
Common Physiological Disorders of White/Irish Potato (Solanum tuberosum) Tubers Produced in Swaziland: A Review
*Corresponding Author(s):
Kwanele A NxumaloHorticulture Department, University Of Swaziland, PO Luyengo, M205, Swaziland
Tel:+268 7612 8026,
Email:aknxumalo@uniswa.sz
Abstract
Physiological disorders of white/Irish potato tubers are abnormalities of the tubers which are not caused by infectious diseases/insects, or animals. Potato tuber abnormalities occur as a result of environmental stress, nutritional deficiencies or excess on the plant. Information was obtained through informal surveys in the four agro-ecological zones of Swaziland, review of existing literature and interviews of key farmers and producers of the potato crop. Physiological disorders of potato tubers encountered in this study included black heart, brown core, cold damage, greening, growth cracks, loose skin, enlarged lenticells, malformation, sprouting and vascular browning. Ways to alleviate various potato tuber disorders are suggested.
Keywords
Physiological Disorders, Irish potato tubers, white potato tubers
INTRODUCTION
The potato also known as the white or ‘Irish’ potato is a member of the solanaceae family thought to have originated in South America in the cool highlands of the Andes, Spanish explorers are believed to have introduced it to Europe and it later spread throughout the world and to the African continent [1,5,6]. In its habitant of origin, it is a cool season perennial. It is susceptible to freezing temperature; optimum temperatures for maximum growth are 10 to 15?C. The plant develops from rhizomes -seed pieces, it is characterized by compound leaves with opposite leaflets. When the plant flower, apical dominance is eliminated and the plant forms lateral branches [5].
The plant grows up to 60 to 120cm in height. The plant is a vine or haulm. Flower colour is cultivar dependent; it can be purple, white or yellow. The potato plant produces fruits similar to tomato and contains seeds which are mostly used for breeding purposes. The plant exhibits a high degree of heterozygosity because it is a polyploidy [7]. The underground portion of the stem has buds which give rise to lateral slender underground stems called stolons. It is the stolons which enlarge to form tubers. Tubers form at the time of flowering and they have buds called ‘eyes’ from where sprouting occurs to give rise to the vegetative plant [1,5,6,8].
In Southern Africa where Swaziland is located potatoes can successfully grow in the highlands or Highveld all year round, elsewhere like the Lowveld during the cool season or winter months [8,9]. Small tubers called ‘sets’ are used for propagation where planting can be on ridges or flatland at a depth of 15 to 30cm at a spacing of 90x30cm or 50x30cm depending on cultivar. The most suitable soils to grow potatoes are sandy loams which are deep, fertile, friable and well drained. The ideal pH is 4.8 to 6.5. Tuberization is promoted by Short Days (SD) and cool temperatures. Compact and poorly drained soils result in production of deformed tubers which is a physiological disorder. High temperatures inhibit tuberisation and it has been reported that tubers are not formed at 30?C and above [1,5,6,8].
Swaziland although still a developing country is classified under the middle income group, however it is still faced with poverty problems and struggling with the HIV and AIDS pandemic [10]. There are high hopes of the 2022 vision by that time the country would have leap flogged to a near fully developed country. The backbone of the country’s economy is largely dominated by agriculture whose contribution to the Gross Domestic Product (GDP) is about 11% [11]. Potato is a horticultural crop, (horticulture is a subsector of the agricultural industry) which is a popular vegetable in Swaziland. During production potato tubers are affected by a number of diseases and physiological disorders which affect produce quality. Most of the physiological disorders are often caused by environmental stress resulting from unpredictable and uncontrollable weather conditions and or mineral deficiencies usually micronutrients [12,13].
Physiological or non-pathogenic disorders in general
Consumers of pre-packed and loose potatoes increasingly desire potatoes of good quality and skin finish. These should have a bloom, which is good and shiny skin with no blemishes. Attention has been focussed on postharvest skin diseases with little attention on physiological disorders. Black heart, brown centre and hollow heart are the most commonly reviewed disorders of potato. Sprouting, netting and loose skin are some of the physiological disorders affecting potato skin finish thereby affecting marketing for either seed or for home consumption.
The objective of this review was to identify common physiological disorders of potato produced under Swaziland environmental conditions in the four agro-ecological zones and suggest ways of alleviating these adverse conditions by situational/environmental manipulation and use of locally available resources.
METHODOLOGY
Agro-ecological zones of Swaziland
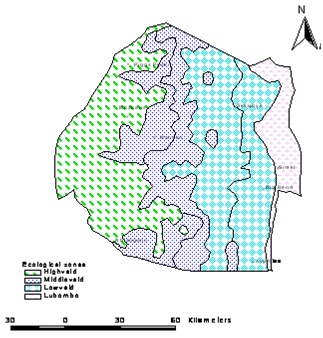
Figure 1: Ecological zones of Swaziland.
The Middleveld is characterized with temperatures ranging from 2.5 to 37.2°C [19]. This region has fertile valleys which favour intensive farming. It has the most diversely cultivated and heavily populated area in the country [11]. Protected nature reserve areas include Mantenga and Milwane [20]. Further east, there is the Lowveld with the largest area coverage of 40% of the country and is drought prone [19]. There is the Western Lowveld which is underlain by sandstone/claystone and the Eastern Lowveld which is underlain by basalt [19]. It has a vegetation of shrubs, and mean temperature ranges from 2.6 to 41.8?C with the bulk of commercial farms growing crops under irrigation, including the three sugar estates in the country and citrus fruit plantations. The nature reserves in the area are: Mlawula, Hlane, Shewula, Mbuluzi, Simunye and Nisela game reserves [11,18]. The fourth region is the escarpment called Lubombo plateau with an altitude of 600m above sea level and climatic conditions similar to the Middleveld [19]. Given the mountainous topography of the Lubombo plateau, only one eighth of the land is arable and the rest is suitable for animal grazing [19].
Common potato tuber physiological disorders encountered in Swaziland’s four agro-ecological zones are reviewed in this paper.
FINDINGS AND DISCUSSION
|
Name of physiological disorder |
Plant part affected |
Symptom |
Cause |
Management |
||
|
1. Black heart |
Tubers- internal |
• The inside of the tubers are greyish black to black and the affected tissue is normally well defined • The affected tissue is hard and leathery |
• Develops as a result of oxygen shortage or an oversupply of CO2 which causes the tissue to die, especially if the oxygen shortage is accompanied by high temperatures • Black heart can also occur in the potato field if high temperatures follow excessive rain which causes an oxygen shortage in the soil • When harvested tubers are left on the land for a long time • When seed planted by hand lie in furrows for a long time before being covered by soil, especially when it is very hot |
After harvesting do not leave tubers in the sun on trailers under tarpaulins • Ensure sufficient ventilation during storage and transportation • Avoid fields with poor drainage if rain is received in the after season. • Make sure that lifted tubers are picked up as soon as possible • Cover seed tubers in furrows as soon as possible after planting
|
||
|
2. Brown core and hollow heart |
Tubers-internal |
The cavities can appear lengthwise or diagonal in the tuber and also have irregular shapes. |
Brown core develops when tubers are very small and temperature is low (<15?C), especially during tuber initiation up to the tuber reaching 50 g. Cells die, turn brown and can easily tear apart. |
Select cultivars that are less prone to the disorder. • Avoid over-irrigation • Avoid a low plant population • If brown centre occurs regularly in early plantings, consider planting later when temperatures are slightly higher • Endeavour to maintain uniform conditions throughout the season by managing fertilisation and irrigation |
||
|
3. Cracking/Growth cracks |
Tubers-external |
• Growth cracks vary in depth and length, but normally occur in the length of the tuber. |
Growth cracks occur during varying soil moisture conditions; • Tubers crack when a dry period is followed by heavy rains or over-irrigation. Moisture uptake causes a quick increase in tuber moisture and growth and consequently an increase in tuber size • Uneven plant population, over fertilisation with nitrogen and nutrient imbalances contribute to the occurrence of growth crack • Application of large amounts of nitrogen at one time after tuber formation |
• Avoid cultivars that are prone to crack if growth cracks regularly occur and cause loss in income • Where possible ensure uniform growing conditions, even plant population, good irrigation scheduling and good fertilisation practices |
||
|
4. Enlarged lenticels |
Tubers-external |
· Raised, white callus-like tissue on the surface of the tubers · The size may vary from inconspicuous to large |
Enlarged lenticels are formed under conditions of oxygen shortage and excessive moisture in the soil surrounding the tubers, or in storage as the cells beneath the lenticels expand and the cell mass breaks through the corky layer. |
• Avoid fields that are prone to water logging • Reduce irrigation two weeks prior to harvesting by irrigating when the plant available water is 40-50%
|
||
|
Tubers-internal |
Exposure for short periods to temperatures around 2?C - 0?C can result in grey or reddish tissue. The tissue can also turn dark grey or black. |
Exposure of tubers to temperatures that vary from just above freezing point (2?C) or below freezing-point. |
Do not leave harvested tubers on the land over night, especially if low temperatures are expected. • Avoid cultivars that tend to bear shallow in regions with low temperatures at the end of the growing season • Do not store seed potatoes on the farm if the correct conditions are not available. Isolate stacks by covering it with grass and/or hessian bags, but preferably store it in a storeroom with temperature control and ventilation • If seed potatoes must be transported in winter, the consignment must be properly covered and it must be done during the day • Ensure that mechanisms are in place for proper temperature control during cold storage • Harvest plantings with tubers close to the soil surface before the first cold front is expected |
||
|
6. Greening |
Tubers-external |
• Greening of the tissue immediately under the skin • The intensity may differ and may also be accompanied by sunburn |
Greening occurs when tubers are exposed to light (sun or artificial). |
• Avoid cultivars that are prone to bear shallowly • Avoid high nitrogen application, especially with cultivars that are prone to form long stolons • Plant in well prepared soils • If possible, irrigate to prevent cracks occurring during dry weather conditions • If tubers are exposed after foliage die-off, it can be ridged to cover it with soil. • Do not expose tubers to light for long periods after harvesting • Use packaging materials that provide sufficient protection against light transmission if potatoes are exposed to light for more than a few days. White paper transmits more light than brown paper |
||
|
7. Internal brown fleck |
Tubers-internal |
Irregular brown flecks that primarily occur in the vascular bundle ring. |
The primary cause of brown fleck is localised shortages in Calcium (Ca). Environmental factors that lead to brown fleck include air and soil temperature, soil types and soil moisture.
|
Ensure that sufficient Ca is available for uptake where and when tubers are formed. Where granular fertilizer is used, the Ca must be available in the whole tuber zone. • Opting for planting times to avoid high temperatures during tuber formation and harvesting, reduces the risk associated with brown fleck Avoid stress conditions at the beginning of the growing season through proper fertilisation and irrigation practices |
||
|
8. Loose skin |
Tubers-external |
• The skin is totally or partially removed from the tubers leading to the underlying tuber tissue being exposed. |
• Excessive application of nitrogen (N) late in the season and wet soil may delay ripening and promote loose skin. |
• Top-growth must be completely dead 14 to 21 days prior to harvesting to promote skin setting • Ensure that the harvester is properly set to prevent tuber damage |
||
|
9. Malformation |
Tubers-external |
Tubers form secondary tubers, some on short stolons and others directly on an eyelet. |
• Malformed tubers form when an interruption in growth is experienced during the growing season as a result of high temperature, often accompanied by moisture stress and nutrient shortage |
• Avoid production in periods when heat waves generally occur • Avoid cultivars that are not adaptable to high temperatures, or plant it during cooler times of the year • Ensure uniform growing conditions |
||
|
10. Netting |
Tubers-external |
• Shallow fissures that give the skin a distinctive net like appearance. |
It is related to climatic conditions and physiological factors in the skin of young tubers. • Moisture stress leads to an increased intensity of the disorder |
Where possible tubers should not be exposed to varying soil moisture conditions. |
||
|
11. Sprouting |
Tubers-external |
Potatoes start to sprout when it is harvested, especially when tubers have been left in the soil for long. |
Sprouting is promoted by high temperature before harvesting. Sprouting on the land is normally an indication that the cultivar is not adapted to the cultivation practices of a specific area. |
Evaluate new cultivars for at least three years in the production area and follow normal cultivation practices to identify cultivars that are not totally adapted. |
||
|
12. Thumb nail cracks |
Tubers-external |
• Mechanical impact manifests often as thumb nail cracks on tubers. |
• The cracks can develop after a slight impact or injury and initially only occur in the skin (periderm) of the tuber without bruising to the underlying tissue. |
• To prevent the occurrence of thumb nail cracks, tubers should not be harvested or handled when it is cold. The temperature of tuber tissue should be >9?C • The crop should be fertilised optimally to ensure that sufficient Ca and Mg are present in the cell-walls |
||
|
13. Vascular browning |
Tubers-internal |
• Vascular browning of variable intensity develops at the stolon end, but in serious cases it can extend throughout the whole vascular ring • Vascular browning appears as speckled, light brown to brown and even dark brown stripes |
Physiological vascular browning is generally associated with a combination of low soil moisture and sudden foliage die-off as a result of chemicals, frost or mechanical removal • Vascular browning can also be caused through infection by tuber-borne pathogens such as Verticillium and Fursarium and leaf roll virus |
Irrigation prior to foliage die-off decreases the incidence of vascular browning, irrespective of the method of foliage die-off. |
Interviews with farmers on potato physiological disorders
Brown core and hollow heart physiological disorders are very common in the Highveld of Swaziland, especially in places like Motshane, Nkhaba, Sgangeni, Nkoyoyo and Mnyokane that are very prone to frost. Growth cracks are more experienced from potatoes grown in the Lower Middleveld and Vuvulane in the Lowveld where there is potato production in the cool season. In 2015 during the El Nino drought, even farmers in the Highveld and the upper Middleveld also experienced a lot of this physiological disorder on their produce due to excessive heat received and shortage of irrigation water. Enlarged lenticels are most seen from potato produced in the Highveld and Middleveld, especially in those places that have waterlogged soils. These places in include: EZulwini, EBuka, Mnyokane, Motshane to name a few. Famers in the Highveld that plant potatoes in late March just before the winter season begin in May experience enlarged frost damage to the plants. Most of them have shifted to planting the potatoes in February so that when frost comes, the plants will be able to withstand it.
Greening physiological disorder is common to potatoes produced in all the 4 agro-ecological zones of Swaziland, especially following heavy rains that wash the soil and expose the potato tubers. This physiological disorder can also be experienced on potatoes are that about to be harvested especially when you have planted varieties like Mondial and Sifra. Exposing potatoes to sunlight can also lead to the occurrence of this physiological disorder, as most of our farmers do not have proper storage facilities that can deter light to enter the harvested produce.
Internal brown fleck is common where potatoes are grown in soils lacking calcium. This is more common in the Highveld where there is lot of nutrient leaching due to excessive rains in most cases. Loose skin and thumbnails damage are most common to potatoes that are harvested using machinery. These physiological disorders are also prone to places like the Highveld, especially in soils lacking calcium and magnesium. Malformation is not very common to potatoes grown in the Highveld, but is it common to potatoes grown in the Lowveld and lower Middleveld especially if the farmers did not plant during the winter season.
Netting and Sprouting are more common to most potatoes grown in the Lowveld. This is due to the fact that this region often receives excessive heat and dry spells, thus promoting the occurrence of these physiological disorders. If the farmers did not adhere to the recommended planting time of potatoes, these physiological disorders become more prevalent. Vascular browning is most common to all potatoes produced in the 4 agro-ecological zones of Swaziland as it is caused by soil pathogens in most cases.
Commonly grown potato varieties in Swaziland’s four agro-ecological zones were Mondial (most common/popular), Sifra, Avalanche and BP1. Susceptibility of a given variety to a particular physiological disorder is dependent on Genetics (G) X Environment/ Agro-ecological zone (E) X Management (M). Severe weather events due to climate change are likely to worsen the physiological disorders of potato tubers.
Encountered disorders are described and various ways to alleviate them are suggested and discussed. It is proposed going forward that the disorders are quantified and losses due to these disorders are determined and their impact on the industry recorded.
Growth cracks
Thumb nail cracks
Enlarged lenticels
Greening
Preventative steps to control this physiological disorder include proper seeding depth, hilling to cover exposed tubers, reducing the time potatoes are exposed to natural light and eliminating exposure to artificial light during storage. If possible, irrigate to prevent cracks occurring during dry weather conditions. Avoid high nitrogen application, especially with cultivars that are prone to form long stolons. Variety choice should also be considered. Use brown packaging materials that provide sufficient protection against light transmission if potatoes are to be exposed to light for more than a few days during marketing [7,24,31,32].
Blackheart
Blackheart is a tuber condition that results from asphyxiation of the tissues of the tuber. This condition occurs in storage or transit when tubers are subjected to very high temperatures are stored where the ventilation is so poor that the supply of oxygen is inadequate, or results from a combination of both factors [33,34]. An oversupply of carbon dioxide whereby the oxygen supply levels are reduced can be another cause [25,26]. Black heart can also occur in the potato field if high temperatures follow excessive rain which causes an oxygen shortage in the soil. The internal symptom is a dark discoloration, greyish to purplish or inky black. Generally, this discoloration is restricted to the heart of the tuber. Usually, the discoloured tissues are sharply set off from healthy tissues. They are firm and leathery if they have dried out [31,35]. In advanced stages affected tissues dry-out and shrink, forming cavities. When tubers are cut soon after injury, the exposed tissues are of normal colour. Shortly after exposure to air, however, they turn pink, then grey, purplish, or brown, and finally become black. Sometimes only grey or brown discolouration is found. This occurs when tubers have been heated to above 55°C or when they have been deprived of all oxygen for considerable periods of time, as in waterlogged soils or in flooded storage pits. The external symptoms of blackheart, which rarely occur, are moist areas on the tuber surface. These areas may be purplish for a short time but, characteristically, these discoloured areas are brown or black [1,29].
To control blackheart, it is recommended that high-temperature storage should be avoided and storage areas should be well ventilated. Seed tubers in furrows should be covered as soon as possible after planting and poorly draining fields should be avoided. After harvesting potatoes during hot weather, the tubers should be promptly removed from the soil surface. To prevent oxygen shortages, tubers should not be stored in solid piles higher than 1.8 meters, even at low temperatures [1,7,24,25,29].
Internal brown spot
Other factors that are implicated with this defect are lack of available soil moisture, particularly late in the growing season; lack of water at some critical stage in the growth of the plant because of either poor soil texture or alternating wet and dry weather; and excessive evaporation of moisture from the foliage. Internal brown spot can also develop during storage if the calcium content is low and if tubers were harvested under high temperature conditions [24,28,32,].
To control this physiological disorder, favourable cultural conditions, such as proper irrigation practices and covering the tubers with five centimetres or more of soil, reduce the severity of internal brown spot. Sufficient calcium must be available for uptake where and when tubers are formed. Opting for planting times to avoid high temperatures during tuber formation and harvesting, and ensuring calcium uptake is not limited by an imbalance between nutritional elements or unfavourable pH reduces risks associated with internal brown spot [24,25, 28,31,32].
Malformation
To control secondary growth (knobby tubers, there is need to; provide uniform growing conditions through out the growing season. Planting of potatoes should be done during the cooler times of the year. Avoid production in periods when heat waves generally occur usually during mid-summer. Irrigation should be maintained uniformly throughout the growing season especially in the warm areas like the Lowveld of Swaziland. Avoid planting those varieties that tend to be excessively knobby, such as Mondial and Sifra and plant varieties (BP1 and Avalanche) that produce round tubers [24-27].
Cold damage
Brown core/centre and hollow heart
Brown centre and hollow heart arise at a higher incidence when growing conditions abruptly change during the season, such as when potato plants recover too quickly after a period of environmental or nutritional stress. When the tubers begin to grow rapidly, the tuber pith can die and/or pull apart, leaving a void in the centre. Brown centre and hollow heart effects likely form during tuber initiation but could also form during tuber bulking [24,37]. Incidence of brown core and hollow heart also increases with periods of stress because of high or low moisture level, more so if the heavy water applications follow a period of stress because of low moisture levels. If the disorder occurs during the early part of the season, then it is most often preceded by brown centre and forms in the stem-end of the tuber, while late-forming hollow heart [28,37].
Brown core and hollow heart can negatively affect tuber quality as these disorders make cut fresh market tubers unattractive and can result in most potatoes failing to make the grade. To prevent these physiological disorders it is important to select varieties that are known to be resistant and by planting when soil temperature reaches adequate levels to lessen the occurrence of these physiological disorders. Potato should be larger and less aged to reduce brown centre and hollow heart because of increased stem number per seed piece. Other practises that can reduce incidence of brown core and hollow heart include: achieving recommended stand establishment, avoiding planting skips, and applying multiple split fertiliser applications especially nitrogen [24,28,37].
Vascular browning
Spouting
Netting
Loose skin
Management of skin physiological disorders
CONCLUSION
REFERENCES
- Miguel, C., 1985. Production and Utilization of Potatoes in the World.Academic press, London, UK.
- Jackson SD (1999) Multiple signalling pathways control tuber induction in potato. Plant Physiology 119: 1-8.
- Karim MR, Hanif MM, Shahidullah SM, Rahman AHMA, Akanda AM, et.al. (2010) Virus free seed potato production through sprout cutting technique under net-house. African Journal of Biotechnology 9: 5852-5858.
- Masarirambi MT, Mandisodza FC, Mashingaidze AB, Bhebhe E (2012) Influence of Plant Population and Seed Tuber Size on Growth and Yield Components of Potato (Solanum tuberosum). International Journal of Agriculture and Biology 14:545-549.
- Yamaguchi M (1983) World Vegetables. Principles, Production and Nutritive Values, AVI Publishing, Westport, Connecticut, USA.
- Peirce LC (1987) Vegetables: Characteristics, Production and Marketing. John Willey &Sons Inc., New York, USA.
- Bohl WH, Johnsoneds SB (2010) Commercial Potato Production in North America: The Potato Association of America Handbook. The Potato Association of America, Ann Arbor, Michigan, USA.
- Norman JC (1992) Tropical Vegetable Production. Arthur H Stockwell Ltd. Elms Court, Ifracombe, Devon, UK.
- Rice RP, Rice LW, Tindall HD (1987) Fruit and Vegetable Production in Africa. MacMillan Publishers Ltd. London and Basingstoke, UK.
- Zwane PE, Masarirambi MT (2009) Kenaf (Hibiscus cannabinus) and allied fibres for sustainable development in Swaziland. Journal of Agriculture & Social Sciences 5: 35-39.
- Thompson CF (2009) Swaziland Business Year Book. Christina Forsyth Thompson, Mbabane, Swaziland, South Africa.
- Masarirambi MT, Mhazo N, Oseni TO, Shongwe VD (2009) Common Physiological Disorders of Tomato (Lycopersicon esculentum) Fruit Found in Swaziland. Journal of Agriculture & Social Sciences 5: 35-39.
- Masarirambi MT, Oseni TO, Shongwe VD, Mhazo N (2011) Physiological disorders of Brassicas/Cole crops found in Swaziland: A review. African Journal of Plant Science 5: 8-14.
- Verma P (2009) Physiological Disorders of Vegetable Crops. In: Russo VM (ed.). Alfa Beta Technical Solutions. International Journal of Vegetable Science 16: 1-170.
- Ceponis MJ, Cappellini RA, Lightner GW (1987) Disorders in cabbage, bunched broccoli and cauliflower shipments to the New York market, 1972-1985. Plant Disease 71: 1151-1154.
- Chiang MS, Chong C, Landry BS, Crete R (1993) Cabbage (Brassica oleracea subsp Capitata L.) In: Kaloo G, Bergh BO (eds.). Genetic Improvement of Vegetable Crops. Pergamon Press, Oxford, UK.
- Jarvis WR, McKeen CD (1991) Tomato Diseases. Agriculture Canada Publication, Ottawa, Canada.
- Government of Swaziland (2007) Swaziland Review. Ministry of Commerce and Industry. Government of Swaziland, Mbabane, Swaziland, South Africa.
- Dlamini GM, T Lupapa (1995) Swaziland: Country report to the FAO International technical conference on plant genetic resources. FAO, Rome, Italy.
- Government of Swaziland (2005) The Swaziland National Biodiversity Strategy and Action Plan. Government of Swaziland, Mbabane, Swaziland, South Africa.
- Peet MM (1992) Fruit cracking in tomato. Hort Technology 2: 216-223.
- Wien HC (1997) The Physiology of Vegetable Crops. CAB International, New York, USA.
- Dorais M, Papadopouos AP, Gosselin A (2001) Greenhouse tomato fruit quality. Wiley online library, Horticulture Reviews 5: 239-319.
- Potatoes South Africa (2016) Physiological tuber disorders. Potatoes South Africa, Pretoria, South Africa.
- Hiller LK, Thornton RE (2008) Managing Physiological Disorders. In: Johnson DA, Powelson ML (eds.). Potato Health Management: Plant health management series. APS Press, USA. Pg no: 261.
- Hiller LK, Koller DC, Thornton RE (1985) Physiological Disorders of Potatoes. In: Li PH (ed.). Potato Physiology. Academic Press, New York, USA. Pg no: 586.
- Stark JC, Love SL (2006) Potato Production Systems. Educational Communications, University of Idaho, Moscow, USA.
- Selman LN, Andrews N, Stone A, Mosley A (2008) What’s Wrong with my Potato Tubers? Diagnosing tuber abnormalities in western Oregon and Washington. Oregon State University, Oregon, USA.
- Snowden AL (1990) A Colour Atlas of Post-harvest Diseases and Disorders of Fruits and Vegetables: General introduction and fruits. Wolfe, London, UK.
- Zotarelli L, Dittmar PJ, Roberts PD, Noling JW, Wells B (2012) Potato Production. In: Zotarelli L, Dittmar PJ, Roberts PD, Noling JW, Wells B (eds.). Vegetable Production Handbook of Florida, University of Florida, Institute of Food and Agricultural Sciences, Gainesville, USA.
- Paul L (1985) Production and Storage of Potatoes. John Wiley and Sons, London, UK.
- Zaag PV, Demagante AL, Ewing EE (1990) Influence of plant spacing on potato (Solanum tuberosum) morphology, growth and yield under two contrasting environments. Potato Research 33: 313-323.
- Lipton WJ (1967) Some effects of low-oxygen atmospheres on potato tubers. American Potato Journal 44: 292-299.
- Reeve RM (1968) Further histological comparisons of back spot, physiological internal necrosis, black heart and hollow heart in potatoes. American Potato Journal 45: 391-401.
- Stevenson W, Loria R, Franc G, Weingartner D (2001) Compendium of potato diseases. American Phytopathological Society, St. Paul, MN, USA.
- Rich AE (1983) Potato Diseases (1stedn). Academic Press, New York, USA.
- Bussan AJ (2007) The Canon of Potato Science: 45. Brown Centre and Hollow Heart. Potato Research 50: 395-398.
- Hooker WJ (1981) Compendium of Potato Diseases. APS Press, St. Paul, MN, USA.
- Rich AE (1981) Verticillium Wilt In: Hooker WJ (ed.). Compendium of Potato Diseases. International Potato Center, St. Paul, MN, USA. Pg no: 62-63.
- Davis JR, Huisman OC, Westermann DT, Hafez SL, Everson DO, et al. (1996) Effects of green manures on Verticillium Wilt of potato. Phytopathology 86: 444-453.
- Sonnewald S, Sonnewald U (2014) Regulation of potato tuber sprouting. Planta 239: 27-38.
- Lulai EC (2007) The Canon of Potato Science: 43. Skin-set and wound-healing / suberisation. Potato Research, 50: 387-390.
- Bussan AL, Sabba RP, Drillas MJ (2009) Tuber maturation and potato storability: Optimising skin set, sugars and solids. University of Wisconsin-Extension, Cooperative Extension Cooperative Extension Publishing, Madison, Wisconsin, USA.
- British Potato Council (2006) Preserving potato skin finish during storage: Research Review. British Potato Council, UK.
Citation: Nxumalo KA, Masarirambi MT, Mabuza M, Muziri T, Masarirambi T (2017) Common Physiological Disorders of White/Irish Potato (Solanum tuberosum) Tubers Produced in Swaziland: A Review. J Agron Agri Sci 1: 001.
Copyright: © 2018 Kwanele A Nxumalo, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

