
Congenital Cutis Laxa Presenting With Hiatal Hernia in the Third Trimester of Pregnancy
*Corresponding Author(s):
Rui DiogoPediatric Intensive Care Service, Coimbra Hospital And University Centre, Coimbra, Portugal
Email:ruialdiogo@gmail.com
Abstract
Inherited cutis laxa disorders result from monogenic defects that impair elastic fiber assembly and can manifest in newborns with excessive skin folds and systemic involvement.
We present a case of a newborn diagnosed with hiatal hernia in the third trimester of pregnancy. At birth, generalized wrinkled skin was noticed, more prominent in the eyes, face, and limbs, compatible with a congenital cutis laxa. An intermittent hiatal hernia with a dynamic compression of the left atrium by the herniated stomach prompted an exploratory laparotomy, which identified a hiatal hernia with an intra-thoracic gastric fundus and pyloric stenosis. Reduction of the herniated stomach, correction of diaphragmatic hiatus, anti-reflux procedure, and pyloromyotomy were performed, with a good outcome. Skin biopsy revealed elastic fiber heterogeneity in the reticular dermis, but whole exome sequencing was inconclusive. The patient is currently three months old and is thriving well. Peripheral pulmonary stenosis is being closely monitored.
Introduction
Cutis laxa (CL) disorders are a group of rare heterogeneous connective tissue diseases, inherited or acquired, characterized by a reduced number or size of elastic fibers, resulting in loose and redundant skin folds and less skin elasticity [1]. The diagnosis of CL is primarily clinical [2].
Congenital cutis laxa (CCL) is a systemic disorder with diverse clinical presentations and inheritance patterns. A common trait related to skin laxity is skin folds, which are more prominent around the eyes, face, neck, armpits, and thighs, giving an aged appearance [3]. The generalized paucity of elastic fibers is responsible for the systemic involvement of this disease, including gastrointestinal, cardiovascular, and pulmonary manifestations.
CCL can have an autosomal dominant, autosomal recessive, or X-linked inheritance [4]. Autosomal dominant cutis laxa (OMIM #123700) may present with predominant skin findings throughout the lifespan, but emphysema and aortic aneurysms have been described [5]. Autosomal recessive cutis laxa (ARCL) is a systemic disease with three main subtypes, each with multiple forms [1,4,5]. ARCL type 1 has predominant cardiopulmonary complications. ARCL type 1c (OMIM #613177) may present with emphysema, peripheral pulmonary stenosis, visceral diverticula, and hiatal and diaphragmatic hernias [6]. Patients with ARCL type 2 and 3 have more frequent skeletal and central nervous system manifestations [5,7].
There is a significant overlap between CCL types and other disorders, such as geroderma osteodysplasticum, MACS syndrome (Macrocephaly, Alopecia, Cutis Laxa, Scoliosis), arterial tortuosity syndrome and Ehlers-Danlos syndromes [1,4,5].
Congenital hiatal hernias are rare in pediatrics, with few series reported in the literature [8]. Some types of CCL are associated with congenital hiatal hernias [6]. This report describes a newborn with congenital cutis laxa and a prenatal diagnosis of a hiatal hernia.
Case Presentation
Male newborn, born to a G4P4 mother in her 40s, without previous history of miscarriages, familial malformation syndromes or parental consanguinity. Pregnancy was uncomplicated. However, an ultrasound at 32 weeks gestation revealed an anechogenic formation posterior to the fetus’ heart, suggestive of an intrathoracic stomach, without pulmonary hypoplasia. Magnetic resonance imaging at 35 demonstrated the same structure, contiguous to the esophagus, with a tubular morphology and liquid content compatible with a hiatal hernia (figure 1). Additionally, a fetal echocardiogram at 35 weeks showed an aortic isthmus narrowing (3mm in the sagittal plane, z-score -2.4). The neonate was born at 39 weeks via spontaneous noncomplicated vaginal delivery. Because of the known gastric hernia, nasotracheal intubation was tried immediately after birth. However, the baby showed no respiratory compromise, with spontaneous breathing and normal oxygenation, so further attempts were not performed. He was in good condition at birth with Apgar scores of 9 at one minute and 10 at five and ten minutes. On examination, generalized wrinkled and redundant skin were noted, more prominently involving the face, eyes, and limbs (figure 2), giving an aged appearance. Additional findings included small palpebral fissures, palmar hyperlinearity, inverted nipples, and increased inter-nipple distance.
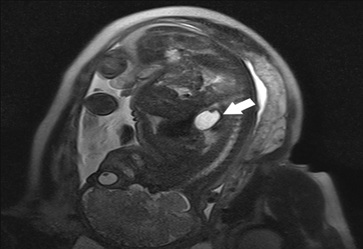 Figure 1: Fetal Magnetic Resonance Imaging (MRI) at 35 weeks: A structure with fluid signal is in a medial location (arrow) posterior to the heart, with a tubular morphology and contiguity with the esophagus. This structure measures 35 x 20 x 25 mm and has a thin, regular wall with folds inside, compatible with a herniated stomach.
Figure 1: Fetal Magnetic Resonance Imaging (MRI) at 35 weeks: A structure with fluid signal is in a medial location (arrow) posterior to the heart, with a tubular morphology and contiguity with the esophagus. This structure measures 35 x 20 x 25 mm and has a thin, regular wall with folds inside, compatible with a herniated stomach.
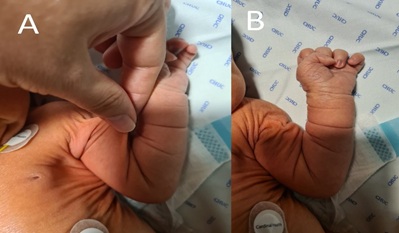 Figure 2: Skin laxity with prominent folds of the newborn. A. Skin stretching. B. Skin takes time to snap back to its original position because of compromised elasticity.
Figure 2: Skin laxity with prominent folds of the newborn. A. Skin stretching. B. Skin takes time to snap back to its original position because of compromised elasticity.
Investigations
Upon admission to the pediatric intensive care unit, thoracoabdominal radiography (figure 3) and abdominal ultrasound demonstrated a partially intra-thoracic stomach, suggesting of a hiatal hernia with preserved diaphragm continuity. Echocardiogram revealed aortic isthmus narrowing with a maximum systolic gradient of 12 mmHg. The neonate was under continuous cardiorespiratory monitoring, and blood pressure, diuresis, and temperature were evaluated every four hours. He was breathing spontaneously with no signs of respiratory distress and had a nasogastric tube in passive drainage.
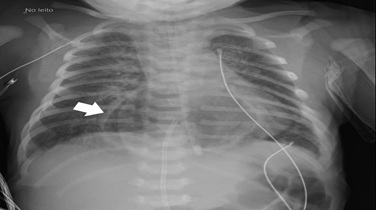 Figure 3: Thoracic and abdominal radiography on day 1 showing herniated intrathoracic stomach (arrow).
Figure 3: Thoracic and abdominal radiography on day 1 showing herniated intrathoracic stomach (arrow).
For preoperative evaluation, imaging was repeated on day two of life. Abdominal ultrasound and upper gastrointestinal series (figure 4) revealed the stomach now in anatomic position, intra-abdominal, with regular gastric emptying and significant gastro-oesophageal reflux. Diaphragmatic ultrasound disclosed a reduced thickness of the left hemidiaphragm and a reduced diaphragmatic excursion on this side (figure 5). Given these results, enteral feeding was started on day three and was initially well tolerated.
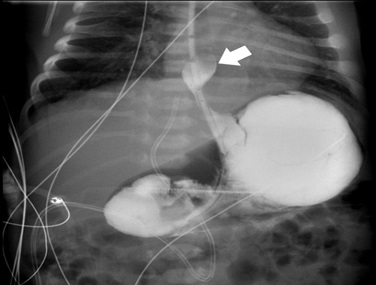 Figure 4: Upper gastrointestinal series on day 2 with an intra-abdominal stomach and significant gastroesophageal reflux (arrow).
Figure 4: Upper gastrointestinal series on day 2 with an intra-abdominal stomach and significant gastroesophageal reflux (arrow).
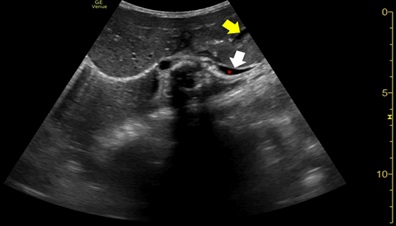 Figure 5: Diaphragmatic ultrasound on day 2 showing left hemi diaphragmatic reduced thickness and excursion (white arrow). Mild left pleural effusion is also seen (red asterisk). An almost empty intra-abdominal stomach can also be noticed (yellow arrow) in the anatomic position.
Figure 5: Diaphragmatic ultrasound on day 2 showing left hemi diaphragmatic reduced thickness and excursion (white arrow). Mild left pleural effusion is also seen (red asterisk). An almost empty intra-abdominal stomach can also be noticed (yellow arrow) in the anatomic position.
However, on day seven, frequent episodes of tachypnoea and desaturation after meals and a 20-mmHg systolic differential between upper and lower limbs were noticed, with poor perfusion. Echocardiogram demonstrated a dynamic left atrium, which was being compressed by the stomach (figures 6 and 7), with repeat diaphragmatic and abdominal ultrasound (figure 8) and thoracoabdominal contrast radiography (figure 9) showing recurrence of the hiatal hernia, prompting cessation of enteral feeds.
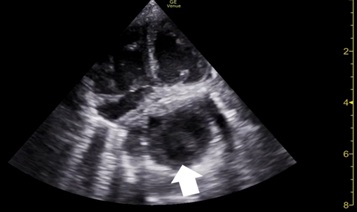 Figure 6: Echocardiogram on day 7, after a meal, showing left atria compression by the stomach (arrow).
Figure 6: Echocardiogram on day 7, after a meal, showing left atria compression by the stomach (arrow).
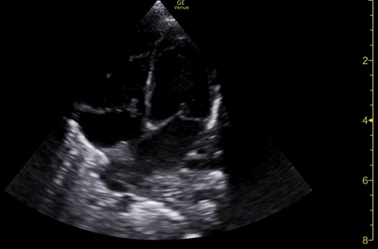 Figure 7: Echocardiogram also on day 7, but now on food break, showing resolution of the left atria compression.
Figure 7: Echocardiogram also on day 7, but now on food break, showing resolution of the left atria compression.
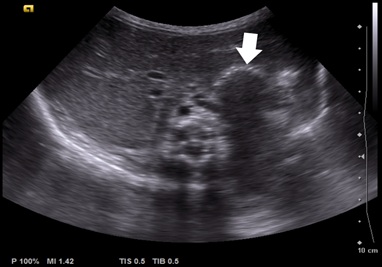 Figure 8: Abdominal ultrasound showing large hiatal hernia (arrow).
Figure 8: Abdominal ultrasound showing large hiatal hernia (arrow).
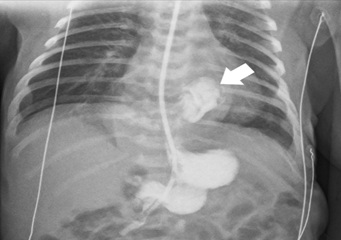 Figure 9: Abdominal ultrasound showing large hiatal hernia (arrow).
Figure 9: Abdominal ultrasound showing large hiatal hernia (arrow).
Neurological and ophthalmologic examinations and cranial ultrasound were normal.
The dermatologist performed a skin biopsy on day five, which demonstrated a significant rarefaction of elastic fibers in the papillary dermis, highlighted by the Verhoeff stain. The fibers were regularly distributed in the reticular dermis but had variable diameters, a convoluted aspect, and poorly defined limits. These morphological changes were pathological and compatible with the clinical diagnosis of cutis laxa.
A CentoXome® whole exome sequencing with copy number variant analysis and mitochondrial DNA analysis was performed, and no clinically relevant variants related to the described phenotype were detected.
Diferential Diagnosis
At birth, widespread redundant skin led to the suspicion of an inherited cutis laxa disorder. The generalized connective tissue laxity, namely in the peri-esophageal connective tissue, could also explain the gastric hernia diagnosed in the third trimester of pregnancy. Considering the three inheritance patterns of congenital cutis laxa disorders and the absence of family history, a recessive variant or a de novo mutation could explain the phenotype. Skin involvement is predominant in autosomal dominant cutis laxa, and X-linked cutis laxa manifests with urogenital tract diverticula and skeletal exostoses [1], which are absent in our patient. The neonate’s clinical manifestations are most compatible with an ARCL, and, considering its three subtypes, ACRL type 1 is the one that better suits the hiatal hernia phenotype [6].
We considered other causes for skin laxity in this patient. Geroderma osteodysplasticum can present with generalized wrinkly skin. However, the patient lacked other features, such as joint laxity and typical craniofacial characteristics like a broad forehead, hypertelorism, flat midface, sagging cheeks, and anteverted ears [5]. We also considered Ehlers-Danlos syndrome. However, the patient did not have hyperelastic skin (which snaps rapidly back after stretching), joint hypermobility, or generalized tissue fragility, which are characteristics of this condition [4].
Treatment
On day 11 of life, the patient underwent an exploratory laparotomy, which showed a paraesophageal hernia with an intra-thoracic gastric fundus, a loose diaphragm at oesophageal hiatus, and a hypertrophied pylorus. The surgeons reduced the herniated stomach into the peritoneal cavity, corrected the diaphragmatic hiatus using non-absorbable sutures, and performed a Boix-Ochoa anti-reflux procedure and a Fredet-Ramstedt pyloromyotomy.
Enteral feeding by nasogastric tube was started on day two post-surgery and progressed steadily to exclusive breastfeeding. The echocardiogram showed resolution of the aortic isthmus narrowing and left atrium compression. However, a peripheral pulmonary stenosis with a peak systolic gradient of 30 mmHg to the right branch (mild stenosis) and 40 mmHg to the left branch (moderate stenosis) was noticed. The baby was discharged home on day 22 (eleven-day post-surgery) when full sucking feeds and a good weight gain were secured.
Outcome
At two months of age, cardiac catheterization to assess the degree of pulmonary arteries stenosis was attempted, however, femoral venous access was complicated by skin and vessel laxity. As the infant is growing and developing appropriately, the procedure was deferred at this time. He is now three months old and continues to be followed by a multidisciplinary team, including pediatric gastric surgeons and cardiologists.
Discussion
CCL is caused by defects in elastin synthesis or assembly of extracellular matrix components and is recognized clinically by excessive skin folds [5]. While skin biopsy is not mandatory [2], in most cases, it is still performed, particularly in the case of inherited CL. In this case, the diagnosis was made immediately after birth, and the skin biopsy showed morphologic changes in the elastic fibers of the reticular dermis, compatible with the diagnosis. The hiatal hernia and peripheral pulmonary stenosis also resulted from this abnormality of elastic fibers.
The newborn's hiatal hernia was diagnosed in the third trimester of pregnancy and was the first manifestation of his systemic disease. Bedside echocardiography, diaphragmatic, and abdominal ultrasounds during the first week of life showed intermittent hiatal hernia with intermittent compression of the left atrium. The increased gastric size after meals and consequent worsening of the stomach's protrusion to the chest cavity may be responsible for the desaturation, probably related to severe gastro-oesophageal reflux and subsequent aspiration. The blood pressure differential noticed between the upper and lower limbs was probably a consequence of the stomach's compression of the left atrium.
Kothari et al. [9] reported the first neonate with CL and a hiatal hernia diagnosed after birth. The patient also had a gluteal hernia and ventricular septal defect. No genetic diagnosis was described. ARCL type 1 presents systemic complications, such as emphysema, diaphragmatic defects, and arterial tortuosity [1]. Letard et al. [10] reported 2 cases of ARCL type 1b (OMIM #614437) with multiple malformations leading to termination of pregnancy. One fetus had a hiatal hernia confirmed by autopsy, combined with pyelectasis, multiple artery tortuosity, and skeletal deformities. ARCL type 1c is more frequently associated with hiatal and diaphragmatic hernias and can also present with peripheral pulmonary stenosis [6,11,12]. Although the whole exome sequencing was inconclusive in our patient, and there is no history of consanguinity, his phenotype is most compatible with an ARCL type 1c. An exome revaluation will be considered after 12 months or if there are phenotypic changes.
CCL has limited treatment, and the management is supportive, according to the systemic manifestations [1,5]. In CCL, wound healing is not impaired, so plastic surgery for skin folds may be considered, but relapsing is frequent [1,2]. Our patient is asymptomatic and has mild to moderate peripheral pulmonary stenosis, so echocardiography is performed monthly. Should gastrointestinal or respiratory symptoms emerge, abdominal ultrasound/ upper gastrointestinal series and lung CT may be performed to assess hiatal hernia recurrence and emphysema, respectively.
Conclusion
Congenital cutis laxa is a systemic disorder that can have prenatal manifestations and hiatal hernias can result from the abnormality of elastic fibres characteristic of this condition. The intermittent hiatal hernia with dynamic compression of the left atrium presented a diagnostic challenge in this case. Cardiovascular involvement is common, so echocardiographic follow-up is recommended in congenital cutis laxa syndromes.
Consent
Written informed consent was obtained from the patient’s mother for publication of this case report.
Disclosure
This clinical case was written based on clinical observation without any funding.
References
- Berk DR, Bentley DD, Bayliss SJ, Lind A, Urban Z (2012) Cutis laxa: a review. J Am Acad Dermatol 66: .e1-17
- Gara S, Riley CA, Litaiem N (2023) Cutis Laxa. StatPearls. StatPearls Publishing
- Dyer JA (2012). Chapter 137. Lipoid Proteinosis and Heritable Disorders of Connective Tissue. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K. eds. Fitzpatrick's Dermatology in General Medicine. The McGraw-Hill Companies 8e.
- Mohamed M, Voet M, Gardeitchik T, Morava E (2014) Cutis Laxa. Adv Exp Med Biol 802: 161-184.
- Beyens A, Boel A, Symoens S, Callewaert B (2021) Cutis laxa: A comprehensive overview of clinical characteristics and pathophysiology. Clin Genet 99: 53-66.
- Callewaert BL, Urban Z (2023) LTBP4-Related Cutis Laxa. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle 1993-2024.
- Van Maldergem L, Dobyns W, Kornak U (2023) ATP6V0A2-Related Cutis Laxa. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle 1993-2024.
- Embleton DB, Tuncer AA, Arda MS, Ilhan H, Cetinkursun S (2019) Congenital hiatus hernia: A case series. North Clin Istanb 6:171-175.
- Kothari P, Arun G, Gowri S, BharatiK K, Dharap S (2005) Case Report- Infantile congenital cutis laxa with multiple hernias and ventricular septal defect. Indian Journal of Surgery 1: 67.
- Letard P, Schepers D, Albuisson J, Bruneval P, Spaggiari E, et al. (2018) Severe Phenotype of Cutis Laxa Type 1B with Antenatal Signs due to a Novel Homozygous Nonsense Mutation in EFEMP2. Mol Syndromol 9:190-196.
- Pottie L, Adamo CS, Beyens A, Lütke S, Tapaneeyaphan P, et al. (2021) Bi-allelic premature truncating variants in LTBP1 cause cutis laxa syndrome. Am J Hum Genet 108: 1095-1114.
- Urban Z, Hucthagowder V, Schürmann N, Todorovic V, Zilberberg L, et al. (2009) Mutations in LTBP4 cause a syndrome of impaired pulmonary, gastrointestinal, genitourinary, musculoskeletal, and dermal development. Am J Hum Genet 85:593-605.
Citation: Diogo R, Leal B, Dinis A, Dionisio T (2024) Congenital Cutis Laxa Presenting With Hiatal Hernia in the Third Trimester of Pregnancy. J Neonatol Clin Pediatr 11: 119.
Copyright: © 2024 Rui Diogo, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

