
Chronic Atypical Cerebral Malaria: A Case Study
*Corresponding Author(s):
Mukul Chandra GopeMedical Science, Rajendra Institute Of Medical Sciences, Ranchi, Jharkhand, India
Tel:+918757839603,
Email:drmukulchandragope@gmail.com
Abstract
Malaria, often presents in atypical forms in malaria endemic zones. Repeated mosquito bites as well as infrequent or incomplete antimalarial measures, complicate the clinical picture of patients. Patients often develop tolerance, attributable to development of naturally acquired immunity against the parasite. The present case is an example of atypical cerebral malaria of very long duration (six years), having episodic headache as chief presenting symptom. Intermittent mild fever along with sore throat also was accompanied to the chief symptom. Additionally, CT scan failed to show any detectable abnormality. The CT scan shows that, brain parenchyma, all cortical sulci, all ventricles, basal cistern are normal. There is no mid line shift. Cerebellum and brain stem also appear normal. On the basis of clinical findings as well as chronic history of the patient, a presumptive diagnosis of malaria came in my mind, based on the fact that, malarial parasites are often detected in peripheral blood when patient has fever (with the rise of body temperature, plasmodium migrate to peripheral blood and become detectable). In the present case, patient was experiencing 'evening rise' (8:00 PM onwards) of temperature, hence her blood smear (taken during daytime) failed to detect any malarial parasite. Furthermore, the history of patient includes anti-malarial measures. Moreover, such chronic cases often fail to reveal malarial parasite due to previous partially-effective or under-treatment with antimalarials.
Keywords
INTRODUCTION
Background and History
The test for malarial retinopathy is usually found positive in severe cases of malaria, often those which eventually lead to coma. Such cases are not very difficult to diagnose as their peripheral blood smear are usually positive for the parasite. Additionally, MR can detect retinal hemorrhages or white patches etc, suggestive of malaria. In the present case, the patient has normal fundus. There are no residua suggestive of previous retinal involvement. She has 6/6 vision on right eye and 6/9 on the left. These finding actually explain the anonymity of atypical malaria. Hence, absence of MR does not rule out malaria.
The presumptive diagnosis as 'malaria' for the present case has been done on the basis of
• Residence of patient, in a malaria endemic zone
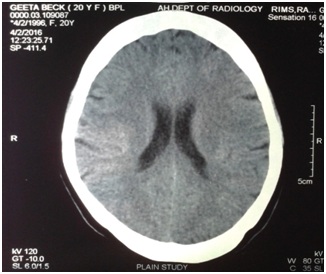
• Long standing headache with normal CT scan (Figures 1-3) and normal hematological report
• Icterus, suggestive of hemolysis and hyperbilirubinemia
• Intermittent mild fever (suggestive of possible acquired immunity against malaria)
• Ineffective routine analgesics
• History of incomplete antimalarial measures
• Cessation of fever with oral Chloroquine only (without adding an antipyretic)
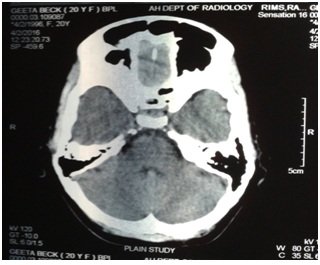
Figure 1: CT Scan showing normal cerebellum and brain stem.
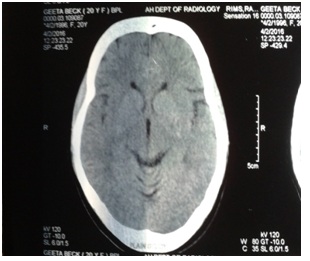
Figure 2: CT scan showing normal parenchyma, no midline shift.
Cessation of fever on 2nd day of starting Chloroquine only (without adding an antipyretic) strongly suggests malaria. In India, the guidelines for the treatment of malaria suggests that, every case presenting with headache and fever, should be given a complete dose of Chloroquine, if the patient belong to a malaria endemic zone (blood should be collected for investigation but no prior test for a malarial parasite is required).
Furthermore, in the present case, rapid antigen for malaria, revealed negative result. The reason of a negative rapid antigen test is still obscure, but, it may suggest a mutant strain of plasmodia being possibly involved. In this context, moreover, the atypical features of malaria are often observed in Falciparum malaria, early stages of infection, patients at extremes of age, immune-compromised patients, Patients undergoing treatment for malaria, recurrent attacks of malaria specially in endemic zones, pregnancy etc.
The atypical symptoms of Malaria can be seen as:
Atypical fever
Headache
Jaundice
Treatment Regime
Primary phase of treatment
• 1 gram stat (i.e., 4 tablets of 250 mg base)
• 500 mg (2 tablets) after 6 hours
• 500 mg (2 tablets) after 24 hours of 1st dose
• 500 mg (2 tablets) after 48 hours of 1st dose
Fever disappeared on second day of starting Chloroquine. Episodic headache was persisting along with sore throat.
Secondary phase of treatment
Final phase of treatment
Definitive phase of treatment
Discussion
The innate immunity is naturally present in the host and it does not require any previous exposure to a malarial parasite. The malarial parasite passes through a complex life cycle involving liver and red blood cells of the host [13]. Here, it is worth to note that, the parasite causes expression a great variety of proteins at different stages. Furthermore, more importantly, these proteins also keep changing often. As a result, it is infact only 'partial' or 'short-lived' immunity that the host acquires by a natural infection. Furthermore, development of such immunity will not be able to protect the individual against a new infection [14]. It seems very difficult or even impossible to develop an effective vaccine against malaria since, there is complex interplay between parasitic proteins and the immune system of the host [15-17]. Natural or innate immunity to malaria usually protects an individual by either exhibiting an inherent refractoriness for establishment of the infection or by exerting an immediate inhibitory response against the parasite [18-22]. Additionally, protection against malaria or against its severe manifestations can also be due to alterations in the structure of hemoglobin or some enzymes involved in the pathogenesis of malaria. People residing in high endemic zone of malaria often exhibit these traits [23-24]. Moreover, for example, duffy negativity, in red cells, being widely prevalent in Africa, protects against "P. vivax" infection, and may be responsible for the virtual elimination of this parasite from the continent. Certain thalassemias reduce the chances of infection by 50%. Similarly, homozygote hemoglobin C, can prevent upto 90% cases of malaria. Additionally, hemoglobin E, and ovalocytosis carrier status have also been reported to confer protection against "P. falciparum" or "P. vivax". Last but not the least, Glucose 6-phosphate dehydrogenase deficiency (50% protection) and sickle cell hemoglobin (90% protection) confer protection against severe malaria and related mortality [25-26].
At present the patient is in good health and there is no episode of headache since last six months (till date). Referring to such outcome of the patient, it can be suggested that for a patient belonging to a malaria endemic zone if presents with fever, should be given oral Chloroquine (without any anti-pyretics) first, (instead of going through a battery of tests) to observe its effect on fever. Any change in the pattern of fever or its cessation is suggestive of malaria. Chloroquine is a safe drug and also in use for prophylaxis against malaria for travelers visiting to malaria endemic zones. Mefloquine is another alternative for the same. The antimalarial used 'Artemether' has long elimination half-life, hence should be given under close monitoring. Appropriate dose modification is required for the cases of renal as well as hepatic failure. Artemether should not be used in the first trimester of pregnancy in situations where other suitable and effective antimalarials are available. Drug interaction should be kept in mind while combining Artemether with other anti-malarials, and appropriate data should be referred for the same [27].
REFERENCES
- http://icmr.nic.in/Publications/hpc/PDF/Annexure%2011.pdf
- Smith T, Genton B, Baea K, Gibson N, Taime J, et al. (1994) Relationships between Plasmodium Falciparum infection and morbidity in a highly endemic area. Parasitology 109: 539-549.
- Frevert U, Engelmann S, Zougbede S, Stange J, Bruce Ng, et al. (2005) Intravital observation of plasmodium berghei sporozoite infection of the liver. PLoS Biol 3: 192.
- Srinivas (2005) Malaria fever. Clinical Features, Malariasite, karnatak, India.
- World Health Organization (2008) Global malaria control and elimination: report of a technical review. World Health Organization, Geneva, Switzerland.
- World Health Organization (2005) World malaria report 2005. World Health Organization, Geneva, Switzerland.
- Doolan DL, Lázar CD, Baird JK (2009) Acquired immunity to malaria. Clin Microbiol Rev 22: 13-36.
- Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C (1999) Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med 5: 340-343.
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI (2005) The global distribution of clinical episodes of Plasmodium Falciparum malaria. Nature 434: 214-217.
- Gallup JL, Sachs JD (2001) The economic burden of malaria. Am J Trop Med Hyg 64: 85-96.
- Smith JD, Chitnis CE, Craig AG, Roberts DJ, Hudson-Taylor DE, et al. (1995) Switches in expression of Plasmodium Falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell 82: 101-110.
- Breman JG, Alilio MS, Mills A (2004) Conquering the intolerable burden of malaria: what's new, what's needed: a summary. Am J Trop Med Hyg 71: 1-15.
- Millington OR, Di Lorenzo C, Phillips RS, Garside P, Brewer JM (2006) Suppression of adaptive immunity to heterologous antigens during Plasmodium infection through hemozoin-induced failure of dendritic cell function. J Biol 5: 5.
- Wykes M, Good MF (2007) A case for whole-parasite malaria vaccines. Int J Parasitol 37: 705-712.
- Freund J, Sommer HE, Walter AW (1945) Immunization against malaria: vaccination of ducks with killed parasites incorporated with adjuvants. Science 102: 200-202.
- Doolan DL, Lázar CD, Baird (2002) Evolutionary and historical aspects of the burden of malaria. Clin Microbiol Rev 15: 564-594.
- Mannoor MK, Weerasinghe A, Halder RC, Reza S, Morshed M, et al (2001) Resistance to malarial infection is achieved by the cooperation of NK1.1(+) and NK1.1(-) subsets of intermediate TCR cells which are constituents of innate immunity. Cell Immunol 211: 96-104.
- Perlmann P, Troye-Blomberg M (2002) Malaria and the Immune System in Humans. In: Perlmann P, Troye-Blomberg M (eds.). Malaria Immunology. Chem Immunol, Karger, Basel, Switzerland. Pg No: 80: 229-242.
- Roetynck S, Baratin M, Vivier E, Ugolini S (2006) NK cells and innate immunity to malaria. Med Sci (Paris) 22: 739-744.
- Stevenson MM, Riley EM (2004) Innate immunity to malaria. Nature Reviews Immunology 4: 169-180.
- Ariyasinghe A, Morshed SR, Mannoor MK, Bakir HY, Kawamura H, et al. (2006) Protection against malaria due to innate immunity enhanced by low-protein diet. J Parasitol 92: 531-538.
- Kumar A, Valecha N, Jain T, Dash AP (2007) Burden of malaria in India: retrospective and prospective view. Am J Trop Med Hyg 77: 69-78.
- Branch O, Casapia WM, Gamboa DV, Hernandez JN, Alava FF, et al. (2005) Clustered local transmission and asymptomatic Plasmodium Falciparum and Plasmodium vivax malaria infections in a recently emerged, hypoendemic Peruvian Amazon community. Malar J 4: 27.
- Bray RS, Gunders AE, Burgess RW, Freeman JB, Etzel E, et al. (1962) The inoculation of semi-immune Africans with sporozoites of Laverania falcipara (Plasmodium falciparum) in Liberia. Riv Malariol 41: 199-210.
- Thomas E Herchline (2016) Malaria. Drugs & Diseases, Infectious Diseases, Medscape, New York, USA.
- Freund J, Thomson KJ, Sommer HE, Walter AW, Pisani TM (1948) Immunization of monkeys against malaria by means of killed parasites with adjuvants. Am J Trop Med Hyg 28: 1-22.
- https://www.medicines.org.uk/emcmobile/medicine/9196#COMPOSITION
Citation: Mukul Chandra Gope (2017) Chronic Atypical Cerebral Malaria: A Case Study. J Pharmacol Pharmaceut Pharmacovigil 1: 004
Copyright: © 2017 Mukul Chandra Gope, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

