
Chemical Constituents from Thalictrum ramosum
*Corresponding Author(s):
Qing-Wen ZhangState Key Laboratory Of Quality Research In Chinese Medicine, Institute Of Chinese Medical Sciences, University Of Macau, Macao SAR, China
Tel:+853 88224879,
Email:qwzhang@umac.mo
Abstract
Thalictrum ramosum is a plant from Thalictrum genus, and is also one substitute of Coptidis Rhizoma in Chinese folk medicine. Phytochemical investigation on the EtOH extract of T. ramosum led to the isolation of 9 compounds. Their structures were elucidated as berberine (1), columbamine (2), thalidastine (3), magnoflorine (4), 1-(4-hydroxy-3-methoxy)-phenyl-2-[4-(1,2,3-trihydroxy-propyl)-2-methoxy]-phenoxy-1,3-propandiol (5), citroside B (6), glochidionionoside A (7), apigenin 6,8-di-C-β-D-xylopyranoside (8) and hydrangeifolin I (9) through 1D and 2D NMR, MS experiments and comparison with literature data. All compounds are isolated from this plant for the first time.
Keywords
INTRODUCTION
ramosum, is one of the substitutes of Coptidis Rhizoma. Recently, we reported some cycloartane triterpene saponins from the plants in Thalictrum genus including this herb [1-3]. Further phytochemical investigations on the n-BuOH-soluble fraction of the EtOH extract of T. ramosum has led to the purification of 9 compounds and their structures were elucidated as: berberine (1), columbamine (2), thalidastine (3), magnoflorine (4), 1-(4-hydroxy-3-methoxy)-phenyl-2-[4-(1,2,3-trihydroxy-propyl)-2-methoxy]-phenoxy-1,3-propandiol (5), citroside B (6), glochidionionoside A (7), apigenin 6,8-di-C-β-D-xylopyranoside (8), hydrangeifolin I (9) by means of UV, IR, MS, NMR, HMBC, HSQC, 1H-1H COSY, ROESY, etc.
EXPERIMENTAL
General experimental procedures
Plant material
Isolation and purification
The n-BuOH solution was concentrated and gives a residue (344 g), which was separated by a silica gel column using CHCl3-MeOH (1:0 → 1:1) as eluent, affording 14 fractions (Fr. 1-14). Compound 1 was obtained by recrystallization of Fr. 7. Fr. 8 (8.6 g) was purified by a silica gel column using EtOAc-MeOH (10:1→5:1) as eluent to afford Fr. 8.1 - Fr. 8.7 (0.7 g). The same elution method was applied to Fr. 8.7 to afford Fr. 8.7.1 which was further purified by Sephadex LH-20 chromatography with MeOH to afford compound 2 (2.6 mg). Fr. 12 was purified by an ODS chromatographic method elueted with MeOH-Water (10%→90%) to afford Fr. 12.1 - Fr. 12.4. Fr. 12.3 was further purified by Sephadex LH-20 chromatography using MeOH-Water (90:10) as eluent to afford Fr. 12.1 - Fr. 12.3. Compound 4 (22.5 mg) and 9 (24.1 mg) was isolated from Fr. 12.3.3 by Prep-HPLC using CH3CN-H2O (15:85) as eluent.
Fr. 9 (25 g) was purified by a silica gel column using CDCl3-MeOH (10:1→1:1) as eluent to afford Fr. 9.1 - Fr. 9.7. Fr. 9.6 was purified by an ODS chromatographic method using MeOH-Water (10% → 90%) as eluent to afford Fr. 9.6.1 - Fr. 9.6.3. Prep-HPLC was applied to Fr. 9.6.2 using CH3CN-H2O (25:75) as eluent to afford compound 3 (50 mg). Fr. 9.5 was purified by an ODS chromatographic method using MeOH-Water (10% → 90%) and then further purified by Sephadex LH-20 chromatography using MeOH-H2O (90:10) as eluent to afford Fr. 9.5.1.1 and Fr. 9.5.1.2. Prep-HPLC was applied to Fr. 9.5.1.1 using CH3CN-H2O (18:82) as eluent to afford compound 6 (12.1 mg) and 7 (2.0 mg). Compound 5 (10.1 mg) was isolated from Fr. 9.5.1.2 by Prep-HPLC using CH3CN-H2O (13:87) as eluent. Fr-14 was chromatographedon a macropourous resin column using EtOH-Water (0% → 95%) as eluent to afford Fr. 14. 1 - Fr. 14.6. Fr. 14.4 was purified by an ODS chromatographic method using MeOH-Water (10% → 90%) as eluent to afford Fr. 14.4.1 - Fr. 14.4.6. Prep-HPLC was applied to Fr. 14.4.5 using CH3CN-H2O (12:88) as eluent to afford compound 8.
RESULTS AND DISCUSSION
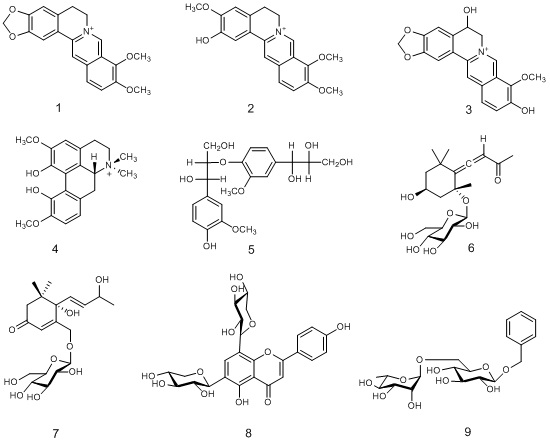
Compound 1 yellow crystals: C20H18NO4+, ESI-MS m/z336 [M]+,1H-NMR (DMSO-d6, 400 MHz) δ:9.87 (1H, s, H-8), 8.91 (1H, s, H-13), 8.19 (1H, d, J = 9.1 Hz, H-11), 7.98 (1H, d, J = 9.1 Hz, H-12), 7.77 (1H, s, H-1), 7.08 (1H, s, H-4), 6.17 (2H, s, OCH2O), 4.93 (2H, t, J = 6.2 Hz, H-6), 4.10 (3H, s, 9-OCH3), 4.07 (3H, s, 10-OCH3), 3.21 (2H, t, J = 6.2 Hz, H-5). 13C NMR (DMSO, 100 MHz) δ: 150. 4 (C-10), 149.8 (C-3), 147.7 (C-2), 145.4 (C-9), 143.7 (C-8), 137.5 (C-14), 133.0 (C-12a), 130.6 (C-4a), 126.8 (C-11), 123.5 (C-12), 121.4 (C-8a), 120.4 (C-14a), 120.1 (C-13), 108.4 (C-4), 105.4 (C-1), 102.5 (OCH2O), 61.9 (9-OCH3), 57.0 (10-OCH3), 55.2 (C-6), 26.3 (C-5). These data were consistent those of berberine in the literature [8]. Thus, compound 1 was elucidated as berberine.
Compound 2 yellow powder: 1H NMR (CD3OD, 400 MHz) δ: 9.74 (1H, s, H-8), 8.63 (1H, s, H-13), 8.09 (1H, d, J = 9.1 Hz, H-11), 7.99 (1H, d, J = 9.1 Hz, H-12), 7.55 (1H, s, H-1), 7.01 (1H, s, H-4), 5.95 (2H, m, H-6), 4.20 (3H, s, 9-OCH3), 4.10 (3H, s,10-OCH3) 3.96 (3H, s, 2-OCH3), 3.23 (2H, m, H-5). 13C NMR (CD3OD, 100 MHz) δ: 152.5 (C-9), 151.9 (C-2), 148.3 (C-3), 146.3 (C-8), 145.7 (C-10), 140.0 (C-13a), 135.3 (C-12a), 128.5 (C-4a), 128.1 (C-12), 124.4 (C-11), 123.3 (C-14), 121.1 (C-13), 120.7 (C-8a), 113.2 (C-4), 112.0 (C-1), 62.5 (9-OCH3), 57.7 (10-OCH3), 57.5 (3-OCH3), 56.7 (C-6), 27.8 (C-5). These data were consistent those of columbamine in the literature [9]. Compound 2 was elucidated as columbamine.
Compound 3 yellow powder: 1H NMR (DMSO-d6, 300 MHz) δ: 9.89 (1H, br s, H-8), 8.97 (1H, s, H-13), 7.90 (1H, br s, H-11), 7.90 (1H, br s, H-12), 7.85 (1H, s, H-1), 7.16 (1H, s, H-4), 6.20 (2H, s, OCH2O), 5.11 (1H, d, J = 13.0 Hz, H-5), 5.01 (1H, br s, H-6a), 4.84 (1H, d, J = 13.0 Hz, H-6b), 4.08 (3H, s, 9-OCH3). 13C NMR (DMSO-d6, 75 MHz) δ: 149.7 (C-3), 149.6(C-10), 148.6 (C-2), 145.0 (C-8), 141.3(C-9), 136.4 (C-14), 132.6 (C-12a), 132.2 (C-11), 131.3 (C-4a), 123.7 (C-12), 122.4 (C-8a), 120.5 (C-14a), 119.8 (C-13), 108.1 (C-4), 105.4 (C-1), 102.3 (OCH2O), 61.4 (C-6), 62.9 (9-O CH3), 60.8 (C-5). These data were consistent those of thalidastine in the literature [10]. So, compound 3 was elucidated as thalidastine.
Compound 4 yellow amorphous powder: C20H24NO4+, ESI-MS m/z 342 [M+H]+, 1H NMR (CD3OD, 300 MHz) δ : 6.63 (1H, d, J = 7.2 Hz, H-9), 6.40 (1H, d, J = 7.2 Hz, H-8), 6.40 (1H, br s, H-3), 3.81 (3H, s, 10-OCH3), 3.72 (3H, s, 2-OCH3), 3.68 (1H, m, H-6a), 3.38 (1H, m, H-5β), 3.18 (3H, br s, N-OCH3β), 3.06 (1H, m, H-4α), 2.90 (1H, m, H-5α), 2.85 (1H, m, H-7β), 2.72 (3H, br s, N-OCH3α), 2.48 (1H, m, H-4β), 2.30 (1H, m, H-7α). 13C NMR (CD3OD, 75 MHz) δ : 153.0 (C-2a), 151.7 (C-10), 150.7 (C-1), 149.7 (C-11), 126.0 (C-7a), 123.5 (C-11a), 123.4 (C-11b), 121.0 (C-6b), 117.0 (C-8), 115.8 (C-3a), 110.5 (C-9), 109.3 (C-3), 70.9 (C-6a), 62.2 (C-5), 56.3 (2-OCH3), 55.9 (10-OCH3), 53.8 (N-CH3α), 43.5 (N-CH3β), 31.6 (C-7), 24.6 (C-4). The above spectral data were identical to those of magnoflorine reported in the reference [8]. Thus compound 4 was determined to be magnoflorine.
Compound 5 amorphous light-yellowish powder: C20H26O9, ESI-MS m/z 433[M+Na]+, 1HNMR (CD3OD, 500 MHz) δ : 7.04 (1H, d, J = 1.8 Hz, H-2), 7.02 (1H, d, J = 1.7 Hz, H-2′), 6.90 (1H, d, J = 8.2 Hz, H-5′), 6.85 (1H, dd, J = 8.2, 1.6 Hz, H-6′), 6.84 (1H, dd, J = 8.2, 1.6 Hz, H-6), 6.74 (1H, d, J = 8.1 Hz, H-5), 4.84 (1H, d, J = 5.8 Hz, H-7), 4.56 (1H, d, J = 6.0 Hz, H-7′), 4.36 (1H, dt, J = 5.7, 3.8 Hz,H-8), 3.87 (1H, dd, J = 12.0, 3.7 Hz, H-9a), 3.83 (3H, s, 3-OMe), 3.83 (3H, s, 3′-OMe), 3.80 (1H, dd, J = 12.0, 3.7 Hz,H-9b), 3.67 (1H, dt, J = 5.9, 4.1 Hz,H-8′), 3.50 (1H, dd, J = 11.2, 4.0 Hz, H-9′a), 3.38 (1H, dd, J = 11.2, 4.1 Hz,H-9′b); 13C NMR (CD3OD, 500 MHz) δ: 151.7 (C-3′), 148.7 (C-3), 148.6 (C-4′), 147.0 (C-4), 137.7 (C-1′), 134.2 (C-1), 121.0 (C-6), 120.5 (C-6′), 118.7 (C-5′), 115.6 (C-5), 112.3 (C-2′), 111.8 (C-2), 86.2 (C-8), 77.4 (C-8′), 75.1 (C-7′), 74.1 (C-7), 64.2 (C-9′), 62.2 (C-9), 56.5 (3′-OCH3), 56.3 (3-OCH3). These data were consistent those in the literature [11]. Therefore, compound 5 was elucidated as 1-(4-hydroxy-3-methoxy)-phenyl-2-[4-(1,2,3-trihydroxy-propyl)-2-methoxy]-phenoxy-1,3-propandiol.
Compound 6 amorphous powder: C19H30O8, ESI-MS m/z 409 [M+Na]+,1H NMR (Pyridine-d5, 500 MHz) δ : 6.04 (1H, s, H-8), 5.14 (1H, d, J = 7.7 Hz, H-1′), 5.10 (1H, m, H-3), 4.48 (1H, dd, J = 11.5, 2.2 Hz, H-6′a), 4.31 (1H, dd, J = 11.5, 5.3 Hz, H-6′b), 3.99 (1H, t, J = 8.1 Hz, H-2′), 3.62 (1H, s), 3.04 (1H, dd, J = 13.6, 2.0 Hz, H-4a), 2.30 (1H, dd, J = 12.5, 2.4 Hz, H-2a), 2.23 (3H, s, H-10), 1.79 - 1.72 (2H, m, H-2b,4b), 1.70 (3H, s, H-13), 1.69 (3H, s, H-12) and 1.21 (3H, s, H-11). 13C NMR (Pyridine-d5, 125 MHz) δ : 212.0 (C-7), 198.1 (C-9), 119.3 (C-6), 101.4 (C-1′), 99.0 (C-8), 79.7 (C-5), 78.7 (C-3′), 78.7 (C-5′), 75.7 (C-2′), 72.2 (C-4′), 63.3 (C-3), 63.0 (C-6′), 50.8 (C-2), 47.9 (C-4), 36.9 (C-1), 32.8 (C-12), 30.3 (C-11), 27.6 (C-10) and 27.0 (C-13). These data were consistent those in the literature [12]. Compound 6 was elucidated as citroside B.
Compound 7 pale yellow amorphous powder: 1H NMR (DMSO-d6, 600 MHz) δ : 6.03 (1H, dd, J = 15.6 Hz, H-7), 5.75 (1H, s, H-4), 5.71 (1H, dd, J = 15.5, 6.1 Hz, H-8), 4.98 (2H, s, H-13), 4.32 (1H, m, H-9), 4.13 (1H, d, J = 7.8 Hz, H-1′), 2.55 (1H, d, J = 16.6 Hz, H-2a), 2.05 (1H, d, J = 16.4 Hz, H-2b), 1.82 (3H, d, J = 1.3 Hz, H-10), 0.94 (3H, s, H-11), 0.92 (3H, s, H-12). 13C NMR (DMSO-d6, 150 MHz) δ : 200.8 (C-3), 166.9 (C-5), 132.6 (C-7), 131.3 (C-8), 127.0 (C-4), 104.5 (C-1′), 80.0 (C-6), 77.9 (C-5′), 77.8 (C-3′), 75.1 (C-2′), 74.8 (C-13), 72.0 (C-9), 71.5 (C-4′), 62.7 (C-6′), 50.7 (C-2), 42.4 (C-1), 23.5 (C-11), 24.6 (C-12) and 19.7 (C-10). These data were consistent those in the literature [13]. Compound 7 was elucidated as glochidionionoside A.
Compound 8 yellow powder: 1H NMR (DMSO-d6, 500 MHz) δ : 13.76 (1H, s, 5-OH), 7.95 (2H, d, J = 8.8 Hz, H-2′, 6′), 6.93 (2H, d, J = 8.8 Hz, H-3′,5′), 6.81 (1H, s, H-3), 4.87 (1H, d, J = 6.8 Hz, H-1), 4.63 (1H, d, J = 9.7 Hz, H-1?). 13C NMR (DMSO-d6, 125MHz) δ : 163.6 (C-2), 102.6 (C-3), 182.1 (C-4), 161.2 (C-7), 121.5 (C-1′), 128.6 (C-2′, 6′), 115.9 (C-3′, 5′), 159.5 (C-4′), 75.2 (C-1″), 71.4 (C-2″), 78.7 (C-3″), 69.7 (C-4″), 70.5 (C-5″), 74.6 (C-1?), 70.9 (C-2?), 78.8 (C-3?), 69.9 (C-4?) and 70.3 (C-5?). These data were consistent those in the literature [14]. Compound 8 was elucidated as apigenin 6,8-di-C-β-D-xylopyranoside.
Compound 9 pale yellow amorphous powder: C19H28O10, m/ z 439 [M+Na]+, 1H NMR (CD3OD, 300 MHz) δ : 7.38 (2H, d, J = 6.7 Hz, H-2, 6), 7.30 (2H, m, H-3, 5), 7.23 (1H, m, H-4), 4.75 (1H, d, J = 11.8 Hz, H-7a), 4.82 (1H, s, H-1''), 4.60 (1H, d, J = 11.8 Hz, H-7b), 4.29 (1H, d, J = 7.6 Hz, H-1'), 3.96 (1H, dd, J = 11.2 Hz, H-6'b), 3.84 (1H, m, H-6'a), 1.23 (3H, d, J = 6.2 Hz, H-6''). 13C NMR (CD3OD, 75 MHz) δ : 138.8 (C-1), 129.3 (C-2, 3, 5, 6), 128.8 (C-4), 103.1 (C-1'), 102.3 (C-1''), 78.0 (C-3'), 76.9 (C-5'), 75.1 (C-2'), 74.0 (C-4''),72.3 (C-3''), 72.2 (C-2''),71.8 (C-7), 71.7 (C-4'), 69.8 (C-5''), 68.1 (C-6') and 18.1 (C-6''). These data were consistent those in the literature [15]. So, compound 9 was elucidated as hydrangeifolin I (Figure 1).
Even though Thalictrum showed different morphological features from Coptidis Rhizoma, many Thalictrum plants have a very close name to Coptidis Rhizoma (horsetail-Coptidis-Rhizoma) and were used as the succedaneum of Coptidis Rhizoma for the treatment of inflammation and infectious diseases for thousands years in China. As is known, bioactivities and functions of herbal medicine are closely related with its chemical composition. The phytochemical investigation in current study and reported literatures showed that the plants of those two genera (Thalictrum and Copdis) contain some same constituents such as berberine-type alkaloid [16-18]. Berberine-type alkaloids showed potent anti-inflammatory and antibacterial activities [19-23]. Compounds berberine (1), columbamine (2) and magnoflorine (3) has also been reported from Coptidis Rhizoma before [10]. Those compounds occurrence in both genera led them to have some same functions and explained why those Thalictrumplants were used as the substitute of Coptidis Rhizoma for the treatment of inflammatory and infectious diseases. Furthermore, together with Thalictrum genus, Coptis genus, the source of Coptidis Rhizoma, also belongs to the subfamily Thalictroideae. Researches on the chemical composition and pharmacological activities should be beneficial for the further development and application of T. ramosum and other plants of Thalictrum genus.
The n-BuOH solution was concentrated and gives a residue (344 g), which was separated by a silica gel column using CHCl3-MeOH (1:0 → 1:1) as eluent, affording 14 fractions (Fr. 1-14). Compound 1 was obtained by recrystallization of Fr. 7. Fr. 8 (8.6 g) was purified by a silica gel column using EtOAc-MeOH (10:1→5:1) as eluent to afford Fr. 8.1 - Fr. 8.7 (0.7 g). The same elution method was applied to Fr. 8.7 to afford Fr. 8.7.1 which was further purified by Sephadex LH-20 chromatography with MeOH to afford compound 2 (2.6 mg). Fr. 12 was purified by an ODS chromatographic method elueted with MeOH-Water (10%→90%) to afford Fr. 12.1 - Fr. 12.4. Fr. 12.3 was further purified by Sephadex LH-20 chromatography using MeOH-Water (90:10) as eluent to afford Fr. 12.1 - Fr. 12.3. Compound 4 (22.5 mg) and 9 (24.1 mg) was isolated from Fr. 12.3.3 by Prep-HPLC using CH3CN-H2O (15:85) as eluent.
Fr. 9 (25 g) was purified by a silica gel column using CDCl3-MeOH (10:1→1:1) as eluent to afford Fr. 9.1 - Fr. 9.7. Fr. 9.6 was purified by an ODS chromatographic method using MeOH-Water (10% → 90%) as eluent to afford Fr. 9.6.1 - Fr. 9.6.3. Prep-HPLC was applied to Fr. 9.6.2 using CH3CN-H2O (25:75) as eluent to afford compound 3 (50 mg). Fr. 9.5 was purified by an ODS chromatographic method using MeOH-Water (10% → 90%) and then further purified by Sephadex LH-20 chromatography using MeOH-H2O (90:10) as eluent to afford Fr. 9.5.1.1 and Fr. 9.5.1.2. Prep-HPLC was applied to Fr. 9.5.1.1 using CH3CN-H2O (18:82) as eluent to afford compound 6 (12.1 mg) and 7 (2.0 mg). Compound 5 (10.1 mg) was isolated from Fr. 9.5.1.2 by Prep-HPLC using CH3CN-H2O (13:87) as eluent. Fr-14 was chromatographedon a macropourous resin column using EtOH-Water (0% → 95%) as eluent to afford Fr. 14. 1 - Fr. 14.6. Fr. 14.4 was purified by an ODS chromatographic method using MeOH-Water (10% → 90%) as eluent to afford Fr. 14.4.1 - Fr. 14.4.6. Prep-HPLC was applied to Fr. 14.4.5 using CH3CN-H2O (12:88) as eluent to afford compound 8.
ACKNOWLEDGEMENT
REFERENCES
- Flora of China (1979) Editorial Committee of the Administration Bureau of Chinese Plant Medicine. Flora of China; Science Press 27: 502.
- Sheng L, Qing SY, Dongke M, Jianyong L, Jia G, et al. (2014) Research progress on chemical constituents and pharmacological activities of alkaloids from plants in Thalictrum L. Drugs & Clinic 29.
- Zuoguang L, Yongming L, Jie C (2001) Survey on Research of Thalictrum Genus Plants in Chemistry and Pharmacody. Journal of Jiangxi College of Traditional Chinese Medicine 93-95.
- Qi C, Anlong X (2000) Antitumor agents from the plants of Thalictrum Journal of the graduates Sun-Yat university (Natural Sciences).
- Meng F-C, Yuan C, Huang X-J, Wang W-J, Lin L-G, et al. (2016) New cycloartane triterpene glycosides from Thalictrum ramosum. Phytochemistry Letters 15: 108-112.
- Zhang X-T, Wang L, Ma S-W, Zhang Q-W, Liu Y, et al. (2013) New cycloartane glycosides from the aerial part of Thalictrum fortunei. J Nat Med 67: 375-380.
- Zhang XT, Ma SW, Jiao HY, Zhang QW (2012) Two new saponins from Thalictrum fortunei. J Asian Nat Prod Res 14: 327-332.
- Jung HA, Yoon NY, Bae HJ, Min BS, Choi JS (2008) Inhibitory activities of the alkaloids from Coptidis Rhizoma against aldose reductase. Arch Pharm Res 31: 1405-1412.
- Shamma M, Dudock BS (1965) Thalictrum Alkaloids II. Thalidastine. Tetrahedron letters6: 3825-3828.
- Xue-gai L, Li-guo Y, Li-xia C, Feng Q (2012) Chemical constituents from the decoction of Coptis chinensis Journal of Shenyang Pharmaceutical University.
- Greca MD, Ferrara M, Fiorentino A, Monaco P, Previtera L (1998) Antialgal compounds from Zantedeschia aethiopica. Phytochemistry 49: 1299-1304.
- Umehara K, Hattori I, Miyase T, Ueno A, Hara S, et al. (1988) Studies on the Constituents of Leaves of Citrus unshiu Chemical and Pharmaceutical Bulletin 36: 5004-5008.
- Otsuka H, Kijima H, Hirata E, Shinzato T, Takushi A, et al. (2003) Glochidionionosides A-D: Megastigmane Glucosides from Leaves of Glochidion zeylanicum (GAERTN.) A. JUSS. Chem Pharm Bull (Tokyo) 51: 286-290.
- Qu L, Ruan J-Y, Jin L-J, Shi W-Z, Li X-X, et al. (2017) Xanthine oxidase inhibitory effects of the constituents of Chrysanthemum morifolium Phytochemistry Letters 19: 39-45.
- Hamerski L, Bomm MD, Silva DHS, Young MCM, Furlan M, et al. (2005) Phenylpropanoid glucosides from leaves of Coussarea hydrangeifolia (Rubiaceae). Phytochemistry 66: 1927-1932.
- Hao DC, Gu XJ, Xiao PG (2015) Research progress on chemical constituents and bioactivity of Thalictrum and its phylogenetic tree. Shizhen Guoyi Guoyao 26: 1731-1733.
- Khamidullina EA, Gromova AS, Lutsky VI, Owen NL (2006) Natural products from medicinal plants: non-alkaloidal natural constituents of the Thalictrum Natural product reports 23: 117-129.
- Kuang YH, Zhu JJ, Wang ZM, Peng XJ (2008) Research progress on chemical constituents and quality control of plants from Coptis Chin Pharm J 143: 1121-1125.
- Jeyakkumar P, Liu HB, Gopala L, Cheng Y, Peng XM, et al. (2017) Novel benzimidazolyl tetrahydroprotoberberines: Design, synthesis, antimicrobial evaluation and multi-targeting exploration. Bioorg Med Chem Lett 27: 1737-1743.
- Duan J-R, Liu H-B, Jeyakkumar P, Gopala L, Li S, et al. (2017) Design, synthesis and biological evaluation of novel Schiff base-bridged tetrahydroprotoberberine triazoles as a new type of potential antimicrobial agents. MedChemComm 8: 907-916.
- Wang Z, Chen Z, Yang S, Wang Y, Huang Z, et al. (2014) Berberine ameliorates collagen-induced arthritis in rats associated with anti-inflammatory and anti-angiogenic effects. Inflammation 37: 1789-1798.
- Yu SM, Cho H, Kim GH, Chung KW, Seo SY, et al. (2016) Berberine induces dedifferentiation by actin cytoskeleton reorganization via phosphoinositide 3-kinase/Akt and p38 kinase pathways in rabbit articular chondrocytes. Exp Biol Med (Maywood) 241: 800-807.
- Zhou Y, Tao H, Li Y, Deng M, He B, et al. (2016) Berberine promotes proliferation of sodium nitroprusside-stimulated rat chondrocytes and osteoarthritic rat cartilage via Wnt/β-catenin pathway. Eur J Pharmacol 789: 109-118.
Citation: Meng F-C, Yuan C, Wang W-J, Huang X-J, Zhang X-T, et al. (2017) Chemical Constituents from Thalictrum ramosum. J Altern Complement Integr Med 3: 043.
Copyright: © 2017 Fan-Cheng Meng, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

