
Clinical and Quality of Life Outcomes among Ixekizumab Treated Psoriasis Patients in a Real-World Setting
*Corresponding Author(s):
William N MalatestinicEli Lilly USA, LLC, Indianapolis, Indiana, 893 S Delaware St Indianapolis, IN 46225, United States
Email:malatestinic_william_n@lilly.com
Abstract
Introduction: Psoriasis is a chronic immune-mediated inflammatory skin condition that has a significant negative impact on the physical, emotional, and psychosocial well-being of those affected. This study aimed to assess the speed of onset and long-term clinical and quality of life (QOL) outcomes among Ixekizumab (IXE) treated plaque psoriasis patients.
Method: A retrospective cohort study was conducted at a single US dermatology referral center. Medical charts were reviewed for adult psoriasis patients starting IXE (index date) between March 22, 2016, and February 28, 2018.Disease severity and QOL data were collected up to one-year pre-IXE initiation and up to 35 months post-IXE initiation. Static Physician Global Assessment (sPGA), Body Surface Area (BSA), and Dermatology Life Quality Index (DLQI) were summarized at 1-month post-index and at 3-month intervals. Logistic regressions were performed to evaluate the 1-month response in relation to long-term sPGA, BSA, and DLQI outcomes.
Results: A total of 153 patients (median age at index: 47.7 years; 65.4% male; 93.5% Caucasian) were included in the study. Majority of patients (69%; n=106) were biologic-experienced prior to IXE initiation. At 1-month post-index 58.8% of patients achieved sPGA (0,1), 55.9% achieved DLQI (0,1), and 66.9% achieved BSA≤1%. Patients with sPGA (0,1) at 1-month post-index had greater odds of remaining sPGA (0,1) and BSA≤1% at 24-month (sPGA 0,1: OR=10.1; 95% CI: 2.1-47.9; BSA≤1%: OR=13.3; 95% CI: 2.2-80.2). Among patients who achieved sPGA (0,1) at 1-month post-index, the observed proportion of patients with sPGA (0,1), DLQI (0,1), and BSA≤1% remained largely the same for the 24-month follow-up.
Conclusion: This real-world study demonstrated that the majority of patients initiating IXE achieved sPGA (0, 1), DLQI (0, 1) and BSA ≤1% targets within the first month of treatment and were able to maintain treatment response for up to 24 months independent of prior biologic exposure.
Keywords
DLQI; Ixekizumab; Psoriasis; Real world study; Treatment response; Quality of life
Key Summary
- Psoriasis is a chronic immune-mediated inflammatory skin condition that has a significant negative impact on the physical, emotional, and psychosocial well-being of those affected. Plaque psoriasis is the most common type accounting for approximately 90% of psoriasis cases
- This study aimed to assess the speed of ixekizumab effectiveness and long-term clinical and quality of life outcomes among ixekizumab treated plaque psoriasis patients
- The current study showed that in a real-world setting, a high level of effectiveness can be observed as early as 1-month following ixekizumab initiation and maintained for up to 24 months
- Findings from this study provide valuable information on the potential correlation of speed of treatment response and long-term outcomes in ixekizumab treated psoriasis patients and in other subgroup populations.
- Future research across multiple centers is needed to corroborate these findings
Introduction
Psoriasis is a chronic immune-mediated inflammatory skin condition that has a significant negative impact on the physical, emotional, and psychosocial well-being of those affected [1,2]. Plaque psoriasis is the most common type accounting for approximately 90% of psoriasis cases [3]. Chronic psoriasis is characterized by recurrent flares, or the sudden and severe worsening of symptoms, often without any obvious cause. In the United States (US), the estimated prevalence of psoriasis is 3.2% among individuals aged 20 years or older with greater prevalence found among Caucasians (2.5%) compared to African Americans (1.3%) [4,5].
The Psoriasis Area and Severity Index (PASI) is considered the gold standard measurement for psoriasis severity, yet it is rarely used in routine clinical practice due to its complexity [6]. Instead, physicians routinely quantify the percentage of Body Surface Area (BSA) affected, use the Physician Global Assessment (sPGA) scale, and patient perception of impairment to different aspects of their health-related quality of life (HRQoL) in order to assess disease activity and severity [6]. The Dermatology Life Quality Index (DLQI) is a validated instrument which indicates the degree of reduction in patient’s HRQoL [1,7].
There is currently no cure for psoriasis, however, based on the severity of the disease, treatments are available to help manage symptoms. These include topical agents, phototherapy, conventional systemic therapies, biologics and over-the-counter preparations. The treatment paradigm for psoriasis tends towards the use of topical agents for mild and moderate disease, systemic agents for moderate and severe disease, and biologics for more severe cases [8]. Current available biologic therapies are inhibitors of and cytokines: TNFα, IL-12, IL-23 and IL-17A, and these have shown efficacy in reducing psoriasis symptoms in moderate to severe cases [9]. While rapid response and long-term effectiveness were indicated to be of high importance among patients with moderate to severe disease as well as among physicians making treatment decisions [2,10], there is evidence that current biologic treatments may lose effectiveness over time [11-13].
The newer biologic therapies are those that inhibit the IL-17 or IL-23 pathways. Ixekizumab (Taltz®, IXE), an IL-17A antagonist, was approved in the US on March 22, 2016 for the treatment of adults with moderate-to-severe plaque psoriasis who are candidates for systemic therapy or phototherapy [14,15]. More recently on March 30th 2020, additional approval was granted in the US for the use of IXE in pediatric patients ages six or older.IXE has demonstrated rapid onset of clinical improvement, high levels of skin clearance, and sustained efficacy in clinical trials and in real-world clinical practice [9,16-22], however, limited information on its speed of onset and long-term impact on patient outcomes and treatment persistence in a real-world setting exist.
The aims of this study were as follows: assess speed of IXE effectiveness; describe the persistency of outcomes among psoriasis patients at a single dermatology referral practice in the US; and to assess clinical and HRQoL outcomes in subgroups of patients who had a sPGA score of 0,1 at 1-month or were exposed to biologic treatment prior to IXE initiation. Findings from this study will provide foundational insights that can be used to inform future broader comparative effectiveness studies of IXE.
Methods
Study design
A retrospective cohort study was conducted at a single US dermatology referral center. Medical chart data were used to identify patients who initiated IXE between March 22, 2016 and February 28, 2018 (i.e. the enrolment period). The index date was defined as the date of IXE initiation. Data up to 35 months after index date and 12 months before index date were collected. The end of study period was defined as February 28, 2019, date of IXE discontinuation, or discontinuation of clinical care, whichever occurred first. All data were collected retrospectively from medical charts and de-identified. The study adhered to the Guidelines for Good Pharmacoepidemiology Practices as well as the Health Insurance Portability and Accountability Act (HIPAA) guidelines for protection of patient confidentiality [23,24]. Ethical approval and waiver of consent was obtained from Advarra, Inc., prior to beginning data collection. Study was performed in accordance with the Helsinki Declaration of 1964, and its later amendments.
Data source and study population
Data were collected from Central Dermatology, a dermatology referral practice encompassing one primary investigator supported by nurse practitioners. Patients with moderate to severe psoriasis are routinely seen at one month and then every three months during active treatment stage after biologic initiation for up to one year; and are seen approximately every six months beyond one year of treatment. sPGA, BSA, and DLQI are regularly collected at clinic visits.
Inclusion criteria for the study population were 18 years of age or above, physician-diagnosed plaque psoriasis, and initiation of IXE during the enrollment period (i.e., between March 22, 2016 and February 28, 2018). Exclusion criteria were receipt of IXE for psoriasis through participation in any clinical trials before or during the enrolment period, and less than 18 years of age at time of IXE initiation. Medical records of psoriasis patients who initiated treatment with IXE during the enrolment period were reviewed consecutively in chronological order, based on IXE initiation date. Patient charts were then screened against study eligibility criteria. The study was reviewed and approved by Advarra Institutional Review Board (IRB), and a waiver of informed consent was granted.
Data collection
The medical records of psoriasis patients who met the eligibility criteria were retrospectively reviewed, data were abstracted up to one year prior to IXE initiation and up to 35 months post IXE initiation using an electronic Data Collection Form (eDCF) created with Microsoft® Access. Patients were seen at different schedules depending on the course of their disease.
Variables
Baseline demographic and clinical information including age, sex, ethnicity, insurance status, and time since psoriasis diagnosis was obtained from medical records at index date. Use of systemic, topical and phototherapy treatments as well as treatment discontinuation, and reason for discontinuation were collected during the pre-index period. Primary treatment outcomes of interest collected during post-index were sPGA, DLQI, and BSA. sPGA is a 7-point scale that classifies the severity of psoriasis from “0=clear” to “6=severe”. DLQI responses were classified into five categories (0-1, 2-5, 6-10, 11-20, and 21-30) from “no effect” to “extremely large effect” [6]. BSA was presented as continuous variables and also categorised using National Psoriasis Foundation (NPF) guidelines as: treat to target (BSA ≤1%), mild (1%<BSA<3%), moderate (3%≤BSA≤10%), and severe (BSA>10%) [25]. These outcomes as well as the specific body regions involved were collected during the post-index period for each documented visit. All patients in our study received the FDA approved dose of IXE (80 mg every two weeks) for moderate-to-severe plaque psoriasis.
Statistical analysis
Analyses were performed based on available patient data at each clinic visit. Descriptive analysis was used to summarize baseline demographic and clinical characteristics, treatment details, and study outcomes. Mean, Standard Deviation (SD), median, and range were summarized for continuous variables, and counts and percentages were summarized for categorical variables.
Disease severity measures were summarized from index date up to 24 months post-index. sPGA, DLQI, BSA and specific body regions involved were summarized by counts and percentages at 1-month post-index, at 3-month intervals for up to 12 months post-index, and at 6-month intervals in the second year following IXE initiation among patients with available data at each post-index time point (±45 days). Percentage of BSA and number of body regions involved were summarized by mean, SD, median, and range. Additionally, two subgroup analyses were performed: 1) comparing early responders, defined as patients who had achieved a sPGA (0,1) at 1-month with those who did not; and 2) comparing patients who had used biologic treatment prior to IXE initiation with patients who were biologic-naïve.
Unadjusted logistic regressions were performed to explore the potential determinants of sPGA, DLQI, and BSA outcomes at 12 and 24 months after IXE initiation. The variables included in the logistic regression for outcomes at 12 and 24 months after IXE initiation were age, sex, weight (< 100 kg; ≥ 100 kg), time since psoriasis diagnosis, BSA and PGA at study index date, body region involved at index date. biologic or methotrexate use any time prior to index date, psoriatic arthritis status at pre-index and documented comorbidity. The outcomes were BSA ≤ 1% versus BSA > 1%, sPGA (0, 1) versus sPGA > 1, and DLQI (0, 1) versus DLQI > 1. Odds ratios (OR) were presented alongside 95% CI. Analyses were performed in SAS 9.4 (SAS Institute, Inc., Cary, NC).
Results
A total of 153 psoriasis patients treated with IXE between March 22, 2016 and February 28, 2018 and who met study eligibility criteria were included. Among patients seen at each individual study follow-up visit, nearly all patients had sPGA (98%), BSA (99%), and DLQI (96%) documented. The median age at IXE-initiation was 47.7 years (range: 19.7-74.5), 65.4% of the study population were male, and 93.5% of patients were Caucasian (Table 1). The median time since psoriasis diagnosis was 11.9 years (range: 0.1-41.1). A total of 106 patients received prior biologic therapy for psoriasis; adalimumab was the most commonly-used biologic prior to IXE initiation (n=40), followed by secukinumab (n=35). Almost half of the study cohort received prior non-biologic systemic treatment (n=69, 45.1%) with the majority receiving methotrexate (n=66, 43.1%); 38.6% (n=59) patients received topical treatment during the pre-index period while only two patients (1.3%) received phototherapy. Among patients who switched to IXE from other biologic therapies, the majority of the patients discontinued their prior biologics due to inadequate response to treatment (TNF-α inhibitor: 90.8%, IL-12/23 inhibitor: 100%, IL-17 inhibitor: 82.9%, Table 2).
|
Demographic characteristics |
All patients (n=153) |
|
Age at study index date (years) |
|
|
Mean (SD) |
47.2 (13.1) |
|
Median (Min-Max) |
47.7 (19.7-74.5) |
|
Sex, n (%) |
|
|
Male |
100 (65.4) |
|
Female |
53 (34.6) |
|
Race/ethnicity, n (%) |
|
|
Caucasian |
143 (93.5) |
|
African-American |
5 (3.3) |
|
Multi-racial |
1 (0.7) |
|
Unknown |
4 (2.6) |
|
Insurance status at study index date, n (%) |
|
|
Private |
143 (93.5) |
|
Medicaid |
1 (0.7) |
|
Medicare |
3 (2.0) |
|
Medicare;Private |
4 (2.6) |
|
None |
2 (1.3) |
|
Time since psoriasis diagnosis (years)a |
|
|
Mean (SD) |
14.8 (10.5) |
|
Median (Min-Max) |
11.9 (0.1-41.1) |
|
Unknown |
34 (22.2) |
|
Treatment during pre-indexb |
|
|
Non-biologic systemic medications, n (%) |
69 (45.1) |
|
Methotrexate |
66 (43.1) |
|
Cyclosporine |
3 (2.0) |
|
Apremilast |
9 (5.9) |
|
Biologic medications, n (%) |
106 (69.3) |
|
TNF-a inhibitor |
66 (43.1) |
|
Etanercept |
2 (1.3) |
|
Infliximab |
9 (5.9) |
|
Adalimumab |
40 (26.1) |
|
Certolizumab |
8 (5.2) |
|
IL-12/23 inhibitor |
14 (9.2) |
|
Ustekinumab |
14 (9.2) |
|
IL-17 inhibitor |
35 (22.9) |
|
Secukinumab |
35 (22.9) |
|
Body surface area (%) |
|
|
Mean (SD) |
5.8 (6.8) |
|
Median (Min-Max) |
4 (0-50) |
|
Unknown |
6 (3.9) |
|
sPGA score |
|
|
Mean (SD) |
3.5 (1.3) |
|
Median (Min-Max) |
4 (0-6) |
|
Topical medications, n (%) |
59 (38.6) |
|
Phototherapy, n (%) |
2 (1.3) |
Table 1: Baseline demographics and clinical characteristics.
Abbreviations: IL=interleukin; n=number; SD = standard deviation,TNF=tumor necrosis factor
aThe study index date is the date of ixekizumab initiation
bPatients may have received more than one treatment concomitantly or sequentially during pre-index period; number of treatments received can be higher than number of patients that received the treatment
|
Reasonsa |
TNF-a inhibitor (n=66)b |
IL-12/23 inhibitor (n=14)b |
IL-17 inhibitor (n=35)b |
|
|
n (%) |
n (%) |
n (%) |
|
Treatment discontinuation |
|
|
|
|
Treatment ongoing at end of pre-index period |
1 (1.5) |
0 (0.0) |
0 (0.0) |
|
Treatment switch/discontinuation |
65 (98.5) |
14 (100.0) |
35 (100.0) |
|
Reason for treatment switch/discontinuationc |
|
|
|
|
Inadequate response to treatment |
59 (90.8) |
14 (100.0) |
29 (82.9) |
|
Adverse event |
2 (3.1) |
0 (0.0) |
1 (2.9) |
|
Insurance coverage |
1 (1.5) |
0 (0.0) |
1 (2.9) |
|
Patient preference |
2 (3.1) |
0 (0.0) |
1 (2.9) |
|
Doctor preference |
26 (40.0) |
4 (28.6) |
15 (42.9) |
|
Side effect profile of biologic |
1 (1.5) |
0 (0.0) |
0 (0.0) |
|
Other |
10 (15.4) |
0 (0.0) |
0 (0.0) |
Table 2: Reasons for treatment choice and treatment discontinuation of biologics in the pre-index period.
Abbreviations: IL=Interleukin; n=Number; TNF=Tumor Necrosis Factor
aPatients may have had more than one reason for treatment choice or discontinuation or switch; number of reasons can be higher than number of patients receiving treatment.
bDenominator: total number of patients receiving the treatment. cDenominator for this variable is number of pateints who discontinue the biologics.
Speed of treatment effect
Disease severity during follow-up was summarized (Figure 1). At index, 7.2%, 20.9%, and 19.6% of patients had sPGA (0, 1), DLQI (0,1), and BSA ≤1%, respectively. By 1-month post-index (n=136), 58.8% of patients achieved clear or almost clear skin (sPGA 0, 1), 29.4% clear skin (sPGA 0), 55.9% DLQI (0, 1) and 66.9% BSA ≤1%. The proportion of patients achieving sPGA (0, 1), DLQI (0, 1), and BSA ≤1% (NPF target) during follow-up are shown in Figure 1. At 3-month post-index, nearly three-quarters (73.5%) of patients had sPGA (0, 1), while 68.4% had DLQI (0,1), and 86% had BSA ≤1%. The median change during 24 months post-index period was approximately 3% reduction for BSA, -3 points for sPGA, and -2 points for DLQI scores for the overall study cohort.
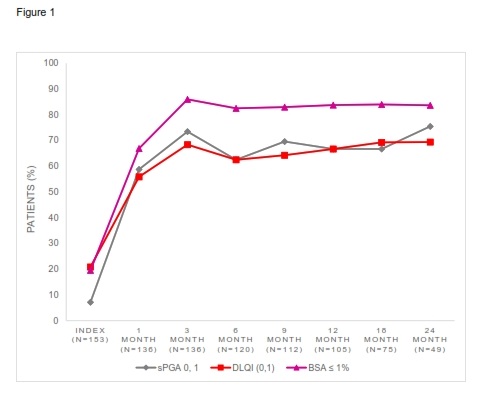 Figure 1: Percentage of patients achieving treatment targets during 24-month post-index, among overall study cohort (n=153).
Figure 1: Percentage of patients achieving treatment targets during 24-month post-index, among overall study cohort (n=153).
Subgroup analysis was done on early responders (achieved sPGA 0, 1 at 1 month) and non-early responders (not achieving sPGA (0, 1) at 1 month). Among 135 patients with 1-month post-index sPGA data available, 59.2% (n=80) of patients were early responders, among which 70% (n=56) achieved DLQI (0, 1), and 85% (n=68) achieved BSA ≤1%. Over 85%, 75%, and 65% of patients met the targets for BSA, sPGA, and DLQI, respectively, at all the time points after 1-month post-index among early-responders (Figure 2). Among non-early responders (n=55), 46.8%, 55.3%, and 74.5% of them achieved the targets for sPGA, DLQI, and BSA at 3-month post-index, respectively. The proportions remained largely the same for 24-month post-index (Figure 2).
Additional subgroup analyses were done among biologic-naïve patients and biologic-experienced patients prior to index date (Figure 2). Of the 109 patients who received biologic treatments for psoriasis at any time prior to IXE initiation, 6.5% had sPGA (0, 1), 25.7% had DLQI (0, 1), and 22% had BSA ≤1% at IXE initiation. 95 patients in the biologic-experienced group had data available at 1-month post-index, and 58.9% had sPGA (0, 1) including 28.4% with sPGA 0; 55.8% achieved DLQI (0, 1); and 66.3% achieved BSA ≤1%. At 3-month, the proportions of patients achieving sPGA, DLQI and BSA targets increased to 72.6% (52.6% had sPGA 0), 68.4%, and 83.2%, respectively. Of 44 biologic-naïve patients, 41 patients had data available at 1- and 3-months post-index. The proportions of patients achieving sPGA, DLQI, and BSA targets were 58.5% and 75.7%, 68.3% and 75.7%, 68.3% and 92.7%, respectively at 1- and 3-months.
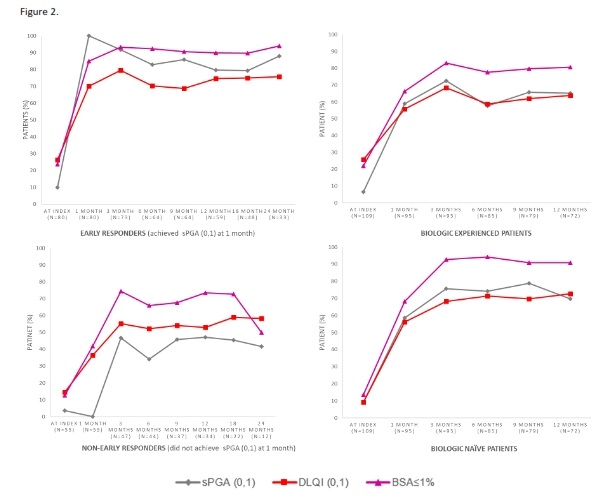 Figure 2: Percentage of patients achieving treatment targets during post-index among subgroup.
Figure 2: Percentage of patients achieving treatment targets during post-index among subgroup.
Long-term treatment effect
Disease severity and quality of life outcomes at 24-month post-index were available for 49 patients from the overall study cohort. Among the 49 patients, 75.5%, 69.4%, and 83.7% had treatment outcomes of sPGA (0, 1), DLQI (0,1), and BSA ≤1%, at 24-month post index respectively. Among early responders, 87.9% of patients had sPGA (0, 1), including 66.7% with sPGA 0; 75.8% had DLQI (0, 1); and 93.9% had BSA ≤1% at 24 months.
Logistic regression model results showed that at 24 months post-index, early sPGA responders at 1-month not only had greater odds of achieving sPGA (0, 1) (OR=10.1; 95% CI: 2.1-47.9; p-value=0.003), but also greater odds of achieving BSA ≤ 1% (OR=13.3; 95% CI: 2.2-80.2; p-value=0.005) than non-early responders (Table 1 in the electronic supplementary material). No association was found for DLQI (0,1) at 24 months. Also, early sPGA responders were more likely to achieve the sPGA (0,1) outcome at 12 months than non-early responders (OR=4.4; 95%CI: 1.7-11.1; p-value=0.002, Table 2 in the electronic supplementary material).
Discussion
This is the first observational study in a referral clinic reporting 1- to 24-months of real-world severity and quality of life outcomes following IXE initiation among patients with moderate-to-severe psoriasis in the US. The current study showed that in a real-world setting, a high level of effectiveness can be observed as early as 1-month following IXE initiation and maintained for up to 24 months. We found the majority of patients met the treatment targets of sPGA (0, 1), DLQI (0, 1) and BSA ≤1% at 1-month after initiating IXE. Most patients not achieving sPGA (0, 1) after 1-month of IXE treatment were still able to achieve response of sPGA (0, 1) by 3 months. Most of the study cohort were on a prior biologic before switching to IXE with mean treatment duration of 9.8 months (SD: 6.7 months). Despite inadequate response to previous biologics, more than half of patients achieved a rapid response at 1-month after IXE initiation, and the proportion of patients meeting treatment targets increased at 3-month and remained relatively stable onward.
The current study found that the majority of patients from the overall study cohort as well as in the subgroups of early responders and non-early responders all maintained high levels of responses of sPGA, DLQI, and BSA outcomes throughout the 24-month follow-up period. These findings were consistent with the 5-year results from the UNCOVER-1 and 2 studies, reported by Leonardi, et al. in which clinical and quality of life responses of IXE were maintained throughout the 5-year period [26,27]. Similar rapid response results were found in other real-world studies outside the US, where significant improvement in disease severity measured by PASI was observed at one-month after IXE initiation and continued to improve at 3-month and at 6-months [9,19]. In the current study, BSA percentage at IXE initiation and a rapid response to IXE at one month were found to be strongly associated with the 24-month IXE treatment outcomes in the study.
The results from the unadjusted logistic regression analyses indicated that 24-months post-index, early sPGA responders at 1-month were more likely to achieve sPGA (0, 1) (at 12 and 24 months post-index) and also BSA ≤ 1% (at 24 months post-index) compared to non-early responders. These exploratory results are promising and should be assessed with larger sample sizes in future studies.
The key strength of the study was the availability of both long-term clinical and patient-reported quality of life data collection, as well as the frequency of outcomes collected at each time point. In the current study, a third (n=49) of the study cohort had 24-month post-index data, and treatment outcomes were collected at 1-month and as frequently as every 3 months during the first year following IXE initiation. This density of follow-up visits is not available from other real-world data sources. For example, the CorEvitas registry, established in April 2015 to collect prospective real-world data on patients with autoimmune diseases, including those with psoriasis who are treated with biologic therapies in the US and Canada, only collects data on clinical (e.g., BSA, Investigator Global Assessment [IGA]) and patient reported outcomes (e.g., DLQI) at six-month intervals [28,29]. This makes it difficult to assess speed of treatment response. This single-arm retrospective chart review study was able to address this knowledge gap. Other strengths of this study include the use of a consecutive sampling approach based on treatment initiation date to reduce selection bias, and the unique study design that allows the collection of both pre-index data of up to 12 months and post-index data of up to 35 months. Such study design allows the exploration of treatment history and its impact on subsequent IXE treatment outcomes. The overall patient population and results in this study were consistent to clinical trials [30], and the two real-world studies conducted in Europe and one in Canada which used PASI as disease severity measure [9,19].
There are several limitations to the current study. First, a single study site was chosen based on the availability of relevant data at the site. However, the outcomes reported in our study were measured by a single physician at the study site. While this averts inter-rater variations, it may introduce bias in the reported results. Second, this study looked at cross-sectional clinical and HRQoL data at each follow-up time point. Although the use of patient medical records offers many potential advantages over other data sources such administrative data, in research studies that require detailed clinical information [31], the use of retrospective data has the inherent limitation of having been collected for purposes independent of the study objectives. The granularity of data available depends largely on the charting practices of the study site. For the changes in disease severity and HRQoL analysis performed in this study, only patients with available data at each study time point were used, as no data imputation was performed; as a result, the sample size declined over time due to infrequent clinic visits or losses to follow-up. The regression analyses performed in the study were unadjusted due to this sample size constraint. The present study also did not assess the washout period between cessation of previous biologic therapy and initiation of IXE. Lastly our focus was to assess the association between one-month response and long-term outcomes rather than evaluating causal determinants of long-term outcomes. Therefore, we did not include other potential determinants in our model. Overall, larger, multi-center studies are needed to address the various limitations in the current study.
Conclusion
In the findings of the current real-world study support the speed of response and persistency of effect seen in IXE clinical trials [26,27]. Results from this study demonstrated that the majority of patients achieved sPGA (0,1), DLQI (0,1) and BSA ≤1% targets within the first month of treatment and were maintained at each follow-up time point up to 24 months. Findings from this study provide valuable information on the potential correlation of speed of treatment response and long-term outcomes in IXE-treated psoriasis patients and in other subgroup populations. Future research across multiple centers is needed to corroborate these findings.
Acknowledgement
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authorship Contributions
Craig Leonard contributed to the acquisition of data, analysis of data, critical revision of the work, interpretation of the data, design and concept. Rei Tao contributed to concept and design of work, acquisition of data, interpretation of data, drafting of the work and critical revision of the work. Solmaz Setayeshgar contributed to analysis of data, interpretation of data and critical revision of work. Suzanne McMullen contributed to design and interpretation of work and critical revision of work. Russel Burge contributed to interpretation of data, critical revision of work and data design of the work. Baojin Zhu contributed to interpretation of data for the work, critical revision of the work and statistical analysis. William N Malatestinic contributed to concept and design of work, interpretation of data, drafting of the work and critical revision of the work.
Funding
Eli Lilly and Company funded the Study and the Journal of Dermatology and Therapy’s Rapid Service Fee.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing, Editorial, and Other Assistance
Not applicable.
Disclosures
Craig Leonardihas received honoraria from Eli Lilly for the following: Advisory Board, Speaker, and Consultant.
Rei Tao, Solmaz Setayeshgar, Sisi Wang, Suzanne. McMullen are employees of ICON plc., a company contracted by Eli Lilly to conduct this study.
Russel. Burge, Baojin. Zhu, and William .N. Malatestinicare employees of Eli Lilly.
Compliance with Ethics Guidelines
Ethical approval was obtained from Advarra, Inc., prior to beginning data collection. Study was performed in accordance with the Helsinki Declaration of1964, and its later amendments. The manuscript does not contain individual person’s data.A Waiver of informed consent was granted.
All analyses and reporting were carried out using de-identified data. This study adhered to the Guidelines for Good Pharmacoepidemiology Practices as well as the Health Insurance Portability and Accountability Act (HIPAA) guidelines for protection of patient confidentiality [23,24].
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- Guenther L, Langley RG, Shear NH, Bissonnette R, Ho V, et al. (2004) Integrating biologic agents into management of moderate-to-severe psoriasis: a consensus of the Canadian Psoriasis Expert Panel. J Cutan Med Surg 8: 321-37.
- Gorelick J, Shrom D, Sikand K, Renda L, Burge R, et al. (2019) Understanding Treatment Preferences in Patients with Moderate to Severe Plaque Psoriasis in the USA: Results from a Cross-Sectional Patient Survey. Dermatology and Therapy 9: 785-797.
- Sarac G, Koca TT, Baglan T (2016) A brief summary of clinical types of psoriasis. North Clin Istanb 3: 79-82.
- Rachakonda TD, Schupp CW, Armstrong AW (2014) Psoriasis prevalence among adults in the United States. J Am Acad Dermatol 70: 512-516.
- Gelfand JM, Stern RS, Nijsten T, Feldman SR, Thomas J, et al. (2005) The prevalence of psoriasis in African Americans: Results from a population-based study. J Am Acad Dermatol 52: 23-26.
- Feldman SR, Krueger GG (2005) Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 64: 65-68.
- Finlay AY, Khan GK (1994) Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol 19: 210-216.
- DiPiro J, Talbert R, Yee G, Matzke GR, Wells BG, et al. (2011) Pharmacotherapy: A Pathophysiologic Approach (8th edn.). Mc-Graw-Hill Medical, USA.
- Chiricozzi A, Burlando M, Caldarola G, Conti A, Damiani G, et al. (2020) Ixekizumab Effectiveness and Safety in the Treatment of Moderate-to-Severe Plaque Psoriasis: A Multicenter, Retrospective Observational Study. Am J Clin Dermatol 21: 441-447.
- Alcusky M, Lee S, Lau G, Chiu GR, Hadker Net al. (2017) Dermatologist and Patient Preferences in Choosing Treatments for Moderate to Severe Psoriasis. Dermatol Ther (Heidelb) 7: 463-483.
- Hsu L, Snodgrass BT, Armstrong AW (2014) Antidrug antibodies in psoriasis: a systematic review. Br J Dermatol 170: 261-273.
- Rompoti N, Sidiropoulou P, Panagakis P, Stratigos A, Papoutsaki M, et al. (2020) Real-world data from a single Greek centre on the use of secukinumab in plaque psoriasis: effectiveness, safety, drug survival, and identification of patients that sustain optimal response. J Eur Acad Dermatol Venereol 34: 1240-1247.
- Smith JA, Wehausen B, Richardson I, Zhao Y, Li Y, et al. (2018) Treatment Changes in Patients With Moderate to Severe Psoriasis: A Retrospective Chart Review. J Cutan Med Surg 22: 25-30.
- FDA. (2016) FDA approves new psoriasis drug Taltz: U.S. Food & Drug Administration.
- Eli Lilly (2017) Taltz-ixekizumab injection, solution. Addendalndex Summary.
- Gordon KB, Colombel JF, Hardin DS (2016) Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N Engl J Med 375: 345-356.
- Sawyer LM, Cornic L, Levin LA, Gibbons C, Moller AH, et al. (2019) Long-term efficacy of novel therapies in moderate-to-severe plaque psoriasis: A systematic review and network meta-analysis of PASI response. J Eur Acad Dermatol Venereol 33: 355-366.
- Blauvelt A, Lebwohl MG, Mabuchi T, See K, Gallo G, et al. (2020) Long-term efficacy and safety of ixekizumab: A 5-year analysis of the UNCOVER-3 randomized controlled trial. J Am Acad Dermatol 85: 360-368.
- Deza G, Notario J, Lopez-Ferrer A, Vilarrasa E, Ferran M, et al. (2019) Initial results of ixekizumab efficacy and safety in real-world plaque psoriasis patients: a multicentre retrospective study. J Manag Care Spec Pharm 33: 553-559.
- Blauvelt A, Shi N, Burge R, Malatestinic WN, Lin C-Y, et al. (2020) Comparison of real-world treatment patterns among patients with psoriasis prescribed ixekizumab or secukinumab. J Am Acad Dermatol 82: 927-935.
- Blauvelt A, Shi N, Burge R, Malatestinic WN, Lin CY, et al. (2020) Comparison of Real-World Treatment Patterns Among Psoriasis Patients Treated with Ixekizumab or Adalimumab. Patient Prefer Adherence 14: 517-527.
- Blauvelt A, Shi N, Burge R, Malatestinic WN, Lew CR, et al. (2019) Comparison of healthcare costs among psoriasis patients initiating ixekizumab, secukinumab, or adalimumab. J Manag Care Spec Pharm 25: 1366-1376.
- Ispe (2008) Guidelines for good pharmacoepidemiology practices (GPP). Pharmacoepidemiol Drug Saf 17: 200-208.
- CDC (1996) The Health Insurance Portability and Accountability Act of 1996 (HIPPA). CDC, USA.
- Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, et al. (2019) Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol 80: 1029-1072.
- Leonardi C, Maari C, Philipp S, Goldblum O, Zhang L, et al. (2018) Maintenance of skin clearance with ixekizumab treatment of psoriasis: Three-year results from the UNCOVER-3 study. J Am Acad Dermatol. 79: 824-830.
- Leonardi C, Reich K, Foley P, Torii H, Gerdes S, et al. (2020) Efficacy and Safety of Ixekizumab Through 5 Years in Moderate-to-Severe Psoriasis: Long-Term Results from the UNCOVER-1 and UNCOVER-2 Phase-3 Randomized Controlled Trials. Dermatol Ther (Heidelb) 10: 431-447.
- Lockshin B, Cronin A, Harrison RW, McLean RR, Anatale-Tardiff L, et al. (2021) Drug survival of ixekizumab, TNF inhibitors, and other IL-17 inhibitors in real-world patients with psoriasis: The Corrona Psoriasis Registry. Dermatol Ther 34: 14808.
- Corrona. Corrona Registries: Psoriasis. 2017.
- Leonardi C, Reich K, Foley P, Torii H, Gerdes S, et al. Efficacy and Safety of Ixekizumab Through 5 Years in Moderate-to-Severe Psoriasis: Long-Term Results from the UNCOVER-1 and UNCOVER-2 Phase-3 Randomized Controlled Trials. Dermatol Ther (Heidelb) 10: 431-447.
- Liang S-Y, Phillips KA, Wang G, Keohane C, Armstrong J, et al. (2011) Tradeoffs of using administrative claims and medical records to identify the use of personalized medicine for patients with breast cancer. Med Care 49: 1-8.
Citation: Leonardi C, Tao R, Setayeshgar S, Wang S, McMullen S, et al. (2021) Clinical and Quality of Life Outcomes among Ixekizumab Treated Psoriasis Patients in a Real-World Setting. J Clin Dermatol Ther 7: 087.
Copyright: © 2021 Craig Leonardi, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

