
Congenital B-Cell Acute Myeloid Leukemia Associated With Down's Syndrome
*Corresponding Author(s):
Dhan Raj BagriAssistant Professor, Sir Padampat Institute Of Neonatal And Pediatric Health, J K Lone Hospital, SMS Medical College, Jaipur, Rajasthan, India
Email:meena.drdhanraj6@gmail.com
Abstract
Introduction: Congenital Leukemia (CL) is extremely rare with an incidence rate of fewer than five cases per 1 million live births in neonates; mostly non-lymphoblastic leukemia in contrast to the predominance of ALL found in later childhood. CL may be associated with Down's syndrome.
Case presentation: A female neonate presented with lethargy, poor feeding, mild hepatosplenomegaly, manifestations suggestive of sepsis, and a negative sepsis workup. Physical examination showed that the patient was febrile and pale, with a facial syndromic appearance suggestive of Down's syndrome. His peripheral blood smear showed severe leukocytosis, thrombocytopenia, and atypical cells. Peripheral blood film examination revealed increased myeloblasts but no nucleated RBC; Flow cytometry confirmed the diagnosis of congenital leukemia. Karyotyping indicated chromosome 21-trisomy.
Conclusions: Congenital Leukemia should be considered as a differential diagnosis in neonates having syndromic features and leukocytosis, TMD should be ruled out by flow cytometry.
Keywords
Acute myeloblastic leukemia; Congenital, Chromosome 21; Down's syndrome.
Introduction
Congenital Leukemia (CL) is an extraordinarily rare condition in newborns, which is typically diagnosed at birth or within the first month of life1. It makes a medical diagnostic challenge, as its clinical features are similar to prenatal/or natal sepsis. Most CLs are myeloid contrasting pediatric leukemia, which is frequently lymphoid in origin; prognosis is evenly poor [1,2]. The course of congenital leukemia ordinarily leads to rapid deterioration and expiry due to infection or hemorrhagic events. We introduce a neonate presenting with clinical manifestations of sepsis; however, the patient was then confirmed as CL.
Case Report
A full-term female child presented to the pediatric emergency department with complaints of a rash over her face and respiratory distress since birth. She was born out of a non-consanguineous marriage with a birth weight of 2.5 kg. Her 25-year-old primigravida mother had a normal antenatal course with normal routine tests including HIV & HBsAg, RPR test, TSH, urine microscopy, and ultrasound were normal. The maternal TORCH profile was normal. The baby had normal APGAR scores.
On examination, her craniofacial configuration was abnormal with a flattened round face, depressed bridge of the nose, upward slanting almond-shaped eyes, small low set ears, with skin folding on the pinna, short neck with skin folding, small hands with palmer crease, small fingers curved towards the thumb and small feet with bilateral wide spaced great toes and second toes - features suggestive of Down's syndrome. The baby had a macular rash with tiny red spots on the face (Figure 1). The anterior fontanelle bulged and the head circumference was 32 cm. The abdomen was mildly distended (abdominal girth – 38 cm) and hepatosplenomegaly on palpation with the liver 4 cm below the costal margin and spleen 3 cm below the costal margin. He was tachypneic with a respiratory rate of 70 per minute. Respiratory system examination revealed subcostal and intercostal retractions and central cyanosis. On auscultation, occasional crepitations were audible. Cardiovascular examination suggested abnormal heart sounds and murmur.
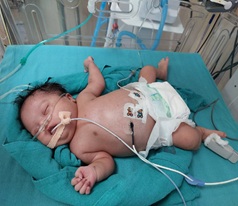 Figure 1: Down's Syndrome
Figure 1: Down's Syndrome
Blood investigations are depicted in table-1. Complete blood counts suggested total leucocyte counts 78x103/Cumm, Serum calcium was 7.3 mg/dl, phosphorus was 3.69 mg/dl, and serum uric acid levels were 7.0 mg/dl, LFT & RFT were normal. 25 LDH was elevated (5032 U/L). TSH was 4.331 micro-IU/ml with normal T3&T4. PT/PTTK INR (1.9) was abnormal (Table 1). Chest X-ray and 2D ECHO suggested a cyanotic congenital heart disease- PDA (3.7 mm, PG – 5.0 mmHg) with the left to right shunting and LALV enlarged.
|
Parameter |
LIVE DAY 1 |
Result-LIVE DAY 3 |
Live day 8 |
Unit |
Reference Interval |
|
RBC |
3.68 |
4.27 |
4.47 |
106 |
(M) 4.5-5.5(F) 3.8-4.8 |
|
Hemoglobin |
14.8 |
15.8 |
16.2 |
g/dL |
(M) 13-17(F) 12-15 |
|
HCT |
39.4 |
45.3 |
45.7 |
% |
(M) 36-51(F) 36-46 |
|
MCV |
107.4 |
106.1 |
102.2 |
fL |
(M) 70-99(F) 83-101 |
|
MCH |
40.2 |
37 |
36.2 |
Pg |
(M) 24-35(F) 27-32 |
|
MCHC |
37.6 |
34.9 |
35.4 |
g/dL |
(M) 33-37(F) 32-35 |
|
RDW-SD |
28 |
25.9 |
26.1 |
fL |
(M) 40-57(F) 40-57 |
|
RDW-CV |
|
|
|
% |
(M)11.6-14(F)11.6-14 |
|
WBC |
78 |
55.87 |
45.09 |
103 μL |
(M)4.5-11.5(F)4-11 |
|
NEUTROPHIL |
|
30% |
57 |
% |
(M)36-72(F)40-80 |
|
LYMPHOCYTES |
|
27% |
13 |
% |
(M)18-48(F)20-40 |
|
MONOCYTES |
|
2% |
1 |
% |
(M)2-11(F)2-10 |
|
Atypical cells |
|
35% |
24 |
% |
(M)1-6 (F)1-6 |
|
Myelo |
|
3% |
2 |
% |
(M)0-2(F)1-2 |
|
Metamyelo |
|
3% |
2 |
% |
|
|
NRBC |
|
35/100WBC |
1/100WBC |
% |
|
|
PLATELETS |
69 |
70 |
65 |
103 |
(M) (F)150-400 |
|
RETICULOCYTE COUNTS |
|
10.05 |
|
% |
(M)0.9-2.2(F)0.76-2.21 |
Table 1: Hla & Advanced Hematology Lab Report
Peripheral Blood Film examination reported normocytic normochromic red cells with moderate anisocytosis & adequate density. 30 n-RBC / 100 WBC were noted. White Blood Cell examination suggested leukocytosis with differentials as – blast + promyelocytes -18%, myelocytes -4%, metamyelocytes 3%, band+ neutrophils -28%, lymphocytes 41 %, monocytes 5% and eosinophils 1%. Platelets were reduced in number.
Peripheral Blood Film examination by an Advanced hematology lab suggested abnormal pathologic features including leukocytosis, lymphocytosis anisocytosis, monocytosis, basophilia, reticulocytosis, blasts, and giant platelets.
Ultrasound brain suggested immature parenchyma with increased echogenicity of the gangliothalamic region suggestive of HIE. Ultrasound abdomen suggested enlarged liver of 74 mm with sludge in the gall bladder with peri gall bladder fluid and portal vein diameter of 4.3 mm. bilateral kidneys were bright with peri renal edema and ascites. Ultrasound chest reported that the Right basal region showed consolidation and air bronchogram, left lobe also showed peripheral consolidation. TORCH (toxoplasmosis, other infections, rubella, cytomegalovirus infection, and herpes simplex) infections were ruled out based on a negative TORCH screen.
A karyotype done to rule out chromosomal abnormalities on the blood sample by stimulated peripheral blood lymphocyte culture revealed 47, XX, +21[20] [ISCN 2020] & presence of an additional copy of chromosome 21, suggestive of trisomy 21, indicative of female child with down syndrome (figure 2).
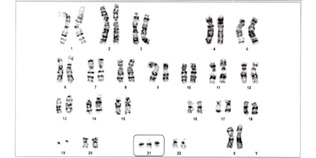 Figure 2: Karyotyping Suggesting Down's Syndrome
Figure 2: Karyotyping Suggesting Down's Syndrome
Supportive care was restricted to intravenous fluids and antibacterial therapy. Flow cytometric immunophenotyping further confirmed congenital B-Cell ALL (figure 3).
 Figure 3: Flow Cytometry-Blast Cells
Figure 3: Flow Cytometry-Blast Cells
Flow Cytometry
Peripheral blood smear shows 40% Blasts. On CD 45 gating the Blasts show the antigenic expression suggestive of Acute myeloid leukemia with megakaryocytic differentiation and aberrant CD 7 (Table 2).
|
|
POSITIVE FOR |
NEGATIVE FOR |
|
|
|
CD 10 |
|
CD 13 |
CD 19 |
|
|
CD 33 |
CD 20 |
|
|
VILA DR |
CD 3 |
|
|
CD 117 |
|
|
|
CD 61 |
GlyA |
|
|
CD 34 |
CD 79a |
|
|
MPO |
cCD 3 |
|
|
|
CD 14 |
Table 2: Flow Cytometry
Discussion
Congenital leukemia (CL) has an incidence of 4.3 - 8.6 per million live births [2,3]. Intrauterine environmental insults, infections, exposure to radiation during pregnancy, and chromosomal derangements are among the etiologies of congenital leukemias. CL is found associated with Down's syndrome, Turner syndrome, Klippel-Feil syndrome, and Ellis-van Creveld syndrome [4,5]; CL was accompanied by Down's syndrome with dysmorphic clinical presentations in our case. Clinical features of leukemia might be apparent at birth with hepatosplenomegaly, ecchymosis, and petechial lesions. About 25-30% of newborns suffering from CL present specific cutaneous infiltrates named leukemia cutis looking like firm red or blue nodules 'Blueberry Muffin' [6,7] Unlike the current case. Most CLs are of the myeloid lineage, unlike pediatric leukemia, which is usually lymphoid in origin (1-3, and 8-11). About half of congenital AMLs are of M4 or M5 in terms of morphology; there is the translocation of the MLL gene at band 11q23 and positive CD14 in these cases. The t (9; 11) translocation is the next common genetic defect detected followed by the t (11; 19) translocation [8, 9].
The differential diagnosis of CL comprises mainly sepsis and intra-uterine infections termed TORCH; other possible reasons consist of hemolytic disease of the newborn (HDN) and transient myeloproliferative disease (TMD) [1-3]. Infections were excluded through serology and culture in our present case, numerous erythrocyte precursors were not detected in the peripheral smear in this current case, unlike HDN. TMD of neonates is seen typically linked with Downs syndrome. They often have associated transient polycythemia and/or thrombocytosis, but hematological abnormalities persisted in our case [10]. The prognosis is poor, with merely 23% of cases surviving at 24 months [11, 12]
Conclusions
We are reporting a newborn with congenital B-cell -AML. Congenital leukemia should be considered in the differential diagnosis of neonates having syndromic features and peculiar blood abnormalities. TMD may be excluded appropriately by flow cytometric analysis.
Declarations
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest/Competing interests
None
References
- Perkins SL (2007) Hematopoietic system. In: Gilbert Barness E (Ed). Potter's pathology of the fetus, infant, and child. 2nd ed. Elsevier 1453-1514.
- Bajwa RP, Skinner R, Windebank KP, Reid MM (2004) A demographic study of leukemia presenting within the first three months of life in the northern health region of England. J Clin Pathol 57: 186-188.
- McCoy JP Jr, Overton WR (1995) Immunophenotyping of con-genital leukemia. Cytometry 22: 85-88.
- Warrier RP, Emami A, Ravindranath Y, Inoue S, Kaplan J (1983) Perinatal leukemia: the surface antigens, cytochemistry, and cytogenetic features. Indian Pediatr 20: 325333.
- Duru C, Pughikumo C, Adeyemi O (2013) Acute myeloblastic-leukemia in a patient with Down syndrome and sickle cell anemia: a case report. Pediatr Ther 3: 167.
- Pui CH, Kalwinsky DK, Schell MJ, Mason CA, Mirro J, et al. (1988) Acute non lymphoblastic leukemia in infants: clinical presentation and outcome. J Clin Oncol 6: 10081013.
- Resnick KS, Brod BB (1993) Leukemia cutis in congenital leukemia: analysis and review of the world literature with a report of an additional case. Arch Dermatol 129: 1301-1306.
- Badhe PB, Sane SY (1992) Congenital leukemia – Organ involvement in six autopsy cases. J Postgrad Med 38: 127.
- Felix CA, Lange BJ (1999) Leukemia in infants. Oncologist 4: 225-240.
- Zipursky A (2003) Transient leukaemia-a benign form of leukemia in newborn infants with trisomy 21. Br J Hematol 120: 930-938.
- Bresters D, Reus AC, Veerman AJ, van Wering ER, van der Does-van den Berg, et al. (2002) Congenital leukemia the Dutch experience and review of the literature. Br J Hematol 117: 513-524.
- Isaacs H Jr (2003) Fetal and neonatal leukemia. J Pediatr Hematol Oncol 25: 348-361.
Citation: Bagri DR, Mehta A, Sharma A, Gulati S, Bairwa R, et al. (2024) Congenital B-Cell Acute Myeloid Leukemia Associated With Down's Syndrome. J Neonatol Clin Pediatr 11: 123.
Copyright: © 2024 Dhan Raj Bagri, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

