
Chronic Allergen Exposure Might Shift Allergic Mechanisms in Horses with Insect Bite Hypersensitivity
*Corresponding Author(s):
Antonia Fettelschoss GabrielDepartment Of Dermatology, University Hospital Zurich, Wagistrasse 12, 8952 Schlieren, Switzerland
Tel:+41 43 253 30 97,
Email:antonia.gabriel@usz.ch
Abstract
Insect bite hypersensitivity (IBH) is a chronic recurrent dermatitis characterized by intense itching and represents the most frequent allergic skin disease in horses. The disease is, on one hand, characterized by allergic pruritus mediated by IL-31; but on the other hand, it is also IgE type I-mediated, however, with involvement of eosinophil-driven delayed type hypersensitivity (DTH) IVb characteristics. In the latter case, the dominant role of eosinophils might also be explained by an accumulation of IL-5-positive Th2 cells, which might be mediated and induced by the chronic allergen exposure during the life of an allergic individual. By differentiating between the two types of hypersensitivity reaction in IBH, we place particular emphasis on a potential shift of the allergic mechanism through chronic allergen exposure during the natural course of disease. Accordingly, we suggest individual allergic state should be considered when treating an allergic patient to enhance treatment success.
INTRODUCTION
IBH (also known as “summer eczema” or “sweet itch”) represents the most common allergy in horses and on average affects more than 10% of the equine population worldwide with varying incidences in different geographical regions ranging from 3% up to 60% [1]. This highly debilitating disease manifests as a chronic seasonally relapsing pruritic dermatitis, and is caused by insect bites mainly of the genus Culicoides [2]. Skin lesions typically occur along the dorsal and ventral bodyline and do look eczema-like, with hyperkeratotic scales, excoriations due to scratching, bloody crust formation, resulting in lichenification. Histopathological hallmarks are oedema, mast cell degranulation, perivascular infiltration of eosinophils, dendritic cells and CD4+ T cells leading to dermal fibrosis and hyperplasia and hyperkeratosis of the epidermis during chronicity [3,4].
The important underlying allergic mechanism of IBH not only involves Interleukin (IL)?31, a Th2 cell–derived cytokine that mediates intense pruritus by targeting peripheral nerves [5], but is also classified as an IgE-mediated or type I allergy [6], where B cells undergo class switch during sensitization process and produce allergen-specific IgE that binds to high affinity FcεRI receptors on pre-sensitized basophils and mast cells, ultimately leading to a release of effector mediators [7]. So far allergen-specific immunotherapy (ASIT) did not show proof of concept in placebo-controlled clinical trials [8,9], despite the clear mechanistic evidence of the IgE-mediated hypersensitivity (type I). Also, IBH-like symptoms could be induced in healthy horses after intradermal transfer of the allergen in combination with not only IgE, but also IgG antibodies from IBH-affected horses [10]. In another study, treatment of histamine H1-antagonist Cetirizine could not reduce dermatitis compared to placebo-control group [11]. This suggests the involvement of additional hypersensitivity mechanisms. Evidence emerged that cell-mediated hypersensitivity, also called type IV allergy or DTH may play a role [12]. Type IV allergies can be subdivided into type a-d, dependent on cell types involved [13]. Already in 1994, hypersensitivity type IV characteristics were described for IBH-affected horses [3], which has been later reconfirmed by showing a close positive correlation of eosinophil levels in blood with IBH severity, while IgE levels did not correlate with severity [1]. Furthermore, eosinophil accumulation is a clinical manifestation of IBH, forming dense perivascular clusters in the deeper dermis and ultimately resulting in acute skin inflammation [14,15].
Therefore, eosinophils are nowadays known to contribute to an additional IBH mechanism by not only playing a key role in the late phase of IgE-mediated hypersensitivity (type I) but also in the cell-mediated hypersensitivity (type IVb) reactions [13].
HYPOTHESIS FOR IBH ALLERGY MECHANISM
In terms of the great relevance of eosinophils in IBH mechanisms, it is worth mentioning its close connection to Th2 cells, more specifically conventional Th2 (cTh2) and pathogenic effector Th2 (peTh2) cells. In humans, it has recently been shown that chronic allergen exposure leads to a shift from allergic IL-5-- Th2 cells (cTh2) towards IL-5+- Th2 cells (peTh2). In detail, cTh2 has been described to mainly express IL-4 and IL-13, thereby promoting B cell class switch towards IgE antibody producing plasma cells, causing type I allergic reactions, but only low levels of IL-5. In contrast, the peTh2 cells are highly positive for IL-5, which is a major source for eosinophil production, recruitment and activation, leading to eosinophil-mediated tissue damage. It has been suggested that chronic antigen exposure promotes the transformation from cTh2 cells into peTh2 cells, thus shifting dominating allergic molecular mechanism of allergy [16].
Also, IBH-affected horses are exposed to their allergens every year. Thus, it is likely that allergic horses also shift from cTh2 towards IL-5-producing peTh2 cells, which can be explained by the strong correlation of eosinophils and IBH severity. We herewith suggest that the early phase of IBH is dominated by a type I allergy including allergen-specific cTh2 cells and IgE, whereas the chronic phase is characterized by a transition of cTh2 cells towards IL-5+- peTh2 cells. The high amounts of T-cell derived IL-5 will lead to enhanced eosinophil production followed by enhanced recruitment into the allergic skin and subsequent cell activation causing tissue damage. By contrast, pruritus remains constant in IBH-affected horses with the natural allergy progression; however, the source may differ, i.e. of histaminic or non-histaminic origin such as IL-31 (Figure 1).
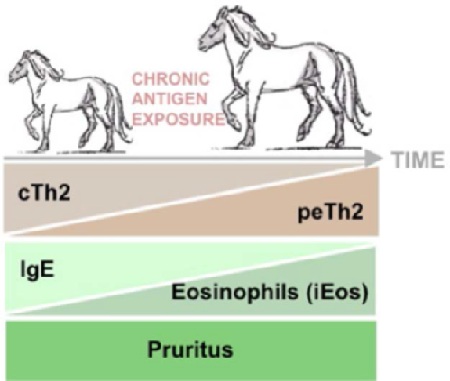 Figure 1: Model of switching allergy mechanisms through chronic allergen exposure during the natural course of IBH.
Figure 1: Model of switching allergy mechanisms through chronic allergen exposure during the natural course of IBH.
Allergic mechanisms of IBH are influenced by the duration of allergen exposure. The early onset of IBH is classified as an IgE-mediated or type I allergy, which is primary implied by the dominant role of conventional Th2 (cTh2) cells. As such, cTh2 expresses IL-4 and IL-13, promoting B cell class switch towards IgE antibody producing plasma cells and causing type I allergic reactions (left). With allergy progression, chronic allergen exposure during the natural course of IBH might drive the differentiation of cTh2 into pathogenic effector (peTh2) cells, which are highly positive for IL-5 and thus a major source for eosinophil production, recruitment and activation (right). By contrast to the mechanistic drive of IgE to eosinophils and cTh2 into peTH2 by chronic allergen exposure, pruritus exacerbates constant itching throughout the course of disease, whereby IL-31 is a key player.
DISCUSSION
IBH is well known and characterized, yet conventional treatment options are not satisfactory and did not always result in an improvement of allergy symptoms.
We currently suggested treating IBH-affected horses by targeting eosinophils using a commercially not yet available virus-like particle- (VLP-) based therapeutic vaccine. Its underlying mechanism involves the successful induction of antibodies against IL-5 [1,17], which critically contributes to eosinophil development, recruitment and activation. Besides, other potential treatment options are nowadays investigated, such as the induction of antibodies against IL-31, a Th2 cytokine-mediating histamine independent allergic pruritus via nerve cells in the skin [5], improved ASIT using recombinant allergens or the selection of IL-10-/Treg-inducing adjuvants [7]. Once, new products will be available for commercial use, it is important to fully understand the mechanism behind IBH in order to define biomarkers for opting for the best treatment outcome in terms of individual allergic horse patients.
Our hypothesis suggests that the underlying allergic mechanisms of IBH are influenced by the duration of allergen exposure. Early allergy might be highly IgE-dependent, whereas IgE might rather become negligible with allergy progression, while instead eosinophils become more dominant in terms of allergy induction (Figure 1). This novel insight suggests that treatment methods e.g. anti-histamines and ASIT will be most beneficial when conducted in young animals with a short history of allergy. On the contrary, more severe cases, already showing chronic features, should be treated with agents decreasing numbers of eosinophils such as corticosteroids or the IL-5-vaccine. Also, when conducting clinical proof of concept studies, patient cohorts should be carefully selected in order to show efficacy of therapies.
Future management of allergic diseases might be determined by taking into account the current dominating allergic response of each allergic individual. Thus, more suitable choices for therapies will improve the owner’s compliance leading to more satisfying therapeutic effects.
REFERENCES
- Fettelschoss-Gabriel A, Fettelschoss V, Thoms F, Giese C, Daniel M, et al. (2018) Treating insect-bite hypersensitivity in horses with active vaccination against IL-5. J Allergy Clin Immunol 142: 1194-1205.
- Schaffartzik A, Hamza E, Janda J, Crameri R, Marti E, et al. (2012) Equine insect bite hypersensitivity: What do we know? Vet Immunol Immunopathol 147: 113-126.
- Kurotaki T, Narayama K, Oyamada T, Yoshikawa H, Yoshikawa T (1994) Immunopathological study on equine insect hypersensitivity ("kasen") in Japan. J Comp Pathol 110: 145-152.
- McKelvie J, Foster AP, Cunningham FM, Hamblin AS (1999) Characterisation of lymphocyte subpopulations in the skin and circulation of horses with sweet itch (Culicoides hypersensitivity). Equine Veterinary Journal 31: 466-472.
- Olomski F, Fettelschoss V, Jonsdottir S, Birkmann K, Thoms F, et al. (2019) Interleukin 31 in insect bite hypersensitivity-Alleviating clinical symptoms by active vaccination against itch. Allergy.
- Hellberg W, Mellor PS, Torsteinsdottir S, Marti E (2009) Insect bite hypersensitivity in the horse: comparison of IgE-binding proteins in salivary gland extracts from Simulium vittatum and Culicoides nubeculosus. Vet Immunol Immunopathol 132: 62-67.
- Jonsdottir S, Cvitas I,Svansson V, Gabriel FA, Torsteinsdottir S, et al. (2019) New Strategies for Prevention and Treatment of Insect Bite Hypersensitivity in Horses. Current Dermatology Reports 8: 303-312.
- Barbet JL, Bevier D, Greiner EC (1990) Specific immunotherapy in the treatment of Culicoides hypersensitive horses: A double-blind study. Equine Vet J 22: 232-235.
- Ginel PJ, Hernández E, Lucena R, Blanco B, Novales M, et al. (2014)Allergen-specific immunotherapy in horses with insect bite hypersensitivity: A double-blind, randomized, placebo-controlled study. Vet Dermatol 25: 29-e10.
- Wagner B, Miller WH, Morgan EE, Hillegas JM, Erb HN, et al. (2006) IgE and IgG antibodies in skin allergy of the horse. Vet Res 37: 813-825.
- Olsén L, Bondesson U, Broström H, Olsson U, Mazogi B, et al. (2011) Pharmacokinetics and effects of cetirizine in horses with insect bite hypersensitivity. Vet J 187: 347-351.
- Cunningham FM, Dunkel B (2008) Equine recurrent airway obstruction and insect bite hypersensitivity: Understanding the diseases and uncovering possible new therapeutic approaches. Vet J 177: 334-344.
- Pichler WJ (2003) Delayed drug hypersensitivity reactions. Ann Intern Med 139: 683-693.
- Benarafa C, Collins ME, Hamblin AS, Cunningham FM (2002) Role of the chemokine eotaxin in the pathogenesis of equine sweet itch. Vet Rec 151: 691-693.
- Riek RF (1953) Studies on allergic dermatitis (Queensland itch) of the horse I. Description, distribution, symptoms and pathology. Aust Vet J 29: 177-184.
- Mitson-Salazar A, Prussin C (2017) Pathogenic Effector Th2 Cells in Allergic Eosinophilic Inflammatory Disease. Front Med (Lausanne) 4: 165.
- Gabriel FA, Fettelschoss V, Olomski F, Birkmann K, Thoms F, et al. (2019) Active vaccination against interleukin-5 as long-term treatment for insect-bite hypersensitivity in horses. Allergy 74: 572-582.
Citation: Lam J, Fettelschoss V, Olomski F, Rhiner T, Birkmann K, et al. (2020) Chronic Allergen Exposure Might Shift Allergic Mechanisms in Insect Bite Hypersensitivity Affected Horses. J Clin Immunol Immunother 6: 017.
Copyright: © 2020 Juwela Lam, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

