
Comparative Study in the Management of Postoperative Pain with High Dose Intravenous Paracetamol versus Tramadol
*Corresponding Author(s):
Tanudeep KaurFellow (Urogynecology), AIIMS, New Delhi, India
Email:tanibedi@gmail.com
Abstract
Background: Pain is an unpleasant sensory and emotional experience causing agony and several side effects in a postoperative patient. Thus effective postoperative pain management has a humanitarian role with additional medical and economic benefits Paracetamol (PCM) has been widely used as an effective analgesic and antipyretic for over a century with an established safety profile, and Tramadol is a commonly used intravenous drug for postoperative pain relief. Till now other NSAIDS and opioids were at the centrestage but with their share of side effects. So this study was undertaken to establish a safer drug for postoperative use with effective pain relief.
Study design: A single blind, randomized comparative study of 100 patients, with 50 patients in each group were administered 1000 mg of Intravenous (IV) PCM infusion versus 50 mg of IV Tramadol postoperatively, and at various intervals. Comparison of various side effects and additional need of analgesics in each group was also sought to establish the most suitable analgesic with minimal side effects which can be used in routine after surgery.
Results: VAS (Visual Analogue Scale) score was significantly more in the PCM group as compared to the Tramadol group. PCM group significantly required additional supplemental doses of analgesics, and there was a greater incidence of side effects like nausea/vomiting and drowsiness in the Tramadol group.
Conclusion: Tramadol has edge over paracetamol as far as mean pain scores on VAS are analyzed, but with a higher incidence of nausea, vomiting and drowsiness.
INTRODUCTION
Pain is a major cause of discomfort, distress and agony in a patient. It impairs the patient physically as well as psychologically [1,2]. It is important to allay pain before it starts or gets worse by using some pain relief method. Pain can cause impaired GI motility, splinting and delayed wound healing in a post operative patient [1]. It increases the symphathetic response of the body with an increase in the heart rate, cardiac work load and oxygen consumption. Prolonged pain can reduce the physical activity and lead to venous stasis thus increasing the risk of deep venous thrombosis and associated pulmonary embolism [2]. For decades together, NSAIDS and opioids have taken centrestage but with their accompanying deleterious side effects like respiratory depression, GI bleeding, renal injury, etc., [3]. Recently, their use has seen a downfall in view of the emerging safer alternatives like IV PCM and Tramadol [4]. IV PCM is a known safe drug worldwide, introduced in USA in 2010, as an effective analgesic and antipyretic for both children and adults, with extremely rare side effects (<1/10,000) [5]. Tramadol on the other hand is a synthetic weak opioid belonging to the aminocyclohexanol group, with a centrally acting analgesic action and effects noradrenergic and serotonergic neurotransmission. It has an action similar to that of morphine 5 mg or the alpha 2 agonist, clonidine 150 µg [6].
AIMS & OBJECTIVES
- • To compare and evaluate the analgesic effects of IV PCM 1000 mg versus 50 mg IV Tramadol on a VAS scale in relieving postoperative pain at various scheduled fixed intervals, 0, 8 and 16 hours
- • To determine any additional need of analgesics apart from fixed doses, along with monitoring of vitals at predefined intervals, which are reflective of the extent of pain relief
- • To note side effects like nausea/vomiting and drowsiness and then compare all these variables statistically
MATERIAL AND METHODS
Study setting: Surgical ward, Department of Obstetrics and Gynecology of a tertiary care teaching hospital, AMC, New Delhi
Study design: Single blind randomized comparative study
Study period: 6 months
Ethical clearance: Institution Ethics Committee, AMC, New Delhi
Sample size: 100 postoperative patients >/=18 years of age, were randomly allocated by computer generated random numbers to two groups with 50 patients each, after an informed and written consent with patients blinded for the type of drug administered. Group1 was given IV PCM1000 mg as an infusion over 15 minutes, and group 2 was given IV Tramadol 50 mg, 2mg/kg slow IV immediately after extubation at 0,8 & 16 hours. The various vital parameters like pulse rate, blood pressure, respiratory rate, intensity of pain on VAS scale [2], additional need of analgesics apart from scheduled intervals for VAS>5, were recorded to ascertain the effect of drugs on pain relief at 0 hours, 30 minutes, 1 hour, 4 hours, 8 hours, 12 hours and 24 hours. Also, side effects like nausea/ vomiting & drowsiness or any other side effect was noted. The maximum total dose for IV PCM was 4 g/day and for Tramadol was 400 mg/day. Anaesthetic protocol was similar for all cases.
VAS scale is a 11cm, 10 point scale, with score 0- no pain, 1-3-mild pain, 4-7-moderate pain and 8-10-severe pain [7]. We included patients who underwent uncomplicated anaesthesia, ASA - class I or II, major surgical operations with either abdominal or flank incision including caesarean section, hysterectomy, laparotomy for rupture/unruptured ectopic, ovarian cystectomy for torsion, myomectomy etc. We excluded patients who were pregnant, lactating, drug or alcohol abusers, known allergics to either drug in study, or with any contraindications to use of opioids. We also excluded patients with history of severe renal, cardiac, depressive, hepatic, neurological, pulmonary or haemorrhagic disorders.
Interpretation of results and statistical analysis
The statistical analysis was performed using SPSS version 19.0. t test and pearson-chi square test was applied to assess the various correlations in the data. Value of P < 0.05 is considered significant and P < 0.0001 as highly significant.
RESULTS
Most of our cases were between 41-43 years of age, 58 females and 42 males with no significant statistical difference between the two groups, rendering them equal for comparison. The average pulse rate, average systolic and diastolic blood pressure difference between the two groups was found to be statistically non significant except at 8 hours in the pulse rate, when it was found to be significantly more in the PCM group [Table 1-3]. The average respiratory rate difference was also non significant between the two groups except at 0 hours, just after extubation and at 24 hours, being significantly more in the Tramadol group [Table 4]. The VAS (Visual Analogue Scale) score was found to be statistically significant, being more in the PCM group at 1hour, 4 hours, 8 hours, 12 hours and at 24 hours, thereby stating that patients in the paracetamol group were not having optimum pain relief as compared to the Tramadol group [Table 5], [Figure 1]. Additional need for analgesics was significantly more in the PCM group between 8-16 hours, 11 patients (22%) in comparison to Tramadol, 1patient (2%) [Table 6], [Figure 2]. Incidence of nausea and vomiting was found to be present in 24/50 (48%) patients in the Tramadol group, compared to none in the PCM group, (highly significant) [Table 7]. Drowsiness was also more in the Tramadol group, 18 (36%) patients with a statistical difference [Table 8], [Figure 3]. We did not observe any other side effects like urinary retention, respiratory depression, pruritis, fever etc., in both the groups.
|
Group 1 |
Group 2 |
t value |
p value |
|
|
N |
50 |
50 |
||
|
At 0 hours (mean ± s.d*.) |
84.96 ± 4.647 |
84.46 ± 7.702 |
0.393 |
0.695 (NS)† |
|
At 30 min (mean ± s.d.) |
84.04 ± 4.323 |
82.58 ± 3.908 |
1.772 |
0.080 (NS) |
|
At 1 hour (mean ± s.d.) |
80.96 ± 4.005 |
81.36 ± 3.968 |
0.502 |
0.617 (NS) |
|
At 4 hour (mean ± s.d.) |
81.56 ± 4.700 |
79.72 ± 5.284 |
1.840 |
0.069 (NS) |
|
At 8 hour (mean ± s.d.) |
83.78 ± 4.648 |
81.44 ± 4.590 |
2.533 |
0.013 (S) |
|
At 12 hour (mean ± s.d.) |
81.08 ± 4.548 |
80.68 ± 4.250 |
1.725 |
0.088 (NS) |
|
At 24 hour (mean ± s.d.) |
79.24 ± 6.019 |
80.00 ± 5.599 |
0.684 |
0.496 (NS) |
Table1: Average Pulse Rate postoperatively.
*-Standard deviation, NS-Non Significant, S-Significant
|
Group 1 |
Group 2 |
t value |
p value |
|
|
n |
50 |
50 |
||
|
At 0 hours (mean ± s.d.*) |
126.60 ± 6.145 |
127.62 ± 7.143 |
.766 |
.446 (NS) † |
|
At 30 min (mean ± s.d.) |
127.72 ± 5.686 |
127.68 ± 6.579 |
.033 |
.974 (NS) |
|
At 1 hour (mean ± s.d.) |
128.96 ± 5.455 |
128.68 ± 6.093 |
.242 |
.809 (NS) |
|
At 4 hour (mean ± s.d.) |
127.92 +\-4.020 |
128.34 ± 6.100 |
.407 |
.685 (NS) |
|
At 8 hour (mean ± s.d.) |
127.92 ± 5.375 |
128.92 ±5.965 |
.881 |
.381 (NS) |
|
At 12 hour (mean ± s.d.) |
127.84 ± 4.137 |
128.98 ± 6.238 |
1.077 |
.284 (NS) |
|
At 24 hour (mean ± s.d.) |
128.96 ± 5.455 |
128.88 ± 5.965 |
.070 |
.944 (NS) |
Table 2: Average systolic blood pressure.
*-Standard deviation, NS-Non Significant, S-Significant
|
N |
Group 1 |
Group 2 |
t value |
p value |
|
At 0 hours (mean ± s.d.*) |
80.32+/-2.810 |
80.56+/-2.651 |
.439 |
.661 (NS) |
|
At 30 min (mean ± s.d.) |
80.56+/-2.357 |
80.40+/-1.069 |
.437 |
.663 (NS) |
|
At 1 hour (mean ± s.d.) |
80.40+/-3.130 |
80.76+/-2.134 |
.672 |
.503 (NS) |
|
At 4 hours (mean ± s.d.) |
80.76+/-1.802 |
80.84+/-2.461 |
.185 |
.853 (NS) |
|
At 8 hours (mean ± s.d.) |
80.40+/-3.130 |
80.48+/-1.182 |
.169 |
.866 (NS) |
|
At 12 hours (mean ± s.d.) |
80.76+/-1.802 |
80.72+/-2.129 |
.101 |
.919 (NS) |
|
At 24 hours (mean ± s.d.) |
80.80+/-1.761 |
80.52+/-1.266 |
.913 |
.364 (NS) |
Table 3: Average diastolic blood pressure.
*-Standard deviation, NS-Non Significant, S-Significant
|
N |
Group 1 |
Group 2 |
t value |
p value |
|
At 0 hours(mean ± s.d.*) |
18.68+/-1.834 |
19.32+/-1.316 |
-.2004 |
.048 (S) |
|
At 30 min (mean ± s.d.) |
18.46+/-1.982 |
18.48+/-1.832 |
-.052 |
.958 (NS) |
|
At 1 hour (mean ± s.d.) |
17.24+/-2.282 |
17.40+/-1.906 |
-.381 |
.704 (NS) |
|
At 4 hours (mean ± s.d.) |
16.96+/-2.070 |
17.02+/-2.290 |
-.137 |
.891 (NS) |
|
At 8 hours (mean ± s.d.) |
18.16+/-1.845 |
17.80+/-1.948 |
.949 |
.345 (NS) |
|
At 12 hours (mean ± s.d.) |
17.00+/-2.777 |
16.52+/-2.735 |
.871 |
.386 (NS) |
|
At 24 hours (mean ± s.d.) |
16.56+/-2.865 |
17.68+/-2.751 |
-1.994 |
.049 (NS) |
Table 4: Average respiratory rate.
*-Standard deviation, NS-Non Significant, S-Significant
|
|
Group 1 |
Group 2 |
t value |
P value |
|
N |
50 |
50 |
||
|
At 0 hours (mean ± s.d*.) |
4.20 ± 0.969 |
4.12 ± 1.722 |
.286 |
.775 (NS) |
|
At 30 min (mean ± s.d.) |
3.54 ± 1.073 |
3.16 ± 1.419 |
1.510 |
.134 (NS) |
|
At 1 hour (mean ± s.d.) |
2.94 ± 0.712 |
2.48 ± 0.953 |
2.735 |
.007 (S)‡ |
|
At 4 hour (mean ± s.d.) |
3.32 ± 1.203 |
2.54 ± 0.994 |
3.535 |
.001 (S) |
|
At 8 hour (mean ± s.d.) |
3.70 ± 0.909 |
3.26 ± 1.103 |
2.177 |
.032 (S) |
|
At 12 hour (mean ± s.d.) |
2.92 ± 1.122 |
2.50 ± 0.995 |
1.981 |
.050 (S) |
|
At 24 hour (mean ± s.d.) |
3.00 ± 0.728 |
2.38 ± o.923 |
3.728 |
.000 (S) |
Table 5: Visual analogue score.
*-Standard deviation, NS-Non significant, S-Significant
|
|
Group 1 |
Group 2 |
t value |
p value |
|
During 0-8 hours |
0.02+/-0.141 |
0.00+/-0.000 |
1.000 |
.320 (NS) |
|
During 8-16 hours |
0.22+/-0.418 |
0.02+/-0.141 |
3.202 |
0.002 (S) |
|
During 16-24 hours |
0.00+/-0.000 |
0.00+/-0.000 |
- |
- |
Table 6: Additional supplemental doses taken.
NS-Non significant, S-Significant
|
Nausea/Vomiting |
Group 1 |
Group 2 |
Total |
|
Absent |
50 (100%) |
26 (52%) |
76 |
|
Present |
0 |
24 (48%) |
24 |
|
Total |
50 |
50 |
100 |
Table 7: Incidence of nausea/ vomiting.
x2 = 31.579; df = 1 ;p=0.000,HS (Highly Significant)
|
Drowsiness |
Group 1 |
Group 2 |
Total |
|
Absent |
46 (92%) |
32 (64%) |
78 |
|
Present |
4 (8%) |
18 (36%) |
22 |
|
Total |
50 |
50 |
100 |
Table 8: Incidence of drowsiness.
x2 = 11.422; df = 1 ; p = 0.001; Significant
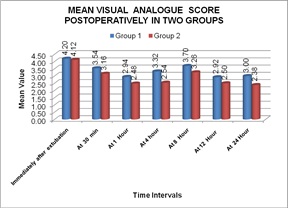 Figure 1: Mean VAS scale.
Figure 1: Mean VAS scale.
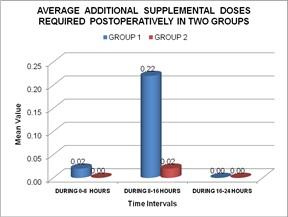 Figure 2: Average additional supplemental doses.
Figure 2: Average additional supplemental doses.
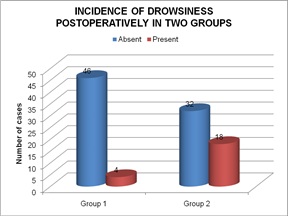
Graph 3: Incidence of drowsiness.
DISCUSSION
After the fallback of NSAIDS, physicians have started opting for postoperative analgesic drugs with lesser adverse effects like IV PCM and IV Tramadol [4]. IV PCM was introduced in 2010 in USA, but was not much in use until recently because of its cost and a lack of adequate clinical trials advocating its efficacy. A review of 14 RCT’s, was done by et al., in favour of its use in 12/14 of them [8]. PCM with its central analgesic action can be given by various routes like oral, rectal, intramuscular, and IV [8]. It is excreted by liver after conjugation. It offers adequate relief in both outpatient and inpatient departments. It’s peak action comes in 1 hour and lasts 4-6 hours [8], has a superior efficacy in a 2gm IV starting dose as compared to 1 gm, as reported by et al., [9]. Also, Tramadol has precedence over other analgesics due to no adverse effects on cardiac and respiratory systems, and can be safely administered to low reserve patients like elderly, frail, smokers, diabetics, obese, with deranged hepatic and renal systems and in whom NSAIDS have been contraindicated [10]. Literature is teeming with comparative studies between IV PCM and Tramadol, but most studies conclude about their efficacy at par with slightly more side effects in the Tramadol group, and an early onset of action with PCM group, though our study had Tramadol exceeding in supremacy for pain relief analysed on a VAS scale, and via need for additional doses of analgesics but side effects like other studies were more in the Tramadol group. Examples are Kela et al., [3] who compared pain relief in postoperative cardiothoracic patients and found effective results with both drugs, with an increased nausea, vomiting in the Tramadol group. Akcali et al., [11] who compared 3 drugs, the third being Lornoxicam apart from PCM and Tramadol, and reported at par results for all 3 in postoperative pain relief. Some other studies clearly reported IV PCM being better than Tramadol like Mohammad et al., [1], Sinatra et al., [12] who in orthopaedic patients post surgery found significantly reduced morphine consumption in 24 hours, when IV PCM was used. Singh B et al., [13] used it for post elective surgery patients and reported results in its favour. Nikoda et al., [14], considered it as an important part of a non opioid multimodal early postpone pain relief therapy. IV PCM works synergistically better over a background of IV Tramadol in post operative period, it was concluded in patients of median sternotomies by Cattabriga et al., [15]. PCM is superior in early post operative recovery, but equal to Tramadol in analgesia, Uysal et al., [16]. Similar results as our study were reported by Dejonckheere M et al., [2], with mean pain scores using VAS being significantly lower with Tramadol ( p= 0.03) but with a higher incidence of nausea and vomiting (p=0.01) in the first 2 postoperative hours, subsiding thereafter. Tramadol also proved better in day care tonsillectomies in children, with lesser need of rescue analgesics, Pendeville et al., [17], in nephrectomy patients, Manne VS et al., [18], due to its quick onset of action. Also, in a comparison of Tramadol versus Nalbuphine by Kiran KS et al., [19], Tramadol proved superior. Emesis of Tramadol was a notable point in various studies like Mohammad et al., [1], Aghamir et al., [20], Kumari Usha Rani et al., [21], Kiran KS et al., [19], D.P. Prosser et al., [22], A.C. Senel et al., [23] and our study. Also, we could not find much data on analysis of need for additional supplemental doses of analgesics and on analysis of vital parameters to determine the effectiveness of analgesics postoperatively except in a study by Mohammad et al., [1]. Thus, still we have to walk a long way before these drugs are a routine in our postoperative wards.
CONCLUSION
IV PCM 1000 mg and IV Tramadol 50 mg both are safe and effective in providing postoperative analgesia, with Tramadol having an edge over PCM, as far as mean pain scores and lesser need of additional analgesics is concerned but with a higher incidence of nausea, vomiting and drowsiness.
ACKNOWLEDGEMENT
I want to thank my parents, Mr Jaspal Singh and Mrs. Kuldeep Kaur for their unending support.
STATEMENT OF AUTHOR CONTRIBUTION
Each of the authors: namely Dr Tanudeep Kaur as first author and corresponding author as well as Dr Ravinder Pal Singh and Dr Anukriti Kumarias co-authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content. They have made substantial contributions to the conception and design of the work; as well as in the acquisition, analysis, and interpretation of data for the work; and in drafting the work and revising it critically; AND in the final approval of the version to be published; AND agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The manuscript in its final form has been read and approved by all the authors and all testify to the honest work represented in the manuscript.
REFERENCES
- Shahid M, Manjula BP, Sunil BV (2015) comparative study of intravenous paracetamol and intravenous tramadol for postoperative analgesia in laparotomies. Anesth Essays Res 9: 314-319.
- Dejonckheere M, Desjeux L, Deneu S, Ewalenko P (2001) Intravenous tramadol compared to propacetamol for postoperative analgesia following thyroidectomy. Acta Anaesthesiol Belg 52: 29-33.
- Kela M, Umbarkar S, Sarkar M, Garasia M (2011) Comparative study of efficacy of IV paracetamol vs IV tramadol for postoperative pain relief after cardiac surgery. Bombay Hospital Journal 53: 582-586.
- Flouvat B, Leneveu A, Fitoussi S, Delhotal-Landes B, Gendron A (2004) Bioequivalence study comparing a new paracetamol solution for injection and propacetamol after single intravenous infusion in healthy subjects. Int J Clin Pharmacol Ther 42: 50-57.
- Duggan ST, Scott LJ (2009) Intravenous paracetamol (acetaminophen) 69: 101-113.
- Lehmann KA (1997) [Tramadol in acute pain]. Drugs 53: 25-33.
- Wewers ME, Lowe NK (1990) A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health 3: 227-236.
- Macario A, Royal MA (2011) A literature review of randomized clinical trials of intravenous acetaminophen (paracetamol) for acute postoperative pain. Pain Pract 11: 290-296.
- Juhl GI, Norholt SE, Tonnesen E, Hiesse-Provost O, Jensen TS (2006) Analgesic efficacy and safety of intravenous paracetamol (acetaminophen) administered as a 2 g starting dose following third molar surgery. Eur J Pain 10: 371-377.
- Scott LJ, Perry CM (2000) Tramadol: A review of its use in perioperative pain. Drugs 60: 139-176.
- Akcali GE, Iskender A, Demiraran Y, Kayikci A, Yalcin GS, et al. (2010) Randomized comparison of efficacy of paracetamol, lornoxicam, and tramadol representing three different groups of analgesics for pain control in extracorporeal shockwave lithotripsy. J Endourol 24: 615-620.
- Sinatra RS, Jahr JS, Reynolds LW, Viscusi ER, Groudine SB, et al. (2005) Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology 102: 822-831.
- Singh B, Singh I, Singh A (2015) The Efficacy of Intravenous Paracetamol Versus Tramadol for Postoperative Analgesia after Elective Surgery. Int J of Medical and Dental Sciences 4: 547.
- Nikoda VV, Makarova VV, Maiachkin RB, Bondarenko AV (2006) [Clinical aspects of analgesia with intravenous paracetamol in the early postoperative period]. Anesteziol Reanimatol 6: 54-58.
- Cattabriga I, Pacini D, Lamazza G, Talarico F, Di Bartolomeo R, et al. (2007) Intravenous paracetamol as adjunctive treatment for postoperative pain after cardiac surgery: A double blind randomized controlled trial. Eur J Cardiothorac Surg 32: 527-531.
- Uysal HY, Takmaz SA, Yaman F, Baltaci B, Basar H (2011) The efficacy of intravenous paracetamol versus tramadol for postoperative analgesia after adenotonsillectomy in children. J Clin Anesth 23: 53-57.
- Pendeville PE, Von Montigny S, Dort JP, Veyckemans F (2000) Double-blind randomized study of tramadol vs. paracetamol in analgesia after day-case tonsillectomy in children. Eur J Anaesthesiol 17: 576-582.
- Manne VSSK, Gondi SR (2017) Comparative Study of the Effect of Intravenous Paracetamol and Tramadol in Relieving of Postoperative Pain after General Anesthesia in Nephrectomy Patients. Anaesth Essays Res 11: 117-120.
- Kiran KSDK, Vyas V, Patil S (2018) Comparative efficacy and safety of intravenous tramadol and nalbuphine for pain relief in postoperative patients. Indian J Pain 32: 96-101.
- Aghamir SK, Mojtahedzadeh M, Alizadeh F, Khalili H, Najafi A, et al. (2005) Propacetamol vs. Tramadol for post–operative pain management after urologic surgery. Internet J Pharmacol 4: 2.
- Rani KU, Zutshi V, Patel M, Marwah S (2016) Analgesic efficacy of intravenous paracetamol versus intravenous tramadol after caesarean section: A single blind randomized controlled study. Int J Reprod Contracept Obstet Gynecol 5: 4285-4289.
- Prosser DP, Davis A, Booker PD, Murray A (1997) Caudal tramadol for postoperative analgesia in pediatric hypospadias surgery. Br j Anaesth 79: 293-296.
- Senel AC, Akyol A, Dohman D, Solak M (2001) Caudal bupivacaine-tramadol combination for postoperative analgesia in pediatric herniorrhaphy. Acta anaesthesiol Scand 45: 786-789.
Citation: Kaur T, Singh R, Kumari A (2020) Comparative Study in the Management of Postoperative Pain with High Dose Intravenous Paracetamol versus Tramadol. J Reprod Med Gynecol Obstet 5: 056.
Copyright: © 2020 Tanudeep Kaur, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

