevalence Of Leptospirosis Among High- Risk Population of Andaman Islands. Am J Trop Med Hyg 278-283.
- Vijayachari P, Sugunan AP, Umapathi T, Sehgal SC (2011) Evaluation of darkground microscopy as a rapid diagnostic procedure in leptospirosis. Indian J Med Res 114: 54-58.
- https://censusindia.gov.in/nada/index.php/catalog/1434/study-description
- LERG (2011) Report of the Second Meeting of the Leptospirosis Burden Epidemiology Reference Group. World Heal Organ.
- Wolff JW (1954) The laboratory diagnosis of leptospirosis. Springfield, Ill: Thomas.
- Tubiana S, Mikulski M, Becam J, Lacassin F, Lefèvre P, et al. (2013) Risk Factors and Predictors of Severe Leptospirosis in New Caledonia. PLoS Negl Trop Dis 7.
- http://andssw1.and.nic.in/ecostat/2020/averagerainfall.pdf
- Singh SS, Vijayachari P, Sinha A, Sugunan AP, Rasheed MA, et al. (1999) Clinico-epidemiological study of hospitalized cases of severe leptospirosis. Indian J Med Res 109: 94-99.
- Vimal Raj R, Vinod Kumar K, Lall C, Vedhagiri K, Sugunan AP, et al (2018) Changing trend in the seroprevalence and risk factors of human leptospirosis in the South Andaman Island, India. Zoonoses Public Health 65: 683-689.
- Artus A, Schafer IJ, Cossaboom CM, Haberling DL, Galloway R, et al. (2022) Seroprevalence, distribution, and risk factors for human leptospirosis in the United States Virgin Islands. PLoS Negl Trop Dis 16: 1-21.
- Naing C, Reid SA, Aye SN, Htet NH, Ambu S (2019) Risk factors for human leptospirosis following flooding: A meta-analysis of observational studies. PLoS One 14: 1-15.
- Narkkul U, Thaipadungpanit J, Srisawat N, Rudge JW, Thongdee M, et al (2021) Human, animal, water source interactions and leptospirosis in Thailand. Sci Rep Nature Publishing Group UK 11: 1-13.
- Dreyfus A, Ruf MT, Mayer-Scholl A, Zitzl T, Loosli N, et al (2021) Exposure to Leptospira spp. and associated risk factors in the human, cattle and dog populations in Bhutan. Pathogens 10: 1-16.
- Calvopiña M, Vásconez E, Coral-Almeida M, Romero-Alvarez D, Garcia-Bereguiain MA (2022) Leptospirosis: Morbidity, mortality, and spatial distribution of hospitalized cases in Ecuador. A nationwide study 2000-2020. PLoS Negl Trop Dis 16: 1-16.
- Vijayachari P, Sugunan AP, Singh SS, Mathur PP (2015) Leptospirosis among the self-supporting convicts of Andaman Island during the 1920s-The first report on pulmonary haemorrhage in leptospirosis? Indian J Med Res 142: 11-22.
- Sehgal SC, Sugunan AP, Vijayachari P (2003) Leptospirosis disease burden estimation and surveillance networking in India. Southeast Asian J Trop Med Public Health 2: 170-177.
- Sahira H, Jyothi R, Bai JTR (2015) Seroprevalence of Leptospirosis among Febrile Patients: A Hospital Based Study. Journal of Academia and Industrial Research (JAIR) 3: 481-494.
Citation: Fatema A, Vijayachari P (2023) Change in Clinico-Epidemiological Pattern of Leptospirosis in Andaman & Nicobar Islands: A Retrospective Analysis of the Past Decade (2010-2020). J Community Med Public Health Care 10: 126.
Copyright: © 2023 Ambreen Fatema, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

