
Journal of Plant Science Current Research Category: Agriculture
Type: Research Article
Comparative study of M. oleifera and M. ovalifolia survival rates in Central Namibia
*Corresponding Author(s):
Charles NtahonshikiraDepartment Of Pathobiology, University Of Namibia, Windhoek, Namibia
Tel:+ 264 612063815,
Email:cntahonshikira@unam.na
Received Date: Jun 03, 2017
Accepted Date: Oct 11, 2017
Published Date: Oct 25, 2017
Abstract
The objective of this study was to compare the survival rates of Moringa oleifera and Moringa ovalifolia grown at the Neudamm Experimental Farm of the University of Namibia in the Central Namibia rangeland. This part of Namibia being arid and semi-arid, the growing of drought resistant fodder trees to aid in the provision of animal fodder or supplement is essential and paramount to livestock farmers. Many trees including Moringa species go into dormancy during winter season. After winter, survived plants sprout while others die off permanently due to severe winter cold. It is upon this background that both Moringa species were grown to evaluate their survival rates for three winter seasons. All trees were counted after each winter season for the first four months and recorded the number of survived and dead trees. These fodder plants may be used to boost the animal production sector of Namibia if they are proven to withstand the harsh environmental conditions, namely, very cold winter and constant drought. The results revealed that M. oleifera and M. ovalifolia survived at different rates though they are from the same Moringaceae family and were grown under the same conditions. On average, M. oleifera had 107 total survived trees, which is equivalent a total survival rate of 89.19%; while, M. ovalifolia had 38 total survived trees with a total survival rate of 87.98% for the three winter (2014, 2015 and 2016) seasons. Statistically, there was no significant difference (P > 0.05) between M. oleifera and M. ovalifolia total survival trees after the 2014 and 2016 winter seasons; however, there was significant difference (P < 0.05) between the two species survived trees after the 2015 winter season. Hence, M. oleifera is proven to have higher survival rate under these adverse climatic and environmental conditions compare to M. ovalifolia despite the latter being a native of Namibia.
Keywords
Arid, Moringa oleifera; Moringa ovalifolia; Semi-arid; Survival rate; Winter season
INTRODUCTION
All environments are stressful to plants in one way or another. The significance of a stress to plant survival depends on how long an environment has existed and, therefore, on how long plant taxa have had to evolve adaptations to the environmental stressor [1]. Climate plays an important role in the growth, development and survival of plants. It governs the extent of plants’ area of distribution and sets limits for their survival. This is seen on the large scale in the global distribution of the various types of vegetation according to zonal climate and soil type, and on a smaller scale in the distribution of plant species and communities according to local conditions [2]. Frost, soil moisture and light intensity all play a role, not only in shaping the trees, but also in determining whether a tree can grow in a particular environment or not [3].
Namibia is a semi-arid and drought-pruned country [4,5]. The vegetation patterns of Namibia mostly depend on the type of climate in a particular part of the country. In the central, west and south, the vegetation types are Acacia tree-and-shrub savannah and dwarf shrub savanna [6]. Drought-resistant plants like M. oleifera, which Morton [7] described as a boon to arid lands can grow and survive in such environments. Moringa is widely adapted to the tropics and subtropics [8]. Moringa oleifera is a fast-growing and drought-resistant tree that is native to the Southern foothills of Himalayans in Northern India [9]. Moringa ovalifolia, a native tree to Namibia and Angola is well adopted to its native semi-arid and drought-pruned environment physiologically, which can be seen in its roots systems as discussed by Morlu Korsor et al., and Ministry of Agriculture, Water and Forestry [10,11].
Moringa is the only genus from the Moringaceae family [12,13]. Table 1 shows the taxonomic classification of Moringa.
Namibia is a semi-arid and drought-pruned country [4,5]. The vegetation patterns of Namibia mostly depend on the type of climate in a particular part of the country. In the central, west and south, the vegetation types are Acacia tree-and-shrub savannah and dwarf shrub savanna [6]. Drought-resistant plants like M. oleifera, which Morton [7] described as a boon to arid lands can grow and survive in such environments. Moringa is widely adapted to the tropics and subtropics [8]. Moringa oleifera is a fast-growing and drought-resistant tree that is native to the Southern foothills of Himalayans in Northern India [9]. Moringa ovalifolia, a native tree to Namibia and Angola is well adopted to its native semi-arid and drought-pruned environment physiologically, which can be seen in its roots systems as discussed by Morlu Korsor et al., and Ministry of Agriculture, Water and Forestry [10,11].
Moringa is the only genus from the Moringaceae family [12,13]. Table 1 shows the taxonomic classification of Moringa.
| Kingdom | Plantae | Plants |
| Subkingdom | Tracheobionta | Vascular plant |
| Super division | Spermatophyta | Seed plant |
| Division | Magnoliophyta | Flowering plant |
| Class | Eudicots | Dicotyledons |
| Subclass | Rosids | ---- |
| Order | Brassicales | ---- |
| Family | Moringaceae | Horse-radish tree family |
| Genus | Moringa | Horse-radish tree |
Source: [12,14]
There are 13 known species of the sole genus Moringa, which belonging to the Moringaceae family. These species are divided into three groups based on the shapes of their trunks: slender trees, bottle trees and tuberous shrubs. Moringa oleifera of India, Moringa concanensis of India, Pakistan and Bangladesh, and Moringa peregrina of Arabia, Red Sea area, Egypt, Sinai, Israel and Sudan belong to the slender trees. Moringao valifolia of Namibia and Angola, Moringa drouhardii and Moringa hildebrandtii of Madagascar; Moringa stenopetala of Ethiopia and Kenya belong to the bottle trees. And the last group include Moringa arborea of Kenya; M. rivae of Kenya and Ethiopia; Moring aborziana of Kenya and Somalia, Moringa pygmaea of Somalia; Moringa longituba of Kenya, Ethiopia and Somalia; and Moringa ruspoliana of Kenya, Ethiopia and Somalia which belong to tuberous shrubs and trees of Northeast Africa [9,12,13,15,16]. Although Moringa has such many species, M. oleifera is the most adapted plant worldwide compared to other species. It has also been established that of the known species, M. oleifera is also the most widely known [16]. Many studies have been done on its uses and numerous beneficial properties in the plant kingdom [9,17]. On the other hand, M. ovalifolia roots, bark and wood are eaten by goats and trees also browsed by giraffe [3]. It is less known and studied in comparison to M. oleifera.
The problem is that Namibia is both arid and semi-arid country with very cold winter season that occurs every year and affects rangeland productivity especially trees and grasses that support the livestock production. Sijssens [18] discussed thatthe cultivation of plantations of drought-tolerant fodder shrubs should become a priority in the Namibian rangelands to improve their productivity. However, Namibia being described as the driest climate country in Sub-Saharan Africa by Schalkwyk and Agra Professional Services [19,20], trees cultivation and survival are challenged by the harsh climatic conditions (winter and drought). Therefore, M. oleifera, which is very easy to establish and highly adaptable tree [21] would serve the purpose of Namibia’s rangeland improvement through its incorporation into rangelands along with M. ovalifolia, which is to some extent slower growing and less productive at first but very highly drought-tolerant tree.
There are 13 known species of the sole genus Moringa, which belonging to the Moringaceae family. These species are divided into three groups based on the shapes of their trunks: slender trees, bottle trees and tuberous shrubs. Moringa oleifera of India, Moringa concanensis of India, Pakistan and Bangladesh, and Moringa peregrina of Arabia, Red Sea area, Egypt, Sinai, Israel and Sudan belong to the slender trees. Moringao valifolia of Namibia and Angola, Moringa drouhardii and Moringa hildebrandtii of Madagascar; Moringa stenopetala of Ethiopia and Kenya belong to the bottle trees. And the last group include Moringa arborea of Kenya; M. rivae of Kenya and Ethiopia; Moring aborziana of Kenya and Somalia, Moringa pygmaea of Somalia; Moringa longituba of Kenya, Ethiopia and Somalia; and Moringa ruspoliana of Kenya, Ethiopia and Somalia which belong to tuberous shrubs and trees of Northeast Africa [9,12,13,15,16]. Although Moringa has such many species, M. oleifera is the most adapted plant worldwide compared to other species. It has also been established that of the known species, M. oleifera is also the most widely known [16]. Many studies have been done on its uses and numerous beneficial properties in the plant kingdom [9,17]. On the other hand, M. ovalifolia roots, bark and wood are eaten by goats and trees also browsed by giraffe [3]. It is less known and studied in comparison to M. oleifera.
The problem is that Namibia is both arid and semi-arid country with very cold winter season that occurs every year and affects rangeland productivity especially trees and grasses that support the livestock production. Sijssens [18] discussed thatthe cultivation of plantations of drought-tolerant fodder shrubs should become a priority in the Namibian rangelands to improve their productivity. However, Namibia being described as the driest climate country in Sub-Saharan Africa by Schalkwyk and Agra Professional Services [19,20], trees cultivation and survival are challenged by the harsh climatic conditions (winter and drought). Therefore, M. oleifera, which is very easy to establish and highly adaptable tree [21] would serve the purpose of Namibia’s rangeland improvement through its incorporation into rangelands along with M. ovalifolia, which is to some extent slower growing and less productive at first but very highly drought-tolerant tree.
MATERIALS AND METHODS
Study Site
A Moringa orchard of 0.21 hectares (ha) (0.11 ha for M. oleifera and 0.10 ha for M. ovalifolia) was established in 2014 at the agronomy site of the Neudamm Experimental Farm of the University of Namibia. The farm is about 30 km east of Windhoek, with an area of 10, 187 hectares of land. It was carried out over two growing seasons starting from February 2014 and ending in January 2017. Neudamm Campus is located at 22° 30′ 07″S and at17° 22′ 14″E, and at an altitude of 1762 meters above sea level. The temperature ranges between a minimum of -7°C and a maximum 44°C [22].The farm received an annual average rainfall of 713 mm, 314 mm and 280 mm in 2014, 2015 and 2016 respectively [23], which are low due to severe drought that was declared as an in emergency in 2013 by the President of Namibia [24].
The vegetation of central Namibia is characterized as open savanna vegetation with a continuous grass and herb layer and a more or less dense shrub layer.Thorny shrubs, mostly Acacia species, may form very dense stands with only little understorey growth [5,6]. Neudamm Farmbeing in central Namibia, the vegetation is classified as highland savanna (semi-arid savanna) and characterized by grasses, shrubs and trees that are well spread over the farm. An annual grass like Melinis repens and perennial grass like Schmidtia pappophoporoides, Anthephora pubescens and Brachiaria nigropedata are well represented on the farm. Different types of trees like Acacia brownii, Acacia erioloba, Acacia mellifera as well as shrubs like Greviaflava are found on Neudamm Farm. The estimated carrying capacity is about 12 hectares per large stock unit or 45kg per hectare biomass [5,25-27].
The vegetation of central Namibia is characterized as open savanna vegetation with a continuous grass and herb layer and a more or less dense shrub layer.Thorny shrubs, mostly Acacia species, may form very dense stands with only little understorey growth [5,6]. Neudamm Farmbeing in central Namibia, the vegetation is classified as highland savanna (semi-arid savanna) and characterized by grasses, shrubs and trees that are well spread over the farm. An annual grass like Melinis repens and perennial grass like Schmidtia pappophoporoides, Anthephora pubescens and Brachiaria nigropedata are well represented on the farm. Different types of trees like Acacia brownii, Acacia erioloba, Acacia mellifera as well as shrubs like Greviaflava are found on Neudamm Farm. The estimated carrying capacity is about 12 hectares per large stock unit or 45kg per hectare biomass [5,25-27].
Field preparation and seedlings’ transplantation
The Moringa orchard was established on an old cereal production land. As such, clearing of grass and ploughing [28] were done concurrently on the field using a tractor, which incorporated the grass and vegetative residues into the soil. Since it was a rainy season, the field was left for the grass and vegetative residues to rot within a month before transplanting as suggested by Onwueme & Sinha [29]. After a month, holes were dug at 50 cm diameter and depth for the purpose of loosening the soil and retaining moisture in root zones as well as enabling seedlings roots to develop rapidly. The holes were left open for rain to fall, after which seedlings were transplanted the following day in the morning and at sunset to avoid sun stress. The organic matter in soils consists of the recent remains of plants, microbes, and animals and the resistant compounds resulting from the rotting or decomposition processes [30]. In order to add organic fertilizers to the soil [28], five kilograms (kg) of cow manure was thoroughly mixed with topsoil and used for transplanting the seedlings in the field as recommended by Fuglie et al.,[31].The total of 120 M. oleifera and 64 M. ovalifolia seedlings (both two-months old) were transplanted as demonstrated by Fayolle et al., [32] in February 2014 on the field at spacing distances of 2.5 m × 2.5 m and 3 m × 3 m for M. oleifera as suggested by Fuglie et al. and Radovich [8,31]. For M. ovalifolia spacing distances were 3 m × 3 m, 3.5 m × 3.5 m, 4 m × 4 m and 4.5 m × 4.5 m between rows and plants, respectively. For M. oleifera, the field was divided into two plots with four blocks within each plot. Fifteen seedlings were transplanted in each block, which resulted in total of 120 trees. Moringa ovalifolia, on the other hand had four spacing distances with four blocks which had 16 plants each, resulting to a total of 64 seedlings. The four blocks were meant to determine the appropriate spacing distances since little is known about its domestication. The seedlings had average heights of 19 cm for M. oleifera and 13 cm for M. ovalifolia at transplanting. Transplanting was completed within 2 weeks.It is worth noting thatthis study was focused on the survival rates of the two Moringa species as suggested by Fayolle et al.,[32] after winter seasons; other parameters such as density and fertilizer treatment levels were not considered although they were used.
Watering of trees
Watering was done once a week during the first summer growing season (2014/2015) because the trees were younger with shallow root systems and needed water at closer intervals. During the second and third summer growing seasons (2015/2016), watering was done once fortnightly because the trees were bigger with well-established tap root systems, thus, needing less water to survive. As the plants continue to grow, roots elongate from topsoil to subsoil in which the supply of water, nutrients, oxygen and other growth factors are different. The topsoil frequently becomes depleted of water in dry periods, whereas there is still enough water in the subsoil, resulting to greater dependence of plants on the subsoil for water and nutrients [30]. During winter, watering was done once every two weeks for the three seasons (2014, 2015 and 2016 winter) to avoid freezing of the trees and subsequent mortality. Fuglie et al., [31] discussed that M. oleifera trees do not need much watering, except in very dry conditions when it must be done regularly for the first two months and afterwards only when the trees are obviously suffering.
Application of fertilizers
According to Foth [30], plants need certain essential nutrients such as N, P, K, Ca, Mg, S, Cu, Zn and so on to complete their life cycle. Therefore, superphosphate fertilizer with a P content of 83 g/kg (Wonder superphosphate granular, AGRO-SERVE (Pty) Ltd., Bryanston, South Africa) and nitrogen fertilizer with an N content of 280 g/kg (Limestone Ammonium Nitrate - LAN), WONDER HORTICULTURAL PRODUCTS (Pty) Ltd., Silverton, South Africa) were applied at the rate of 0 g, 100 g, 200 g and 300 g a month after transplanting to boost the root- and leaf-system development [8] since winter was to begin two months ahead. Both Moringa species were cultivated in the same field of 0.21 ha, but divided into two parts in which M. oleifera was planted on 0.11 ha and M. ovalifolia on 0.10 ha.
Data collection procedures and analysis
Data collection included counting of trees for survival rates after each winter season. All trees were counted to determine the survival rates and sprouting rate after winter seasons as well as the duration of dormancy of each Moringa species. In Namibia, rainfall is strongly seasonal since more than 99% of rainfall occurs in the summer (October to April) months [4].
Data collected were analysed using General Linear Models (GLM) Pairwise Comparisons in which the number of survived Moringa trees were used as dependent variables while Moringa species were used as independent variables to test the level of survival rates based on species. Means comparison was used to derive descriptive statistics. The Statistical Package for Social Sciences (SPSS® version 23) was used to carry out the statistical analysis while Microsoft Excel® version 13 were used to compare the two Moringa species survival rates as well as the years in figures.
Data collected were analysed using General Linear Models (GLM) Pairwise Comparisons in which the number of survived Moringa trees were used as dependent variables while Moringa species were used as independent variables to test the level of survival rates based on species. Means comparison was used to derive descriptive statistics. The Statistical Package for Social Sciences (SPSS® version 23) was used to carry out the statistical analysis while Microsoft Excel® version 13 were used to compare the two Moringa species survival rates as well as the years in figures.
RESULTS AND DISCUSSION
The results of this research include the survival and sprouting rates of M. oleifera and M. ovalifolia after three winter seasons (2014, 2015 and 2016). The results clearly indicate the rate at which sprouting occurred based on the level of maturation of the trees in consecutive years.
Soil composition of the field
The results of soil analysis are found in table 2. The soil was analysed at the Ministry of Agriculture, Water and Forestry’s soil laboratory in Windhoek, Namibia. The results show the soil pH, Electrical Conductivity or Soluble Salts (EC), Organic Matter (OM), Phosphorus (P), Potassium (K), Calcium (Ca), Magnesium (Mg), Sodium (Na) and Soil Texture. Johnston et al., [33] stated that phosphorus must be in adequate, readily available reserves in the soil; however, most un-manured soils contain too little readily available phosphorus to meet the large demand of crops, particularly during certain growth periods such as root development of seedlings and flowering. Gibbon et al., [34] pointed out that soils in semi-arid regions are very low in OM content (often less than 0.5%), which confirm the results of this study (0.87% and 0.65% for 0-30 cm and 30-60 cm depth respectively). The OM contentof this study was lower than 1.65% found in Nigeria (atropical country)but had higher pH levels of 7.22 and 7.67 than 5.55 as obtained by Adegun et al. [28]. Fuglie et al., [31] emphasized that Moringa trees tolerate a wide range of soil conditions, but prefer a neutral to slightly acidic pHs of 6.3-7.0. Different crops need different pH levels but grow best if the soil they are planted in is maintained at an optimal pH [35]. The particle size analysis revealed that the soil is sandy (84.2% at 0-30 cm depth and 82.1% at 30-60 cm depth) with low silt and clay (< 10% each), which corroborates the results found by Foth and Gibbon et al. [28,34].
| Type of Analysis | Sample No. | ||
| Units | 0-30 cm Depth | 30-60 cm Depth | |
| pH | 7.22 | 7.67 | |
| Electrical Conductivity or Soluble Salts (EC) | µS/cm | 80 | 87 |
| Organic Matter (OM) | % | 0.87 | 0.65 |
| Phosphorus (P) | ppm | 24.60 | 12.30 |
| Potassium (K) | ppm | 295 | 384 |
| Calcium (Ca) | ppm | 572 | 586 |
| Magnesium (Mg) | ppm | 95 | 107 |
| Sodium (Na) | ppm | 5 | 8 |
| Texture | ---- | Loamy sand | Loamy sand |
| Sand | % | 84.2 | 82.1 |
| Silt | % | 8.2 | 9.7 |
| Clay | % | 7.6 | 8.1 |
Table 2: Soil nutrients and properties.
Survival rates of Moringa species after 2014 winter season
Figure 1 presents the survival rates of M. oleifera and M. ovalifolia trees after 2014 winter season. Although all 120 M. oleifera and 64 M. ovalifolia seedlings were transplanted in early 2014 (late January to early February) from a nursery to the field, they were all affected by the winter frost from May to September. Afterwards, summer sets in from October to April [4,5] and temperatures rose to the level that could support both Moringa species growth and development as discussed by Larcher and Metcalfe et al., [2,36]. Survived tree sprouting was observed in October 2014 in which M. oleifera had 66 sprouted trees compared to M. ovalifolia with 10 sprouted trees. The sprouting process continued up to January 2015 with the highest of 85 in M. oleifera and 52 for M. ovalifolia trees respectively. The survival of a young plant is critical because the root system is small [30]. As newly planted trees, many of their roots systems were not fully established and therefore succumbed to the winter frost to which they are susceptible. Metcalfe et al., [36] agreed that plants are dependent on the root systems for their very life, for water and nutrients, as well as for anchorage in the soil. Moringa oleifera had higher survival rates in the four months (55% in October, 57.5% in November, 63.33% in December and 70.83% in January). In comparison, to the Contrary, M. ovalifolia had lower survival rates of 10 (15.63%) in October, 29 (45.31%) in November, 50 (78.13%) in December and 52 (81.25%) in January. Comparatively, M. oleifera had a total survival of 85 out of 120 trees, which was 70.83%, while M. ovalifolia had 52 out of 64 trees, an equivalent of 81.25%. This clearly indicates that M. ovalifolia had higher survival rate than M. oleifera after 2014 winter season. As exotic plant to Namibia, M. oleifera survival rates supports Morton and Fuglie’s et al., [7,9] statement that it is a boon of arid lands and drought-resistant plant. However, M. oleifera dead trees were all replaced through direct sowing of seeds but M. ovalifolia trees were not replaced due to the lack of seeds. 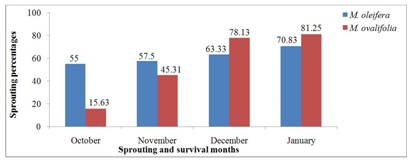

Figure 1: Moringa trees sprouting and survival rates after 2014 winter season.
A Pairwise Comparison test was conducted to determine the statistical difference between M. oleifera and M. ovalifolia survival rates after the 2014 winter season (Table 3). The survived trees (sprouted trees) were used as dependent variables while the Moringa species were used as independent variables for the analysis. There result shows no significant difference (P < 0.05) between M. oleifera and M. ovalifolia survival rates of trees after 2014 winter season, an indication that both Moringa species had similar survival rates as seen in figure 1.
A Pairwise Comparison test was conducted to determine the statistical difference between M. oleifera and M. ovalifolia survival rates after the 2014 winter season (Table 3). The survived trees (sprouted trees) were used as dependent variables while the Moringa species were used as independent variables for the analysis. There result shows no significant difference (P < 0.05) between M. oleifera and M. ovalifolia survival rates of trees after 2014 winter season, an indication that both Moringa species had similar survival rates as seen in figure 1.
| (I) Moringa spp. | (J) Moringa spp. | Mean Difference (I-J) | Std. Error | Sig.a | 95% Confidence Interval for Differencea | |
| Lower Bound | Upper Bound | |||||
| M. oleifera | M. ovalifolia | 6.585 | 15.855 | 0.692 | -32.210 | 45.380 |
| M. ovalifolia | M. oleifera | -6.585 | 15.855 | 0.692 | -45.380 | 32.210 |
a-Adjustment for multiple comparisons: Least Significant Difference (equivalent to no adjustments).
The descriptive statistics for M. oleifera and M. ovalifolia survival rates after the 2014 winter season is found in table 4 in which the number of cases, mean, standard deviation, standard error of the mean, minimum and maximum were considered. M. oleifera had a mean of 61.67 with a maximum of 70.83% survival rate, while M. ovalifolia had a mean of 55 with a maximum of 81.25% survival rate. A study conducted by Korsor et al., [10] with M. oleifera and M. ovalifolia seedlings had 81.56% survival rate for M. ovalifolia and 93.33% for M. oleifera, which concurs with the present study M. ovalifolia survival rate but higher than M. oleifera in this study.
The descriptive statistics for M. oleifera and M. ovalifolia survival rates after the 2014 winter season is found in table 4 in which the number of cases, mean, standard deviation, standard error of the mean, minimum and maximum were considered. M. oleifera had a mean of 61.67 with a maximum of 70.83% survival rate, while M. ovalifolia had a mean of 55 with a maximum of 81.25% survival rate. A study conducted by Korsor et al., [10] with M. oleifera and M. ovalifolia seedlings had 81.56% survival rate for M. ovalifolia and 93.33% for M. oleifera, which concurs with the present study M. ovalifolia survival rate but higher than M. oleifera in this study.
| Moringa spp. | N | Mean | Std. Deviation | Std. Error of Mean | Minimum | Maximum |
| M. oleifera | 4 | 61.67 | 7.01 | 3.52 | 55.00 | 70.83 |
| M. ovalifolia | 4 | 55.08 | 30.92 | 15.46 | 15.63 | 81.25 |
Figure 2 shows a global humidity index map in which Namibia is shown as semi-arid and arid country. Figure 3 shows pictures of M. oleifera at the beginning and the end 2015 winter; while figure 4 are pictures of M. ovalifolia at the beginning and end of 2015 winter season. The pictures clearly how much damage winter imposes on the trees every season. This led to mortality of many trees after winter.
Figure 2: World Humidity Index Zone (Courtesy of United Nations Environment Program).

Figure 3: M. oleifera at beginning (A) and end (B) of winter.


Figure 4: M. ovalifolia at beginning (A) and end (B) of winter.
Survival rates of Moringa trees after 2015 winter season
Figure 5 presents the survival rates of M. oleifera and M. ovalifolia trees after the 2015 winter season. Out of 120 Moringa oleifera trees, 117 survived and sprouted which corresponds to 97.50% survival rate at the end of October 2015. The situation remained unchanged until end of January 2016 when it was noticed that the three trees that did not sprout had died of winter frost/cold and were replaced immediately. A research with M. oleifera in Ihumwa and Ruvu, Tanzania assessment of 30-months old trees showed the survival rates of 98.67% and 92% respectively [17]. The present study concurs with their research findings even though the period of this research in Namibia was under complete drought conditions. Moringa ovalifolia trees had a gradual sprouting and survival rate of 30 (57.69%), 36 (69.23%), 40 (76.92%) and 43 (82.69%) out of 52 trees from October 2015 to January 2016 respectively. In comparison, M. oleifera had 97.50% while M. ovalifolia had 82.69% survival rates. The survival rates of the two Moringa species after the 2015 winter season was carefully monitored from October 2015 to January 2016, when it was noticed that sprouting was faster compared to October 2014 to January 2015 when the trees were younger and only started sprouting in November. Larcher [2] pointed out that perennial plants in the first year form a rosette and underground storage organs to ensure a rapid start for development in the second year. It was observed that M. ovalifolia trees can take longer (6-10 months) in dormancy than M. oleifera (4-6 months) and still sprout. This survival ability might be attributed to M. ovalifolia tuberous-root system that stores food for its future uses during stress as discussed by Korsor et al. [10]. Billings [1] reported that most perennials plants, both dicotyledonous and monocotyledonous, have large underground roots and rhizomes systems in which carbohydrates and other compounds are stored. Metcalfe et al., [36] pointed out that plants roots often also perform an important function in storing food for future use. They also emphasized that slow drop in temperature kills less plants, which was the case with the beginning of the 2015 winter season in comparison to the 2014 winter season.
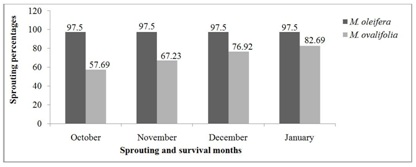
Figure 5: Moringa trees sprouting and survival rates after 2015 winter season.
Table 5 presents a GLM Pairwise Comparison test of Moringa species survival rates after 2015 winter season. The survived trees (sprouted trees) were used as dependent variables while the Moringa species were used as independent variables for the analysis. The test result reveals a significant difference (P < 0.05) between M. oleifera and M. ovalifolia survival rates. Although planted in the same orchard and environmental conditions, each Moringa species survived the 2015 winter season at different rate (Figure 5).
| (I) Moringa spp. | (J) Moringa spp. | Mean Difference (I-J) | Std. Error | Sig.b | 95% Confidence Interval for Differenceb | |
| Lower Bound | Upper Bound | |||||
| M. oleifera | M. ovalifolia | 25.868* | 5.404 | 0.003 | 12.645 | 39.090 |
| M. ovalifolia | M. oleifera | -25.868* | 5.404 | 0.003 | -39.090 | -12.645 |
Based on estimated marginal means
*-The mean difference is significant at the .05 level.
b-Adjustment for multiple comparisons: Bonferroni.
The descriptive statistics of Moringa species survival rates after the 2015 winter season is found in table 6. The statistics shows the means of 97.50 and 71.63 with maxima of 97.5% and 82.69% survival rates for M. oleifera and M. ovalifolia, respectively. Moringa oleifera sprouted at once in 2015 causing it to have zero standard deviation and standard error of the mean, while M. ovalifoliahad a gradual sprouting with 10.80 standard deviation and 5.40 standard error of the mean.
b-Adjustment for multiple comparisons: Bonferroni.
The descriptive statistics of Moringa species survival rates after the 2015 winter season is found in table 6. The statistics shows the means of 97.50 and 71.63 with maxima of 97.5% and 82.69% survival rates for M. oleifera and M. ovalifolia, respectively. Moringa oleifera sprouted at once in 2015 causing it to have zero standard deviation and standard error of the mean, while M. ovalifoliahad a gradual sprouting with 10.80 standard deviation and 5.40 standard error of the mean.
| Moringa spp. | N | Mean | Std. Deviation | Std. Error of Mean | Minimum | Maximum |
| M. oleifera | 4 | 97.50 | 0.00 | 0.00 | 97.50 | 97.50 |
| M. ovalifolia | 4 | 71.63 | 10.81 | 5.40 | 57.69 | 82.69 |
Survival rates of Moringa trees after 2016 winter season
The sprouting and survival rates of M. oleifera and M. ovalifolia trees after 2016-winter season are found in figure 6. This winter season came in the middle of a severe drought that started in 2013 [24] and continued through 2016, which condition created scarcity for water. Both annual and some perennial plants serve as feed sources for the wild and domestic animal grazing and browsing. Moringa ovalifolia roots being so succulent and full of water (no stems) than M. oleifera, resulted in wild animals rooting out 23 trees as source of food and water. Haferkamp et al., [37] emphasized that water is required by all living organisms. In this case, only 20 trees were left on the field out of the 43 trees that survived the 2015-winter season. The result in figure 6 shows earlier sprouting and survival rates of M. oleifera and M. ovalifolia species starting in September with 93.33% and 42.11%, October 99.17% and 89.47%, and November 99.17% and 100% respectively. This earlier sprouting and survival rates of both Moringa species after the 2016 winter season is an indication that older trees have higher tendencies of survival and faster sprouting rates compared to younger trees. As observed on the field, older trees (three years old) sprouted within three to five days whenever temperatures improved even in the middle of the winter season and died off when temperatures worsened. It implies that temperature is cardinal to plants growth and survival just as moisture as stated by Haferkamp [37].
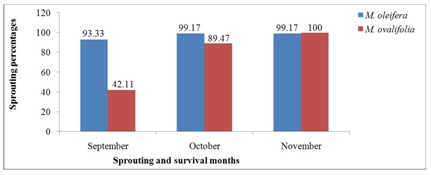
Figure 6: Moringa trees species sprouting and survival rates after 2016 winter season.
A GLM Pairwise Comparison test of Moringa species survival rates after the 2016 winter season is seen in table 7. During the analysis, the survived (sprouted) trees were used as dependent variables while the Moringa species were used as independent variables. The test result shows that there was no significant different (P < 0.05) between M. oleifera and M. ovalifolia survival rates after the 2016 winter season. This may be attributed to the ages of the trees (Figure 6) in comparison to the first and second winter (2014 and 2015) seasons when the trees were younger with less roots systems establishment as discussed by Foth and Larcher [2,30].
| (I) Moringa spp. | (J) Moringa spp. | Mean Difference (I-J) | Std. Error | Sig.a | 95% Confidence Interval for Differencea | |
| Lower Bound | Upper Bound | |||||
| M. oleifera | M. ovalifolia | 15.023 | 33.520 | 0.670 | -66.997 | 97.042 |
| M. ovalifolia | M. oleifera | -15.023 | 33.520 | 0.670 | -97.042 | 66.997 |
Based on estimated marginal means
a-Adjustment for multiple comparisons: Bonferroni.
Table 8 presents descriptive statistics of Moringa species survival rates after the 2016 winter season which includes the number, means, standard deviation, standard error of the mean, minimum and maximum. The two Moringa species had equal survival rates after the 2016 winter season with maximum of 99.19 % and 100% for M. oleifera and M. ovalifolia, respectively.
| Moringa spp. | N | Mean | Std. Deviation | Std. Error of Mean | Minimum | Maximum |
| M. oleifera | 4 | 72.92 | 48.69 | 24.34 | 0.00 | 99.17 |
| M. ovalifolia | 4 | 57.90 | 46.08 | 23.04 | 0.00 | 100.00 |
Figure 7 presents photographs of M. oleifera (A) and M. ovalifolia (B) sprouting in the middle of the 2016 winter season, while figure 8 shows photographs of M. oleifera roots (A) and M. ovalifolia roots (B) after the 2016 winter. As the trees grew older and bigger with firmed root-system establishment in 2016, they were less affected by winter and whenever temperatures improved, they sprouted immediately even in the middle of winter, which reduced the mortality rates. Moringa oleifera grows rapidly even in poor soils, arid and/or dry lands [7,38], which from observation shows that M. ovalifolia grows in poor soil, arid, and/or dry lands as well since both Moringa species grew under the same adverse environmental conditions.

Figure 7: M. oleifera (A) and M. ovalifolia(B) sprouting in middle of 2016 winter.

Figure 8: M. oleifera roots (A) and M. ovalifolia roots (B) after 2016 winter.
Table 9 shows Moringa species total survived trees and their survival rates after 2014, 2015 and 2016 winter seasons with average total survived trees and total survived rates. On average, M. oleifera had a total of 107 trees that survived, which is equivalent to a total survival rate of 89.19%; while, M. ovalifolia had a total of 38 trees that survived with a total survival rate of 87.98% for the three winter seasons under study. Statistically, there was no significant difference (P > 0.05) between M. oleifera and M. ovalifolia total survival trees in 2014 and 2016, except for 2015 in which they were significantly different (P < 0.05).
| Moringa spp. | 2014TotalSurvivedTrees | 2014% Trees | 2015TotalSurvivedTrees | 2015% Trees | 2016TotalSurvivedTrees | 2016% Trees | Averageof totalSurvivedTrees | % TotalSurvivedTrees |
| M. oleifera | 85 | 70.83 | 117 | 97.50 | 119 | 99.17 | 107 | 89.17 |
| M. ovalifolia | 52 | 81.25 | 43 | 82.69 | 20 | 100 | 38 | 87.98 |
Table 9: Total and percent survived of Moringa trees after 2014, 2015 and 2016 winter seasons.
MORTALITY RATES OF MORINGA TREES DUE TO WINTER SEASON
The mortality rates of the two Moringa species were established based on the amount of trees that succumbed to frost during each winter season (Table 10). McDowell et al., [39] stated that longer winter minimum temperatures can lead to trees mortality, and Parker [40] concurred that freezing temperatures can retard plants growth, which subsequently lead to death. During 2014 winter season, 35 out of 120 M. oleifera trees died and 12 out of 64 M. ovalifolia trees died, resulting to 29.17% and 18.75% mortality rates for M. oleifera and M. ovalifolia, respectively. It was observed that 2014 winter season started with a very complete/severe drop in temperature at once, affecting almost all the trees, which may have contributed to the high mortality rate among the trees in addition to tree age. Metcalfe et al., [36] emphasized that a rapid drop in temperature kills plants more frequently than a gradual drop. In 2015 winter season, three out 120 M. oleifera trees and 9 out of 52 M. ovalifolia trees died, which caused the mortality rates 2.5% for M. oleifera and 17.31 M. ovalifolia. The less mortality can be attributed to the trees size and roots-system establishments as discussed by Gibbon et al., [34]. Besides the root development of the Moringa trees, 2015 winter sets in slowly and affected the trees gradually, which may have contributed to the low mortality rates compared to 2014 winter season. Finally, during 2016 winter season, one out of 120 M. oleifera trees died and there was no death recorded among M. ovalifolia trees. The almost zero mortality rates after 2016 winter are attributed to the well development of the trees and their roots (Figures 7 and 8). Furthermore, the total of 39 M. oleifera trees and 21 M. ovalifolia trees died during the three winter seasons (2014, 2015 and 2016) with the total mortality rates of 32.5% and 32.8%, respectively. Considering M. oleifera as an exotic plant and M. ovalifolia as an indigenous plant, the equal-total survival rates are indications that M. oleifera can survive in tropical and driest climatic conditions as discussed in many literature [7,9].
| Moringa spp. | 2014Dead Trees | 2014% Dead Trees | 2015Dead Trees | 2015% dead Trees | 2016Dead Trees | 2016% dead Trees | Total Dead Trees | Total % Dead Trees |
| M. oleifera | 35 | 29.16 | 3 | 2.5 | 1 | 0.83 | 39 | 32.5 |
| M. ovalifolia | 12 | 18.75 | 9 | 17.3 | 0 | 0 | 21 | 32.8 |
Both M. oleifera and M. ovalifolia survived well even though M. oleiferais exotic to Namibia. (Al et al., 2013) [21] elucidated that originally Moringa is considered as a tree of hot semi-arid regions, which is adaptable to a wide range of environmental conditions; from hot to dry, humid and wet conditions. The tree is tolerant to light frosts, but does not survive as a perennial under freezing condition. Moringa is quite drought tolerant and is well suited for a wide range of adverse environments that would not be suitable for other fruit, nut and tree crops.
CONCLUSION
M. oleifera and M. ovalifolia have the ability to survive under harsh conditions. Both Moringa species need little amount of water to survive, which makes them suitable for arid and semi-arid climates like Namibia. Although many trees, including Moringa in this central part of Namibia are affected by winter frost annually, both species survived and sprouted quickly after winter. As young trees in the first year, M. oleifera had a survival rate of 70.83% while M. ovalifolia had 81.25% after the 2014-winter season. In the second year, M. oleifera had a higher survival rate of 97.50% and M. ovalifolia had 82.69% after the 2015-winter season. Year after year, the strength and vigor of trees’ survival increased, which was seen in the third year with M. oleifera having 99.17% and M. ovalifolia having 100% survival rates, respectively. Therefore, M. oleifera and M. ovalifolia trees can survive well under harsh environmental conditions such as very cold winter and drought, and could be cultivated to improve rangeland productivity in terms of fodder and soil fertility.
ACKNOWLEDGEMENT
This study was financially supported by Namibia Students Financial Assistance Fund (NSFAF) and Namibia-South Africa Bilateral Research Agreement through the National Commission on Research Science and Technology (NCRST). This article is part of a PhD research undertaken by Morlu Korsor at the University of Namibia, Department of Animal Science.
REFERENCES
- Billings WD (1987) Constraints to Plant Growth, Reproduction, and Establishment in Arctic Environments. INSTAAR, University of Colorado. 19: 357-365.
- Larcher W (1995) Physiological plant ecology: Ecophysiology and stress physiology of functional groups, (4th edn). Springer, Berlin, Germany.
- van Wyk B, van Wyk P, van Wyk BE (2011) Photo Guide to Trees of Southern Africa. Briza Publications, Pretoria, South Africa.
- Pallett J (1994) Understanding the Oshana environment. Gamsberg Macmillan Publishers (Pty) Ltd,Windhoek, Namibia.
- Oldeland J, Dorigo W, Wesuls D, Jürgens N (2010) Mapping Bush Encroaching Species by Seasonal Differences in Hyperspectral Imagery. Remote Sens 2: 1416-1438.
- Klaus H, Völkel J (2010) Soil clay minerals in Namibia and their Significance for the terrestrial and marine past Global change research. African Study Monographs.
- Morton JF (1991) The Horseradish Tree, Moringa pterygosperma (Moringaceae): A Boon to Arid Lands?? Springer on behalf of New York Botanical Garden Press 45: 318-333.
- Radovich T (2007) Farm and forestry production and marketing profile for Moringa oleifera. Western SARE & PAR, USA.
- Fuglie LJ (2001) The miracle tree: the multiple attributes of Moringa. Church World Service, Dakar, Senegal.
- Morlu K, Ntahonshikira C, Kwaambwa HM, Bello HM (2016) Comparative performance of Moringa oleifera and Moringa ovalifolia seeds and seedlings establishment in central Namibia. Net Journal of Agricultural Science 4: 35-44.
- Ministry of Agriculture, Water and Forestry, Moringa ovalifolia. Ministry of Agriculture, Water and Forestry, Windhoek, Namibia.
- Arora DS, Onsare JG, Kaur H (2013) Bioprospecting of Moringa (Moringaceae): Microbiological Perspective. Journal of Pharmacognosy and Phytochemistry 1: 193-215.
- Olson ME (2002) Combining Data from DNA Sequences and Morphology for a Phylogeny of Moringaceae (Brassicales). Systematic Botany 27: 55-73.
- USDA (2017) Classification for Kingdom Plantae Down to Species Moringa oleifera USDA, Washington DC, USA.
- Olson ME (2001) Introduction to the Moringa family. In: Fuglie LJ (ed.). The miracle tree: The multiple attributes of Moringa. Church World Service, Dakar, Senegal.
- Price ML (2007) The Moringa tree. ECHO Technical Note, Florida, USA.
- Edward E, Chamshama SAO, Ngaga MCA-N’s, Mndolwa MA (2014) Survival, growth and biomass production of Moringa oleifera provenances at Gairo inland plateau and Ruvu Coastal Region in Tanzania. African Journal of Plant Science 8: 54-64.
- Sijssens P (2014) Evaluation of MCA-N’s Livestock Support Activity. Millennium Challenge Account, Namibia. Pg no: 1-5.
- Schalkwyk R (2014). Introduction to plant products in Namibia.
- Agra Professional Services (2013) Namibia livestock catalogue. Ministry of Agriculture, Water and Forestry, Windhoek, Namibia.
- Al Gunaid Hassan F, Ibrahim MA (2013) Moringa oleifera?: Nature is Most Nutritious and Multi- Purpose Tree. International Journal of Scientific and Research Publications 3: 1-5.
- University of Namibia (2011) Neudamm Agricultural College Farm. University of Namibia. Windhoek, Namibia.
- Lubbe A (2017) Average annual rainfall of Neudamm Experimental Farm. Windhoek, Namibia.
- Korsor M (2013) Northern Namibia: Water and its impact on subsistence rural farming. Anglican Communion Environmental Network. Pg no: 2-3.
- Kahumba A (2010) Comparison of the rehabilitative effects of mechanical and chemical methods of bush control on degraded Highland Savanna rangelands in Namibia. Agrobiodiversity, Namibia.
- Kapu GS (2012) Assessment of rangeland condition and evaluation of the Nutritional value of common grass and browse species at The Neudamm Experimental Farm, Namibia (Master of Science Degree). University of Namibia, Windhoek, Namibia.
- Beukes A (2017) Vegetation of Neudamm Experimental Farm.
- Adegun M, Ayodele O (2015) Growth and yield of Moringa oleifera as influenced by spacing and organic manures in South-Western Nigeria. International Journal of Agronomy and Agricultural Research (IJAAR) 6: 30-37.
- Onwueme IC, Sinha TD (1991) Field crop production in tropical Africa. CTA, Wageningen, The Netherlands.
- Foth HD (1990) Fundamentals of soil science, (8th edn). John Wiley & Sons, Singapore.
- Fuglie L, Sreeja V (2001) Cultivation of Moringa. In: The miracle tree: The multiple attributes of Moringa. Church World Service, Dakar, Senegal. Pg no: 153-158.
- Fayolle A, Ouédraogo D, Ligot G, Daïnou K, Bourland, et al. (2015) Differential Performance between Two Timber Species in Forest Logging Gaps and in Plantations in Central Africa. Forests 6: 380-394.
- Johnston AE, Steen I (2000) Understanding phosphorus and it use in agriculture. European Fertilizer Manufacturers Association.
- Gibbon David, Pain A (1985) Crops of the drier regions of the tropics. Longman, New York, USA.
- Hach (2010) What is pH and how is it measured? A technical Handbook for industry. Hach Company. Loveland, Colorado, USA.
- Metcalfe D, Elkins D (1980) Crop production: Principles and practices, (4th edn). Macmillan Publishers, New York, USA.
- Haferkamp M (1988) Environmental factors affecting plant productivity. Montana Agri Exp Sta, Bozeman, Spain. Pg no: 132.
- Alhakmani F, Kumar S, Khan SA (2013) Estimation of total phenolic content, in-vitro antioxidant and anti-inflammatory activity of flowers of Moringa oleifera. Asian Pac J Trop Biomed 3: 623-627.
- McDowell N, Pockman W, Allen C, Breshears D, Cobb N, et al. (2008) Mechanisms of Plant Survival and Mortality during Drought: Why Do Some Plants Survive while Others Succumb to Drought? New Phytologist 178: 719-739.
- Parker J (1969) Further Studies of Drought Resistance in Woody Plants. Springer on behalf of New York Botanical Garden Press 35: 317-371.
Citation: Korsor M, Ntahonshikira C, Bello HM, Kwaambwa HM (2017) Comparative Study of M. oleifera and M. ovalifolia Survival Rates in Central Namibia. J Plant Sci Curr Res 1: 001
Copyright: © 2017 Morlu Korsor, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Journal Highlights
© 2025, Copyrights Herald Scholarly Open Access. All Rights Reserved!
