Characterization and Antimicrobial Investigation of Synthesized Silver Nanoparticles from Annona muricata Leaf Extracts
*Corresponding Author(s):
Aderonke Similoluwa FolorunsoDepartment Of Chemistry, Louisiana State University, Louisiana, United States
Tel:+234 8136872649,
Email:folorunaderonkesimi@gmail.com
Abstract
Green synthesis is an ecological system for the production of eco-friendly and well characterized metallic nanoparticles using plants. In this research work, the synthesis of silver nanoparticles was from the extract of Annona muricata leaves. The characterization of silver nanoparticles was done with Fourier Transform Infrared spectroscopy (FTIR), UV-vis spectrometry and transmission electron microscope to determine functional groups, shape, size and morphology of synthesized nanoparticle. The anti-antimicrobial potency of the synthesize nanoparticle was investigated via well diffusion method. The UV spectrum of synthesized nanoparticle revealed absorbance at 435.00nm which confirmed the formation of silver nanoparticle. The FTIR analysis shows bands corresponding to -OH, C=O and -NH2 nanoparticle are spherical shape of the functional group. The micrograph obtained from TEM analysis confirmed that the synthesized silver is spherical in shape. The antimicrobial investigation of the silver nanoparticles shows good antibacterial activity due to high zones of inhibition against test bacteria. This work revealed that the synthesized silver nanoparticles possess great antimicrobial potency.
Keywords
INTRODUCTION
Different nanomaterials like copper, nickel, gold, zinc and titanium has been reported [4,5]. Among all silver nanoparticles have demonstrated most effectiveness against bacteria there by showing good antimicrobial efficacy. Diverse approach such as the biological and chemical method for the synthesis of nanoparticles has been reported. Whoever, chemical methods have been reported to be very expensive and produce some toxic byproducts which are dangerous [6,7]. In quest to synthesis stable metal nanoparticles that are not expensive, safe, reliable and less toxic. Green synthesis is the application of chemistry in the scheming, developing and implementing of chemical yields to facilitate the eradication of hazardous substances that are dangerous to man and his environment [8]. Lately, the development of interest in the use of green chemistry approach in metal nanoparticles synthesis has greatly increased [9-12]. An innovative methods referred to as green/biosynthesis have been developed recently where extract of plants such as Coriandrum sativum, Petroselinum crispum [13,14]. Murraya koenigii and Ocimum sanctum were used as metal nanoparticles synthesis [10,15].
Among numerous plants Annona muricata leaves extract, was chosen for this study because of many pharmacological effects including anti-cancer, anti-inflammatory, anti-diabetic, antioxidant and hypotensive activities it possess [16,17]. The results obtained from the phytochemical screening of Annona muricata confirmed the presence of geranyl acetate, camphor, geraniol, coumarins and quercetin 3-glucoronide [18]. Also a lot of organic compounds such as xylopine, isolaureline, chlorogenic acid, chatechin, vomifoliol , roseoside, liliolide and epicatechin isolated from Annona muricata leaf has been reported to exhibit good inhibitory concentration against some bacterial has also been [18]. This study was aimed on the investigation of synthesized silver nanoparticles that is environmental friendly from the leaves of Annona muricata and evaluates their antimicrobial activity against some selected human pathogens.
MATERIALS
Extraction procedure
Characterization of synthesized nanoparticles
Antimicrobial activity of synthesized nanoparticles by well diffusion method
RESULTS AND DISCUSSION
UV-Vis spectroscopy
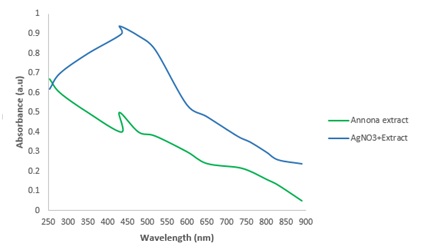
Figure 1: UV-visible absorption spectrum of silver nanoparticle produced.
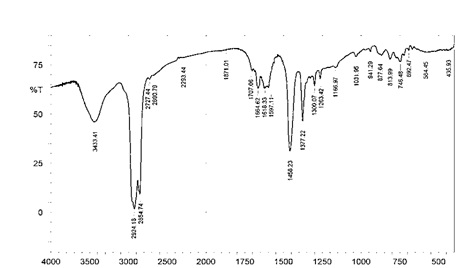
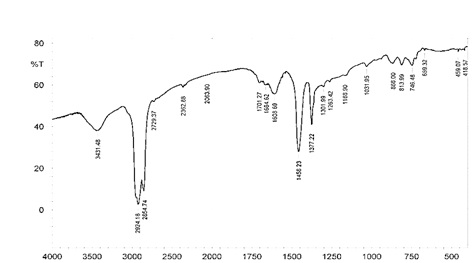
FTIR analysis
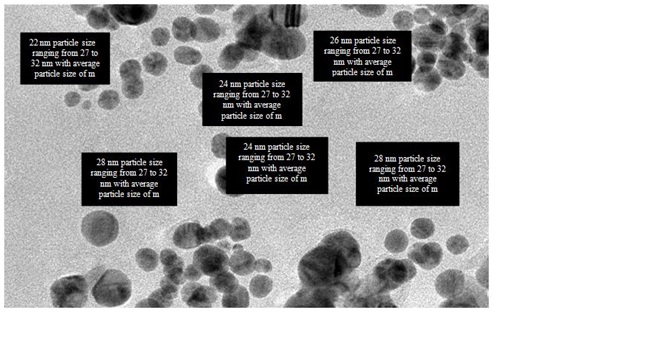
Transmission Electron Microscopic (TEM) analysis
Antimicrobial analysis of synthesized silver nanoparticle
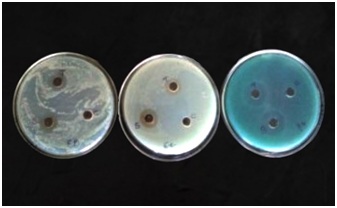
Figure 4: Antibacterial activity of synthesized silver nanoparticles from Annona muricata leaf.
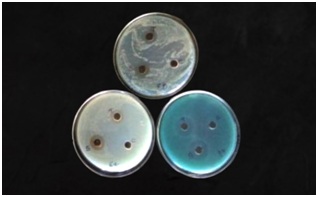
Figure 5: Antifungal activity of synthesized silver nanoparticles from Annona muricata leaf.
|
Bacterial test pathogens |
Inhibition zone of silver nanoparticle(mm) |
|||
|
Concentration (μg) |
Tetracycline |
|||
|
50 |
75 |
100 |
||
|
Pseudomonas aeruginosa |
12 |
17 |
22 |
28 |
|
Klebsiella pneumonia |
10 |
15 |
20 |
23 |
|
Bacillus subtilis |
13 |
16 |
18 |
10 |
|
Fungi test pathogens |
Concentration (μg) |
Chloramphenicol |
||
|
50 |
75 |
100 |
||
|
Aspergillus flaws |
12 |
13 |
15 |
24 |
|
Penicillium camemeri |
5 |
15 |
17 |
20 |
|
Candida albican |
13 |
17 |
19 |
23 |
The synthesized silver nanoparticles showed high inhibition zones against selected bacterial namely; Pseudomonas aeruginosa, Klebsiella pneumonia and Bacillus subtilis especially at 100μg where the Inhibition zones are 22, 20 and 18mm as showed in table 1. Lowest inhibition zone was observed when 50μg of silver nanoparticle was used against Klebsiella pneumonia while highest inhibition zone was observed when 100μg of silver nanoparticle was used against Pseudomonas aeruginosa. The potency of the synthesized nanoparticle increases as concentrations of synthesized nanoparticles used against the test bacterial increase due to the observed zones of inhibition. The antimicrobial assay used in this study shows a better inhibition zones when compared with inhibition zones obtained from the antimicrobial assay used by on Juglan regia extract and that used by on some novel antibiotics [29,30]. The inhibition zones in this study were higher than that obtained from the study of at concentration of 50, 75 and 100μg [28]. The synthesized silver nanoparticles compete better than the control (tetracycline) at all concentration against Bacillus subtilis. This suggests that silver nanoparticles synthesized using Annona muricata can inhibit both gram positive and gram negative bacteria. This is evidence that the synthesized silver nanoparticle exhibit good antibacterial activity.
CONCLUSION
REFERENCES
- Logeswari P, Silambarasan S, Abraham J (2013) Ecofriendly synthesis of silver nanoparticles from commercially available plant powders and their antibacterial properties. Scientia Iranica 20: 1049-1054.
- Gajbhiye M, Kesharwani J, Ingle A, Gade A, Rai M (2009) Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine 5: 382-386.
- Bhatt P, Negi PS (2012) Antioxidant and antibacterial activities in the leaf extracts of Indian Borage (Plectranthus amboinicus). Food and Nutrition Sciences 3: 146-152.
- Gu H, Ho PL, Tong E, Wang L, Xu B (2003) Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano Letter 3: 1261-1263.
- Schabes-Retchkiman P, Canizal G, Herrera-Becerra R, Cangas CZ, Liu HB, et al. (2006) Biosynthesis and characterization of Ti/Ni bimetallic nanoparticles. Optical Materials 29: 95-99.
- Phanjom P, Ahmed G (2015) Biosynthesis of silver nanoparticles by Aspergillus oryzae (MTCC No. 1846) and its characterizations. Nanoscience and Nanotechnology 5: 14-21.
- Vinod VT, Saravanan P, Sreedhar B, Devi DK, Sashidhar RB (2011) A facile synthesis and characterization of Ag, Au and Pt nanoparticles using a natural hydrocolloid gum kondagogu (Cochlospermum gossypium). Colloids Surf B Biointerfaces 83: 291-298.
- Kumar SH, Prasad CH, Venkateswarlu S, Venkateswarlu P, Jyothi NVV (2015) Green Synthesis of Silver Nanoparticles Using Aqueous Solution of Syzygium cumini Flowering Extract and its Antimicrobial Activity. Indian Journal of Advances in Chemical Science 3: 299-303.
- Geethalakshmi R, Sarada DVL (2010) Synthesis of plant-mediated silver nanoparticles using Trianthema decandra extract and evaluation of their anti microbial activities. International Journal Engineering Science and Technology 2: 970-975.
- Christensen L, Vivekanandhan S, Misra M, Mohanty AK (2011) Biosynthesis Of Silver Nanoparticles Using Murraya koenigii (Curry Leaf): An Investigation On The Effect Of Broth Concentration In Reduction Mechanism And Particle Size. Advance Material Letter 2: 429-434.
- Arokiyaraj S, Arasu MV, Vincent S, Prakash NU, Choi SH, et al. (2014) Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L and its antibacterial and cytotoxic effects: An in vitro study. Int J Nanomedicine 9: 379-388.
- Gandhi N, Sirisha D, Sharma VC (2014) Microwave-Mediated Green Synthesis of Silver Nanoparticles Using Ficus elastica Leaf Extract and Application in Air Pollution Controlling Studies. International Journal of Engineering Research and Applications 4: 61.
- Singhal G, Bhavesh R, Kasariya K, Sharma AR, Singh RP (2011) Biosynthesis of silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. Journal of Nanoparticle Research 13: 2981-2988.
- Roy K, Sarkar CK, Ghosh CK (2015) Plant-mediated synthesis of silver nanoparticles using parsley (Petroselinum crispum) leaf extract: spectral analysis of the particles and antibacterial study. Applied Nanoscience 5: 945-951.
- Sathyavathi R, Krishna MB, Rao SV, Saritha R, Rao DN (2010) Biosynthesis of silver nanoparticles using Coriandrum sativum leaf extract and their application in nonlinear optics. Advance Science Letter 3: 138-143.
- Muthu S, Durairaj B (2015) Evaluation of antioxidant and free radical scavenging activity of Annona muricata. European Journal of Experimental Biology 5: 39-45.
- de Sousa OV, Vieira GD, de Jesus R G de Pinho J, Yamamoto CH, Alves MS (2010) Antinociceptive and Anti-Inflammatory Activities of the Ethanol Extract of Annona muricata L. Leaves in Animal Models. Int J Mol Sci 11: 2067-2078.
- Patel S, Patel JK (2016) A review on a miracle fruits of Annona muricata. Journal of Pharmacognosy and Phytochemistry 5: 137-148.
- Espenti CS, Rao KSVK, Rao KM (2016) Bio-synthesis and characterization of silver nanoparticles using Terminalia chebula leaf extract and evaluation of its antimicrobial potential. Material Letter 174: 129-133.
- El-Nour KMMA, Eftaiha A, Al-Warthan A, Ammar RAA (2010) Synthesis and applications of silver nanoparticles. Arabian Journal Chemistry 3: 135-140.
- Bose D, Chatterjee S (2016) Biogenic synthesis of silver nanoparticles using guava (Psidium guajava) leaf extract and its antibacterial activity against Pseudomonas aeruginosa. Applied Nanoscience 6: 895-901.
- Namratha N, Monica PV (2013) Synthesis of silver nanoparticles using Azadirachta indica (Neem) extract and usage in water purification. Asian Journal Pharmaceutical Technology 3: 170-174.
- Agrawal P, Mehta K, Vashisth P, Sudarshan, Bhat P, et al. (2014) Green Synthesis of Silver Nanoparticles and Their Application in Dental Filling Material. International Journal of Innovative Science Engineering and Technology 3: 13038-13052.
- Gavhane AJ, Padmanabhan P, Kamble SP, Jangle SN (2012) Synthesis of silver nanoparticles using extract of Neem leaf and Triphala and evaluation of their antimicrobial activities. Int J Pharm Bio Sci 3: 88-100.
- Khan MZH, Tareq FK, Hossen MA, Roki MNAM (2018) Green Synthesis and Characterization of Silver Nanoparticles Using Coriandrum sativum Leaf Extract. Journal of Engineering Science and Technology 1: 158-166.
- Sapna GY, Sudeep HP, Pratima P, Vineeetha N, Shahida K, et al. (2018) Green synthesis of silver nanoparticles from plant sources and evaluation of their antimicrobial activity. National Conference on 'Advanced Analytical Tools for Materials Characterization 4: 133-139.
- Kumar S, Daimary RM, Swargiary M, Brahma A, Kumar S, et al. (2013) Biosynthesis of silver nanoparticles using Premna herbacea leaf extract and evaluation of its antimicrobial activity against bacteria causing dysentery. International Journal of Pharma and Bio Sciences 4: 378-384.
- Niveditha K, Sukirtha TH (2018) Green Synthesis, Characterization and Antimicrobial Activity of Silver Nanoparticles from Plectranthus amboinicus Plant Extracts. Indian Journal of Medical Research and Pharmaceutical Sciences 5: 41-51.
- Iqbal J, Siddiqui R, Kazmi SU, Khan NA (2013) A Simple Assay to Screen Antimicrobial Compounds Potentiating the Activity of Current Antibiotics. BioMed Research International Pg no: 1-4.
- Butler MS, Buss AD (2006) Natural products--the future scaffolds for novel antibiotics? Biochem Pharmacol 71: 919-929.
Citation: Akintelu SA, Folorunso AS (2019) Characterization and Antimicrobial Investigation of Synthesized Silver Nanoparticles from Annona muricata Leaf Extracts. J Nanotechnol Nanomed Nanobiotechnol 6: 022.
Copyright: © 2019 Sunday Adewale Akintelu, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.