
Complications of Treatment against SARS-CoV-2: Risk of Hepatitis B Reactivation Associated with Corticosteroids
*Corresponding Author(s):
J Varona PérezDepartment Of Internal Medicine, Cruces University Hospital, Barakaldo, Vizcaya, Spain
Email:jimenavarona@hotmail.com
SHORT COMMENTARY
The health crisis triggered by the new unknown SARS-CoV-2 has led to using all available resources to curb the disease. As we have learned more about the virus, more targeted drugs have been prescribed. The inflammation phase is perhaps the most worrisome. It requires therapies that slow down the immune system activation [1]. Corticosteroids are the favorite drug. However, dosages and maintenance regime are still uncertain. We have already started to have some results on its effectiveness [2], which were not available at the beginning. We have used a three-day regimen of 125 or 250 mg methylprednisolone pulses according to clinical-analytical severity in our hospital. However, corticosteroids are not without risks. One of the most frequent risks is the reactivation of infections. Reactivation of the hepatitis B virus is especially important because it is frequent and we can prevent it. In addition, a recent study has linked chronic HBV infection with a decreased clearance rate of SARS-CoV-2 [3]. Therefore, we review the recommendations on the prophylaxis of reactivation of HBV infection in patients receiving corticosteroid pulses.
EASL [4] and AGA [5] advise HBV screening in all patients receiving immunosuppressive therapy. They use HBsAg and anti-HBc. The AEMPS [6] published a summary of recommendations based on the results of the screening tests. If the HBsAg is positive, we should request the viral load. If it is greater than 2000, we must start treatment with Entecavir or Tenofovir. If it is less than 2000, universal prophylaxis is performed with Lamivudine. We use a short immunosuppressive regimen so Lamivudine is sufficient. In longer immunosuppressive regimens, other antivirals are preferred because of HBV's ability to develop resistance to Lamivudine.
When HBsAg is negative, we must look at anti-HBc. If it is positive and the viral load is detectable, the same regimen is used as in patients with HBsAg positive. If anti-HBc is positive and the viral load is undetectable, the approach is more complex and depends on the risk of reactivation. There are three groups according to the annual probability of reactivation of the virus: high (>10%), moderate (1-10%) and low (<1%). Immunosuppressive drugs are classified into one of these three groups [7]. Corticosteroids are difficult to classify because there are many different patterns and clinical trials are lacking. The latest AGA [8] update differentiates two groups. Patients receiving ≤ 20 mg per day of prednisone or equivalent are in the low risk group for reactivation. Doses>20 mg belong to the intermediate risk group. The review does not explain exactly the duration of treatment with corticosteroids. Corticosteroid pulses are not mentioned at any time.
The Journal of Hepatology published a retrospective study in 2020 [9]. It analyzes the risk of outbreaks of hepatitis and HBsAgseroreversion in 12,997 patients receiving at least one dose of systemic corticosteroids. Patients with HBsAg- / anti-HBc +/ anti-HBs- have an annual risk of hepatitis outbreak of 16.2% and resoreversion of 1.8%. This annual risk of seroreversion is included in the intermediate risk group. The investigators concluded that the risk of seroreversionis independent of time and corticosteroid dose. The time of treatment with corticosteroids neither influence outbreaks of hepatitis. But the dose does. Doses higher than 40mg per day increase the risk.
In summary, HBV screening should be performed in all patients with COVID-19 candidate for corticosteroids. We propose a summary of action according to the guidelines of our hospital (Figure 1). There is evidence to recommend prophylactic treatment with Lamivudine in HBsAg- / anti-HBc + / HBV-DNA- patients with high risk of reactivation. If the risk is low, it is not recommended. If the risk is intermediate, prophylaxis can be started or a quarterly control can be done.
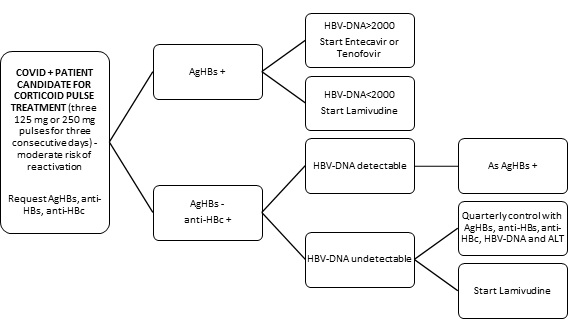 Figure 1: Algorithm of action against COVID+ patients who are candidates for corsticosteroid pulses according to serological findings from HBV screening.
Figure 1: Algorithm of action against COVID+ patients who are candidates for corsticosteroid pulses according to serological findings from HBV screening.
REFERENCES
- Siddiqi HK, Mehra MR (2020) COVID-19 Illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant 39: 405-407.
- Fadel R, Morrison AR, Vahia A, Smith ZR, Chaudary Z, et al. (2020) Early Short Course Corticosteroids in Hospitalized Patients with COVID-19. Clinical Infectious Diseases.
- Zha L, Li S, Pan L, Tefsen B, Li Y, et al. (2020) Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19). Med J Aust 212: 416-420.
- European Association for the Study of the Liver (2017) EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J HepatoL 67: 370-398.
- Perillo RP, Gish R, Falck Ytter YT (2015) American Gastroenterological Association Institute Technical Review on Prevention and Treatment of Hepatitis B Reactivation During Immunosuppressive Drug Therapy. Gastroenterology 148: 221-243.
- https://www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2014/docs/NI-MUH_FV_11-2014-inmunosupresores.pdf
- Cholongitas E, Tziomalos K, Pipili C (2015) Management of patients with hepatitis B in special Populations. World J Gastroenterol 21: 1738-1748.
- Loomba R, Liang TJ (2017) Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts Management Strategies, and Future Directions. Gastroenterology 152: 1297-1309.
- Wong GL, Wong VW, Yuen BW, TseYK, Yip TC, et al. (2020) Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J Hepatol 72: 57-66.
Citation: Varona JP, Rodriguez JMC (2020) Complications of Treatment against SARS-CoV-2: Risk of Hepatitis B Reactivation Associated with Corticosteroids. J Clin Immunol Immunother 6: 030.
Copyright: © 2020 J Varona Pérez, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

