
Contribution of Delayed Metabolic Transition from Rest to Oxidative Steady State on Performance Fatigability in Women with Systemic Lupus Erythematosus
*Corresponding Author(s):
Liana C WootenDepartment Of Health, Human Function, And Rehabilitation Science, School Of Medicine And Health Sciences, George Washington University, 2000 Pennsylvania Ave, NW Suite 2000, Washington, DC, United States
Tel:(202) 994-2000,
Email:lwooten@gwu.edu
Abstract
Severe cardiorespiratory limitations have been documented in patients with Systemic Lupus Erythematosus (SLE). It is hypothesized that these limitations underlie high performance fatigability, which markedly restricts engagement in physical activity, even in instrumental activities of daily living. However, characterization of such performance has been limited to measurements made primarily at peak exercise in SLE population, leaving the ability to sustain a given submaximal exercise intensity (cardiorespiratory endurance) to be less well understood.
Objective
To characterize cardiorespiratory limitations and performance fatigability in women with SLE.
Methods
This is a case- report pilot study of 7 women with SLE [Age 38.3(9.6) years; BMI 26.5(3.9) kg/m2] and 8 sedentary but otherwise healthy controls [Age 38.8(5.5) years; BMI 26.3(6.8) kg/m2] who completed a 5-MET sustained work rate endurance test ending at volitional exhaustion or when 60 minutes of treadmill walking time had accrued. Group measures of total treadmill walk time and VO2 kinetics transition constant (Kt) and amplitude (ΔVO2) were compared among both groups.
Results
There was no significant statistical group differences in cardiorespiratory function at rest. However, test duration [28(9.6) vs 57(7.0) min; p<0.001], Kt [8.5(3.0) vs 13.4(4.4); p=0.019], and ΔVO2 [0.65(0.18) vs 0.85(0.17); p=0.031] were decreased in the women with SLE compared to the controls, even though the energy demand was identical.
Conclusion
The current study suggests that prolonged cardiorespiratory transition from rest to oxidative steady state during physical activity may have contributed to increased performance fatigability in women with SLE. Further studies are needed to explore the mechanistic relationship between patient-perceived fatigue severity and poor cardiorespiratory dysfunction, in women with SLE.
Keywords
Cardiorespiratory; Exercise; Fatigability; Lupus; Metabolic transition, Oxygen uptake kinetics; Performance
Introduction
Significance and Innovation
- The capacity to sustain physical activity at the upper limit of the range of energy requirements for instrumental activities of daily living is restricted in women with SLE.
- The restriction occurs concomitantly with functional cardiorespiratory impairment and prolonged VO2 on-kinetics, suggesting an etiological link between cardiorespiratory dysfunction and performance fatigability.
- The current findings suggests that increased performance fatigability may be mediated through delayed metabolic transition from rest to oxidative steady state during physical activity in women with SLE.
Systemic Lupus Erythematosus (SLE) is a chronic autoimmune disease which produce a wide array of clinical manifestations and organ involvement.[1] Genetics, environmental factors and complex immune pathogenic pathways contribute to the heterogeneity of the clinical manifestations. It is estimated that SLE effects up to 322,000 adults in the United States alone [2], predominantly women between the ages of 15 and 44 years, with peak incidence occurring during the adolescent, childbearing and childrearing, and professional developmental years. [3] Even though the outlook of patients with SLE has improved with earlier diagnosis and improved medical management which resulted in an increase in both SLE prevalence and prolonged life expectancy, which has begun to approach normal ranges. Yet, unmet needs remain in the care of SLE patients in particular the pathogenic mechanism of perceived fatigue.
Fatigue, fever, weight loss, general malaise, arthralgia and arthritis are common in those who have SLE, with the latter two constituting the most commonly presenting manifestations of SLE. [1] Most of these symptoms are readily controlled by medication, with the ultimate goal of controlling disease activity and preventing flares that can cause further damage. However severe fatigue (SLE-related fatigue {SERF}) persists even during times of low disease activity and is one of the most common and debilitating symptoms of SLE, yet it is one of the least understood. SERF affects approximately 90% of people who have the disease with as many as 50% reporting it as their single most debilitating symptom [4], severely restricting physical functioning and the ability to engage in both personal and professional activities. It has been reported that nearly half individuals with SLE working at the time of diagnosis lost their job within an average of 13 years, and while this inability to sustain full-time employment may be a result of multiple factors, severe persistent fatigue was found to be the highest burden having a profound impact on lost work productivity in this population. [5]
Cardiorespiratory function is severely diminished in SLE [6-11] and appears to play a substantial role in the mediation of SERF. [7] Previous studies have provided strong evidence of Functional Aerobic Impairment (FAI) [12,13] in women with SLE , demonstrated by reductions in peak cardiorespiratory function to as low as 38% below levels expected. [7] Use of a quantitative measurement of FAI indicated that the impairment and severity of exercise limitation was likely etiologically mediated rather than the result of physiologic deconditioning such as that occurring in adaptation to a more physically inactive lifestyle. [7] Reduced cardiorespiratory function has also been associated with patient reported fatigue severity and declining physical activity in those with SLE [7,8]. Importantly, this cardiorespiratory function has been shown to be too low to sustain even instrumental activities of daily living in women with mild SLE without inducing fatigue. [7]
The construct “performance fatigue” has been operationally defined as a decline in performance as a result of exertion. (NIH PA-12-227) A phenotype, “performance fatigability”, characterizes the decline in performance over time as a “function of duration, intensity, and/or frequency of activity [or exertion]” (NIH PA-12-227), providing more specific information about functional limitations associated with fatigue. An example of increased performance fatigability, commonly measured and observed by physical therapists in clinical settings, includes decreased distance covered during timed walk tests (e.g. 6-minute walk test [14]) in which the time is held constant. Additional examples of increased fatigability, measured during CardioPulmonary Exercise Testing (CPET), include decreased time, power output, or VO2 at either or both the anaerobic threshold or peak time points. All of these examples demonstrate a decline in performance that can be measured objectively, whether by field testing or during CPET. Women with SLE have demonstrated increased performance fatigability as evidenced by shortened test duration during peak exercise tests [6-8], low anaerobic threshold [7,10] and prolonged oxygen uptake (VO2) on-kinetics during submaximal exercise bouts of specific intensities and durations [11]. Furthermore, significant correlations among FAI scores, patient-reported fatigue scores, and physiological markers of exercise-induced fatigue have been identified [7]. Conversely, quantitative measurements of the ability to sustain a given level of work before reaching a point of volitional exhaustion, which may provide insight into the ability of individuals with SLE to sustain functional activities for prolonged periods, have not been reported in patients who have SLE.
Methods
We examined cardiorespiratory dysfunction and performance fatigability in women with SLE, with a novel approach emphasizing performance during a prolonged treadmill test. Performance fatigability was determined as a function of the time a submaximal bout of work could be sustained before reaching exhaustion, thus fitting within the wider construct as defined by the National Institute of Health (NIH), as a decline in performance (reduction in time) while the intensity is held constant. This study used a case-control design. The associations among performance fatigability, cardiorespiratory function, and the metabolic transition from rest to exercise at the upper limit of VO2 requirements for instrumental activities of daily living were also examined. Our hypothesis is that women with SLE have increased performance fatigability, as measured by prolonged treadmill time during exercise testing [15].
- Inclusion/Exclusion criteria
Women with SLE with no lupus disease activity and sedentary but otherwise healthy controls > 18 years of age were included in the study. None of the women were postmenopausal and none were known to be pregnant. All of the women were physically inactive and indicated they had not routinely participated in physical activity that resulted in perspiring for 10 minutes, more than once per week, for at least six months prior to their participation. Subjects with Systemic Lupus Activity Measure [16] (SLAM) scores ≥ 9, or cardiovascular, pulmonary, renal, neuromuscular, or musculoskeletal complications that would limit cardiorespiratory function, and those taking medications that would either limit or enhance exercise tolerance or aerobic capacity were excluded. Those with fibromyalgia, obstructive sleep apnea, rheumatoid arthritis, or severe anemia (hematocrit < 30%) were also excluded. University of Maryland, Baltimore, Institutional Review Board approval was obtained prior to implementation of the protocol and informed consent was obtained from all participants prior to participation in accordance with the Declaration of Helsinki [17]; the rights of participants were protected.
Procedures
- Exercise testing
Prior to exercise testing, subjects filled out the Fatigue Severity Scale [18] (FSS) questionnaire and then rested for 10 minutes while baseline data was collected. Once the baseline data was obtained and the rest period expired, subjects underwent peak treadmill CPET to determine peak exercise capacity and cardiorespiratory fitness. During the CPET, workload was advanced in three-minute intervals according to the modified Bruce protocol [19]. The test ended at volitional exhaustion defined as subject indication that she must stop exercising due to severe fatigue despite strong encouragement from the testing staff. On a subsequent day, between 40 and 168 hours later, subjects completed a continuous work rate, treadmill exercise test (CWT), during which cardiopulmonary function was again measured in addition to VO2 uptake kinetics. During this test, subjects walked continuously on a treadmill at a single work rate (5-MET) until reaching volitional exhaustion or time limit of 60 minutes. The CWT was performed to determine both measures of kinetics and the subjects’ ability to sustain submaximal physical activity, i.e., performance fatigability measured as minutes of sustained exercise: the longer the time to exercise cessation, the lower the performance fatigability.
During both CPET and CWT, pulmonary gas exchange was measured using a MedGraphics CardiO2 CPET system (Medical Graphics Corp, 350 Oak Grove Parkway, St Paul, MN 55127). The system was comprised of infrared carbon dioxide and zirconium oxygen gas analyzers, digitally interfaced with a bidirectional pneumotachometer and an electrocardiogram (EKG). The system was calibrated according to manufacturer’s specifications prior to each CPET and CWT.
Calculations
- Measures of cardiopulmonary function
Pulmonary gas exchange was assessed continuously by averaging breath-by-breath measurements over every eight breaths, in progressive number bins in which no breath was included in more than one bin. The highest averages during the last 30 seconds of the CPET were determined to be the peak values for the test. Peak respiratory exchange ratio (RER) was calculated as the quotient of volume of carbon dioxide expiration (VCO2) divided by the corresponding VO2. The Anaerobic Threshold (AT), a marker for the onset of exercise-induced fatigue [20], was determined using the V-slope method of Beaver and Whipp [21] applied to the breath-by breath measurements and reported as AT-VO2 and AT-Time. The Physiological Reserve (PR) was calculated as the difference in peak-VO2 and AT-VO2. Expected peak heart rate was determined by the equation [220-subject’s age in years] [19] and measured heart rate was determined by continuous Electrocardiogram (EKG).
Kinetics
Kinetics were measured from data obtained during the CWT portion of the testing procedures, during which pulmonary gas exchange was analyzed breath-by-breath continuously throughout the test and VO2 was plotted on time. VO2 on-kinetics were determined using the monoexponential model developed by Whipp [22]. This model is expressed by the formula VO2 = (ΔVO2 + VO2rest) (1-e-tK), where ΔVO2 is the amplitude of change in VO2 from baseline to end exercise minus any slow component and K is the rate constant. The presence of a slow component was determined using a least squares method and confirmed by the absence of a significant increase in VO2 between the 3rd and 6th minute of exercise [11]. From this model a Mean Response Time (MRT) was estimated as the time taken to achieve 63% of the ΔVO2, and a transition constant (Kt), which normalizes the ΔVO2 and MRT, was computed as the quotient of ΔVO2/MRT [11].
Measure of Performance Fatigability
During the CWT, workload was maintained at a 5-MET intensity, corresponding to 2.0 mph and 9.0% grade on the treadmill. 5-METs is the upper limit of the range of energy requirements for instrumental activities of daily living (range=3- to 5-METs) [23]. Subjects walked at this intensity on a motor driven treadmill until reaching volitional exhaustion as previously defined or a time limit of 60 minutes.
Instrumentation
FAI: The index of Functional Aerobic Impairment (FAI) was calculated using the following formula [12].
FAI =[ (expected peak oxygen consumption - measured peak oxygen consumption)/(expected peak oxygen consumption)] X 100
Expected peak VO2 was determined using the formula 42.3-(0.356 x age in years) for sedentary females and both expected and measured peak VO2 were reported in milliliters of oxygen consumed per kilogram of body weight per minute (ml/kg/minute) [19].
Fatigue Scale
The Fatigue Severity Scale (FSS), is one of the most commonly used and reliable scales for measuring patient-reported fatigue in the SLE population [4,18,24]. The scale consists of 9 statements aimed at detecting how the individual perceives fatigue to limit her or his ability to function physically and socially. The FSS score is determined by grading the responses to the 9 statements on a 7-point likert scale with 1 indicating a strong disagreement, and 7 indicating strong agreement. The average of the statement scores is taken for the final score with a score ≥ 3 indicating clinically significant fatigue and subject perception that fatigue limits physical and social function.
Variables And Statistics
The main variable of interest was the total time duration that exercise was sustained during the CWT, which is a measure of performance fatigability. Additional variables of interest were the FSS, those characterizing the cardiorespiratory response during the CWT including components of the VO2 on-kinetics analyses (Kt and ?VO2), and those obtained during CPET including the time and VO2 at both the AT and peak, peak-RER, peak-HR, and FAI. CPET and CWT results were compared between the groups by independent t-tests. Independent t-tests were also used to compare demographic and FSS scores between the groups. The assumptions necessary for employing one-tailed t-test analyses were both met for total time walked on treadmill during CWT, cardiorespiratory variables, and FSS; the directional hypothesis of inferiority in the women with SLE was identified a priori and there was pre-existing evidence for the direction of the hypothesis provided in previous studies. Baseline, predicted, and demographic variables were analyzed using two-tailed t-test analyses. Regression analyses were utilized to assess associations between FSS scores, measures of performance fatigability, and measures of cardiopulmonary function. For analyses of the kinetic data (Kt and ?VO2), 6 of the 7 SLE subjects were included in the analyses due to missing data for one SLE subject. Statistical significance was accepted at p ≤ 0.05. Central tendencies are reported as means (standard deviations).
Fifteen women, 7 with SLE and 8 sedentary but otherwise healthy controls, similar in age and BMI, participated in the study (Table 1). All women reported to be physically inactive. Of the women with SLE, 5 were on prednisone dosages between 5-10 mg per day (Table 1). All of the subjects met the inclusion criteria and none of the subjects met an exclusion criterion. There were no dropouts over the two days to one week of testing for each subject.
|
|
SLE (n=7) |
Control (n=8) |
p-value |
|
Age (years) |
38.3 (9.6) |
38.8 (5.5) |
0.909 |
|
BMI (kg/m2) |
26.5 (3.9) |
26.3 (6.8) |
0.949 |
|
SLAM score |
1.86 (1.2) |
|
|
|
Prednisone dose (mg/day) |
5 (4.1) |
|
|
Table 1: Demographics and clinical variables of participants.
There were no statistically significant differences in resting VO2 and resting HR between groups at baseline (Table 2). FSS composite score was significantly higher in the women with SLE than in the controls and reached a level of clinical significance (FSS score ≥ 3 indicates clinical significance) only in the group with SLE (Table 2). A clinically significant FAI (≥ 26%) was observed in the women with SLE but not in the controls (Table 2).
|
|
SLE |
Control |
p-value
|
|
Predicted Peak VO2 (ml/kg/min) |
28.7 (3.4) |
28.5 (2.0) |
0.909 |
|
Resting VO2 (ml/kg/min) |
2.0 (0.3) |
2.2 (1.3) |
0.737 |
|
Peak VO2 (ml/kg/min) |
17.8 (2.1) |
26.0 (3.7) |
<0.001* |
|
Predicted Peak HR |
182 (9.6) |
181 (5.5) |
0.909
|
|
Resting HR (bpm) |
70 (13.2) |
78 (11.3) |
0.242 |
|
Peak HR (bpm) |
170 (30.9) |
177 (8.8) |
0.571 |
|
Peak RER |
1.2 (0.09) |
1.19 (0.04) |
0.832 |
|
Peak Time |
13.2 (1.5) |
15.6 (1.2) |
0.002* |
|
AT-VO2 |
11.6 (1.3) |
16.0 (1.6) |
<0.001* |
|
AT-Time |
5.8 (1.3) |
8.3 (1.3) |
0.001* |
|
FAI |
37.4 (7.3) |
8.2 (16.1) |
<0.001* |
|
FSS Composite |
5.5 (1.1) |
2.5 (0.9) |
<0.001* |
Table 2: Resting and Exercise Response, FAI, and FSS.
The women with SLE had significantly attenuated CWT-time (Figure 1) compared to the controls. None of the women with SLE approached the targeted stopping point for sustaining treadmill walking of 60-minutes, whereas all of the women but two in the control group reached the 60-minute stopping point, with one of those two reaching 59 minutes. Additionally, significant associations were found between total walk time and FAI (R2=0.546, p=0.002) as well as FSS composite scores (R2=0.480, p=0.004). Both Kt and ?VO2 were significantly reduced in women with SLE compared to controls (Figures 2a & 2b).
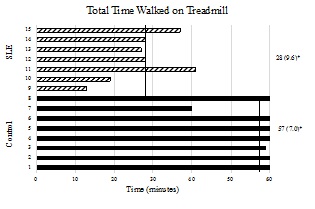 Figure 1: Total time duration time on treadmill during CWT. The x-axis represents time in minutes and the y-axis represents individual subjects by group. Mean values and standard deviations are represented on the right for each respective group. The vertical lines represent group mean values. P-value is 1-tailed. p < 0.001.
Figure 1: Total time duration time on treadmill during CWT. The x-axis represents time in minutes and the y-axis represents individual subjects by group. Mean values and standard deviations are represented on the right for each respective group. The vertical lines represent group mean values. P-value is 1-tailed. p < 0.001.
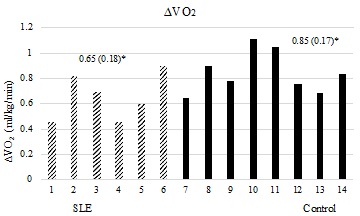 Figure 2a: ?VO2 between groups. The x-axis represents individual subjects by group. Y-axis represents peak ?VO2 in ml/kg/min. Mean values (standard deviations) are represented above for each respective group. P-value is 1 tailed. p=0.031
Figure 2a: ?VO2 between groups. The x-axis represents individual subjects by group. Y-axis represents peak ?VO2 in ml/kg/min. Mean values (standard deviations) are represented above for each respective group. P-value is 1 tailed. p=0.031
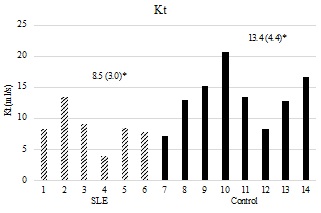 Figure 2b: Kt by group. X-axis representing individuals’ subjects by group. Y-axis representing Kt in ml/sec. Mean values (standard deviations) are represented above for each group. P-value is 1-tailed. p=0.019.
Figure 2b: Kt by group. X-axis representing individuals’ subjects by group. Y-axis representing Kt in ml/sec. Mean values (standard deviations) are represented above for each group. P-value is 1-tailed. p=0.019.
On the CPET, both of the groups attained a mean respiratory exchange ratio (RER) of at least 1.10 and a peak HR of at least 90% predicted (Table 2), indicating a level of exertion that approached a physiologically maximal metabolic demand at volitional exhaustion.19 AT-Time, Peak-Time (Table 2), Peak-VO2, AT-VO2, (Figures 3a & 3b) and physiologic reserve (Figure 4) were significantly diminished in the women with SLE compared to controls. The VO2 demand equaling 17.5 ml/kg/min (5-METs; upper limit for iADLs) was well above the 95% confidence interval for AT-VO2 in women with lupus (CI [10.4-12.8]) and was similar to the upper limit of the 95% confidence interval for AT-VO2 in the controls (CI [14.6-17.3]). At 3-Mets, the VO2 demand of 10.5 ml/kg/min (3-METs, lower limit for iADLs) was within the 95% confidence interval for AT-VO2 in women with lupus (CI [10.4-12.8]) and was well below the 95% confidence interval for AT-VO2 in the controls (CI [14.6-17.3]). (Figure 3B). The association between FSS composite scores and AT-VO2 (R2=0.465, p=0.005), Peak-VO2 (R2=0.576, p=0.001), AT-Time (R2=0.453, p=0.006) Peak-Time (R2=0.410, p=0.010), and physiologic reserve (R2=0.528, p=0.002) were statistically significant.
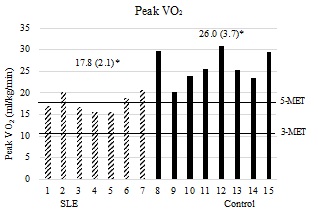 Panel 3a: Peak-VO2 in relation to energy demand. The x-axis represents individual subjects by group. Y-axis represents peak VO2 in ml/kg/min. Mean values (standard deviations) are represented above for each respective group. 3-MET (10.5 ml/kg/min VO2 demand) and 5-MET (17.5 ml/kg/min VO2 demand) intensity bars displayed horizontally. P-value is 1-tailed. p < 0.001
Panel 3a: Peak-VO2 in relation to energy demand. The x-axis represents individual subjects by group. Y-axis represents peak VO2 in ml/kg/min. Mean values (standard deviations) are represented above for each respective group. 3-MET (10.5 ml/kg/min VO2 demand) and 5-MET (17.5 ml/kg/min VO2 demand) intensity bars displayed horizontally. P-value is 1-tailed. p < 0.001
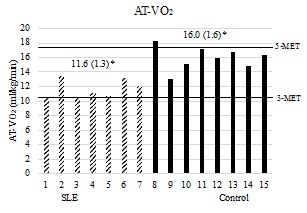 Panel 3b: AT-VO2 in relation to work demand. The x-axis represents individual subjects by group. Y-axis represents AT-VO2 in ml/kg/min. Mean values (standard deviations) are represented above for each group. 3-MET (10.5 ml/kg/min VO2 demand) and 5-MET (17.5 ml/kg/min VO2 demand) intensity bars displayed horizontally. P-value is 1-tailed. p < 0.001
Panel 3b: AT-VO2 in relation to work demand. The x-axis represents individual subjects by group. Y-axis represents AT-VO2 in ml/kg/min. Mean values (standard deviations) are represented above for each group. 3-MET (10.5 ml/kg/min VO2 demand) and 5-MET (17.5 ml/kg/min VO2 demand) intensity bars displayed horizontally. P-value is 1-tailed. p < 0.001
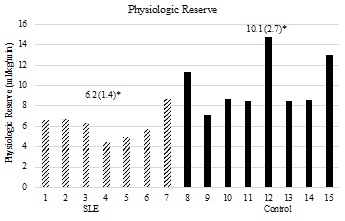 Figure 4: Physiologic VO2 (ml/kg/min) reserve is represented on the y-axis. The x-axis represents individual subjects by group. Mean values (standard deviations) are represented above for each respective group. P-value is 1-tailed. p=0.002.
Figure 4: Physiologic VO2 (ml/kg/min) reserve is represented on the y-axis. The x-axis represents individual subjects by group. Mean values (standard deviations) are represented above for each respective group. P-value is 1-tailed. p=0.002.
Discussion
Previous studies have characterized the relationships between patient-perceived fatigue severity scores and etiologically mediated cardiorespiratory dysfunction in women with SLE [7]. Evidence of cardiorespiratory dysfunction includes reductions in the anaerobic threshold [7,10], and both the VO2 and total exercise duration at the peak time point during CPET in individuals with SLE [6-8].
Specifically, Tench et al reported a Peak VO2 of 23.2 ml/kg/min and a total test duration of 10.4 minutes in a sample of 93 women with SLE, which were both significantly lower than the control group [8] Pinto et al reported a mean Peak VO2 of 24.4 ml/kg/min as well as a total test duration of 11.2 minutes in a sample of 21 adult women with SLE, also significantly reduced compared to controls [6]. Robb-Nicholson et al reported a maximum VO2 of 18.8 ml/kg/min and total test duration of 9.3 minutes in a sample of 23 women with SLE [25]. Reductions in measures at the AT have also been reported [7,10], specifically AT-Time (5.87 minutes) and AT-VO2 (~10.5 ml/kg/min) in women with SLE [7]. The current study supported these findings of diminished aerobic capacity and increased performance fatigability in individuals with SLE compared to controls as evidenced by reductions in both time and VO2 at the AT and peak during CPET.
Adding to the literature, the current study utilized a novel approach to evaluate performance fatigability, assessing the ability to maintain exercise during prolonged treadmill walk testing in combination with the obtained measures obtained during peak exercise testing motioned above. This assessment is beneficial in that the constant work rate intensity demand is more applicable to daily functional activities than perhaps peak testing, as peak efforts are not typically required during normal daily tasks.
The current study provides evidence of increased performance fatigability during prolonged treadmill walking at constant work rate, as evidenced by reductions in total time walked on the treadmill during which work rate was held constant at 5-MET’s for all subjects. Thus, the capacity to sustain physical activity at the upper limit of the range of energy requirements for instrumental activities of daily living [23] was restricted in this group of women with SLE. This restriction, occurring concomitantly with functional cardiorespiratory impairment and prolonged VO2 on-kinetics, suggests a potential etiological link between cardiorespiratory dysfunction and performance fatigability.
The observance of a severe reduction in total walking time on the CWT in the SLE group compared to the controls was striking. At a 5-MET intensity, the women with SLE were able to complete less than half of the 60-minute target interval. One subject was only able to perform a total of 13 minutes before ending the test due to exhaustion, whereas the longest time a subject with SLE could sustain walking was 41 minutes. Of the eight sedentary but otherwise healthy controls, 6 reached the 60-minute target duration, one reached 59 minutes, and one was able to sustain walking for 40 minutes. The comparison noted within these observations underscore a severe performance limitation in the group of women with SLE. The range of energy demands for instrumental activities of daily living are between 3-METS and 5-METs. Even energy demands of 5-METS are considered to be of moderate intensity and thus easily tolerated by most healthy adults. Furthermore, most healthy adults reach their anaerobic threshold, which is a marker of the onset of fatigue, at an energy demand approaching 5-MET’s or higher.
Women with SLE in this study not only reached the anaerobic threshold at a low metabolic demand, but they also reached volitional exhaustion at significantly lower treadmill walking duration. This information provides additional insight into the functional limitations faced by patients with SLE in the community as well as in physical therapy clinical settings.
Previous literature has demonstrated altered VO2 kinetics in individuals with SLE, as evidenced by increased time to steady state in individuals with SLE compared to controls [11]. It was suggested that the findings of an increased oxygen deficit, as well as these prolonged VO2 kinetics, may underlie the performance fatigability in women with SLE [11]. The current findings of this smaller subset add to this literature, demonstrating significant decreases VO2 amplitude change and transition constant in the SLE group when compared to controls. This suggests that increased performance fatigability may in part be mediated through delayed metabolic transition from rest to oxidative steady state during physical activity in women with SLE.
In order to sustain physical activity, adequate muscle oxygenation is necessary for repletion of the ATP used by the cross bridges of the working muscles.20 From the onset of work until an oxidative steady state is reached (matching of oxygen utilization and oxygen demand), anaerobic metabolism must compensate for the oxygen deficit [26]. As a result, metabolic by-product concentrations increase, competing with high-energy phosphates and other ions for cross-bridge and metabolic pump binding sites and reducing cytosol and mitochondrial pH. Accumulation of the by-products impairs muscle contractile and metabolic function, diminishing cardiorespiratory endurance and exacerbating performance fatigability [20].
VO2 on-kinetics can be utilized as a marker of fatigue and impaired exercise tolerance [27] and reflects the rate at which the oxidative system can transition to a steady state of increased energy output, which matches the oxygen demand, during physical activity [28]. The kinetics can be characterized in two domains, the overall rate of the VO2 transition and the magnitude of change in VO2 from rest, or a lower intensity of activity, to steady state. With the metabolic demand held constant, a faster VO2 kinetics results in a lower oxygen deficit, less substrate-level phosphorylation, and thus higher exercise tolerance, whereas slower VO2 kinetics result in a higher oxygen deficit, increased substrate level-phosphorylation, and reduced exercise tolerance [28]. Thus, delayed attainment of a steady state, as seen in this sample of women with SLE, may hamper the ability to perform and sustain physical activity.
Previously, VO2 on-kinetics has been shown to be delayed in women with SLE compared to healthy controls both at similar percentages of the anaerobic threshold and at similar absolute energy demands [11]. VO2 measured at the lung reflects the response to exercise perturbation of both systemic oxygen delivery (indexed as cardiac output; Qt) and muscle oxidative function and oxygen extraction reflected in the arteriovenous oxygen difference (a-vO2) [29].
It has been proposed [30] that maximal VO2 is generally limited by central circulatory delivery of oxygen to the working muscles via the cardiovascular system whereas during submaximal endurance efforts, skeletal muscle oxygen extraction is thought to limit endurance, which is the inverse of performance fatigability. Previous work has suggested that a-vO2 may be diminished during exercise in women with SLE while Qt was suggested to be similar between women with SLE and controls [11]. A prolonged metabolic transition in the working muscles has also been observed, adding further plausibility that muscle oxygen extraction limitations may in part mediate increases in performance fatigability in women with SLE.
Clinically significant patient-reported fatigue was also observed in this subset, similar to previous studies [7,8]. The current study also revealed significant associations between FSS and primary measures of cardiorespiratory fitness (time and VO2 at the AT and peak; physiologic reserve). Additionally, the current study adds to the literature by revealing a significant association between the ability to sustain prolonged walking on the treadmill and fatigue severity scores, furthering the link between etiologic impairments, performance fatigability, and subjective fatigue in this population.
For physical therapists and other rehabilitation and healthcare professionals, understanding the impact that fatigability can have on patients function and presentation in clinic is critical for creating a comprehensive and individualized plan of care. Furthermore, understanding the underlying physiologic limitations is important for developing appropriate and effective interventions. Methods such as energy conservation and prescriptive aerobic exercise planning [1] are suggested to address fatigue and aerobic capacity in this population. Additionally, kinetics have been shown to improve following aerobic exercise training in other populations and these effects seem to occur earlier than those changes seen in maximal VO2 [27]. Further research is needed to understand additional effective interventions as well as optimal dosage of currently effective interventions.
It is worth acknowledging the multifactorial nature of fatigue, as many factors may contribute to increased fatigability during prolonged activity in this population that were not measured in this study. Factors previously associated with fatigue in individuals with SLE include sleep quality, depression, anxiety, mood, and vitamin D deficiency or insufficiency [31]. Therefore, while the current findings are both novel and critical to our understanding of fatigability in women with lupus, they are also preliminary, setting the foundation for which future studies should also investigate potential moderating factors that may contribute to performance fatigability during prolonged activity in individuals with SLE.
Study Limitations
The ability to generalize the results of this study is limited by the small sample size which may not be an accurate representative sample of all the women with heterogeneous clinical syndromes of SLE. Results and conclusions of this study must be delimited to the current sample and experimental conditions. Furthermore, the study employed a cross-sectional design, thus future experimental studies with a larger population are needed to assess the role of delayed metabolic transition as a mediator in performance fatigability in SLE population.
Phase of menstrual cycle and use of oral contraceptive was not controlled in this study. Dean et.al [32] have previously reported that in healthy women, menstrual phase had no effect on Peak-VO2 or lactate threshold. Conversely, Lebrun et al [33] reported a decrease in Peak-VO2 of 4.7% from the follicular to the midluteal phase in a group of healthy women using oral contraceptives, although in a placebo control group an increase of 1.4% occurred across these phases. While, potential introduction of bias introduced by the variance in menstrual cycle and use of oral contraceptives appears minimal, similar effects if found in the current study would account for a bias of no greater than -20% to +06% of 1 SD from the mean Peak-VO2 in the women with SLE (-0.95 to +0.28mL·kg-1·min-1) and -27% to +08% in controls (-1.36 to +0.40mL·kg-1·min1).
Inclusion criteria required subjects to be physically inactive having not performed physical activity causing them to perspire for 10 minutes or longer, one or more times per week, during the 6 months prior to enrolling in the study. This subjective measure of physical activity may have allowed for increased variability in reported physical activity levels due to increased fatigability in the women with SLE. In general, peak-VO2 does not appear to be related to self-assessed physical activity levels, but has shown moderate-strong associations with self-assessed physical function [34]. However, whether VO2 on-kinetics and physical activity level are related remains unknown.
Drug-induced myopathy is one possible side effect of orally administered prednisone, particularly when the agent is fluorinated, or dosages exceed 40mg/d. The women with SLE in the present study were maintained on dosages much less than 40mg/d. Dosages similar to those used by patients in the present study have not been shown to decrease mitochondrial enzyme concentrations nor VO2-peak but a degree of uncertainty regarding prednisone toxicity and the dose-response remains.
Conclusion
The current study demonstrates significant increases in performance fatigability during extended periods of walking in women with SLE and suggests that this performance fatigability may be exacerbated through delayed metabolic transition during physical activity. In women with SLE, the inability to sustain the energy requirements requisite for a 5-MET work rate, which is the upper limit of the metabolic demands of instrumental activities of daily living, accentuates the severity of their functional limitations, potentially reducing health-related quality of life. These findings have direct implications for physical therapists, clinical providers, and caregivers treating individuals with SLE.
References
- Goodman CC, Fuller KS (2015) Pathology: Implications for the physical therapist.
- Helmick CG (2008) Estimates of the prevalence of arthritis and other rheumatic conditions in the United States: Part I. Arthritis Rheum 58: 15-25.
- Danchenko N, Satia JA, Anthony MS (2006) Epidemiology of systemic lupus erythematosus: A comparison of worldwide disease burden. Lupus 15: 308-318.
- Ad Hoc (2007) Committee on Systemic Lupus Erythematosus Response Criteria for Fatigue. Measurement of fatigue in systemic lupus erythematosus: A systematic review. Arthritis Rheum 57: 1348-1357.
- Drenkard C, Bao G, Dennis G, Kan HJ, Jhingran PM (2014) Burden of Systemic Lupus Erythematosus on Employment and Work Productivity: Data From a Large Cohort in the Southeastern United States. Arthritis Care Res 66: 878-887.
- Pinto AJ, Miyake CNH, Benatti FB, Silva CA, Sallum AME, et al. (2016) Reduced Aerobic Capacity and Quality of Life in Physically Inactive Patients With Systemic Lupus Erythematosus With Mild or Inactive Disease. Arthritis Care Res 68: 1780-1786.
- Keyser RE, Rus V, Cade WT, Kalappa N, Flores RH, et al. (2003) Evidence for aerobic insufficiency in women with systemic lupus erythematosus. Arthritis Rheum 49: 16-22.
- Tench C, Bentley D, Vleck V, McCurdie I, White P, et al. (2002) Aerobic fitness, fatigue, and physical disability in systemic lupus erythematosus. J Rheumatol 29: 474-481.
- Forte S, Carlone S, Vaccaro F, Onorati F, Manfredi F, et al. (1999) Pulmonary gas exchange and exercise capacity in patients with systemic lupus erythematosus. J Rheumatol 26: 2591-2594.
- Sakauchi M, Matsumura T, Yamaoka T, Koami T, Shibata M, et al. (1995) Reduced muscle uptake of oxygen during exercise in patients with systemic lupus erythematosus. J Rheumatol 22: 1483-1487.
- Keyser RE, Rus V, Mikdashi JA, Handwerger BS (2010) Exploratory Study on Oxygen Consumption On-kinetics During Treadmill Walking in Women With Systemic Lupus Erythematosus. Arch Phys Med Rehabil 91: 1402-1409.
- Bruce RA, Kusumi F, Hosmer D (1973) Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J 85: 546-562.
- Bruce RA, Kusumi F, Niederberger M, Petersen JL (1974) Cardiovascular mechanisms of functional aerobic impairment in patients with coronary heart disease. Circulation 49: 696-702.
- Human Kinetics Inc (2020) Guidelines for pulmonary rehabilitation programs.
- Schnelle JF, Buchowski MS, Ikizler TA, Durkin DW, Beuscher L, et al. (2012) Evaluation of Two Fatigability Severity Measures in Elderly Subjects. J am Geriatr Soc 60: 1527-1533.
- Liang MH, Socher SA, Larson MG, Schur PH (1989) Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum 32: 1107-1118.
- World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 310: 2191.
- Krupp LB (1989) The Fatigue Severity Scale: Application to Patients With Multiple Sclerosis and Systemic Lupus Erythematosus. Arch Neurol 46: 1121.
- Kluwer W (2018) ACSM’s guidelines for exercise testing and prescription.
- Keyser RE (2010) Peripheral Fatigue: High-Energy Phosphates and Hydrogen Ions. PM&R 2: 347-358.
- Beaver WL, Wasserman K, Whipp BJ (9186) A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol 60: 2020-2027.
- Whipp BJ (1971) Rate constant for the kinetics of oxygen uptake during light exercise. J Appl Physiol 30: 261-263.
- Ainsworth BE (2011) Compendium of Physical Activities: A Second Update of Codes and MET Values. Med Sci Sports Exerc 43: 1575-1581.
- Mattsson M, Möller B, Lundberg IE, Gard G, Boström C (2008) Reliability and validity of the Fatigue Severity Scale in Swedish for patients with systemic lupus erythematosus. Scand J Rheumatol 37: 269-277.
- Robb-Nicholson LC, Eaton H, Daltroy L, Gall V, Wright E, et al. (1989) Effects of aerobic conditioning in lupus fatigue: A pilot study. Rheumatology 28: 500-505.
- Grassi B (2006) Oxygen uptake and kinetics. J Physiol Pharmacol 57: 53-65.
- Grassi B, Porcelli S, Salvadego D, Zoladz JA (2011) Slow VO2 kinetics during moderate-intensity exercise as markers of lower metabolic stability and lower exercise tolerance. Eur J Appl Physiol 111: 345-355.
- Poole DC, Jones AM (2012) Oxygen Uptake Kinetics. in Comprehensive Physiology (ed Terjung R) (John Wiley Sons Inc 2012).
- Principles of exercise testing and interpretation. (Lea & Febiger, 1994).
- Bassett DR, Howley ET (2000) Limiting factors for max O2 uptake and determinants of endurance performance. Med Sci Sports Exerc 32: 70-84.
- Ahn GE, Ramsey-Goldman R (2012) Fatigue in systemic lupus erythematosus. Int J Clin Rheumatol 7: 217-227.
- Dean TM, Perreault L, Mazzeo RS, Horton TJ (2003) No effect of menstrual cycle phase on lactate threshold. J Appl Physiol 95: 2537-2543.
- Lebrun C, Petit M, McKenzie D, Taunton J, Prior J (2003) Decreased maximal aerobic capacity with use of a triphasic oral contraceptive in highly active women: A randomised controlled trial. Br J Sports Med 37: 315-320.
- Bostrom C (2008) Aerobic capacity correlates to self-assessed physical function but not to overall disease activity or organ damage in women with systemic lupus erythematosus with low-to-moderate disease activity and organ damage. Lupus 17: 100-104.
Citation: Wooten LC, Keyser RE, Hasni S, Mikdashi JA (2023) Contribution of Delayed Metabolic Transition from Rest to Oxidative Steady State on Performance Fatigability in Women with Systemic Lupus Erythematosus. J Phys Med Rehabil Disabil 9: 82.
Copyright: © 2023 Liana C Wooten, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

