
COVID Causing Severe Bronchiectasis
*Corresponding Author(s):
Zohra R MalikDepartment Of Internal Medicine, Saint John’s Episcopal Hospital, New York, United States
Tel:+1 9176002733,
Email:zohrarazaq@gmail.com
Abstract
COVID-19 causing severe bronchiectasis in a previously healthy individual with no underlying lung conditions has not been reported in literature yet. We report an unusual case of bronchial dilatation in an adult as evidenced by high-resolution computed tomography caused by COVID-19 virus. The coronavirus (COVID-19), discovered in 2019, has been creating havoc since it first emerged in China and is now spreading worldwide. Its presentation is evolving, causing damage mainly to the respiratory system. Bronchiectasis is an irreversible bronchial dilatation caused by permanent chronic airway inflammation. It is increasingly being recognized with the wider availability of high-resolution computed tomography.
INTRODUCTION
There are no well-defined guidelines for diagnosis of bronchiectasis but computed tomography is one of the best known imaging modalities to diagnose it. Classical definition of bronchiectasis is permanent, irreversible, localized abnormal dilatation of bronchi that ends with fibrosis [1-6]. Bronchiectasis occurs due to repeated stress to the lungs in form of chronic inflammation with failure to clear the mucoid secretions and eventual destruction of the lung parenchyma especially the elastic fibers of the lungs [7]. One of the common causes of bronchiectasis is transmural lung infections such as pulmonary tuberculosis and pneumonia. During pneumonia, dilatations develop with destruction of segmental bronchi. It has also been associated with chronic obstructive pulmonary disease and asthma. There is high percentage of patients with no identifiable cause making the etiological diagnosis difficult and considered idiopathic. These recurrent bronchial dilatations in acute infectious illnesses can be defined as bronchiectasis.
CASE PRESENTATION
We hereby present the case of a 70 years-old male with past medical history of hypertension who presented to the emergency department with cough and myalgia for seven days. Cough was initially dry but later became productive, producing tremendous amount of yellow sputum. At presentation, patient denied chest pain, shortness of breath, fever, nasal congestion, sore throat, headaches, abdominal pain, nausea, vomiting or diarrhea. He is a retired nurse who has never smoked his entire life. He has no allergies and no recent travel. However, patient was in contact with a family member who was positive for COVID-19.
Physical examination was unremarkable. Patient was resting comfortably on 3 liters nasal cannula. Heart sounds were normal. The patient was alert and oriented to time, place and person. Cranial nerves II - XII were grossly intact, strength was 5/5 throughout the upper and lower extremities bilaterally. In the emergency department, he was afebrile, blood pressure of 154/82 mm of Hg, heart rate 80 beats/min, respiratory rate 16/minute and saturation 94%. Laboratory results at the time of presentation were: white blood cell count 3.8×103 [normal 5.2-12.4×103/uL], hemoglobin 13.6g/dl [normal 12-18g/dl], hematocrit 40.7% [normal 42%-52%] and platelet count of 135×103/u [normal 130-400×103/ uL], lactate dehydrogenase was 802 U/L [313-618 U/L], lactic acid 1.6 mmol/l [0.7-2.1 mmol/l], procalcitonin 0.51 ng/ml [normal 0.00-0.05ng/ml], ferritin 252ng/ml [normal 11.1 - 264.00ng/ml].
Blood cultures were negative. An echocardiogram showed ejection fraction of 60-65%. Electrocardiogram showed normal sinus rhythm with premature atrial complexes. Chest x-ray (Figure 1) was remarkable for bilateral opacities in the upper lobes and increased interstitial lung markings.Chest CT (Figure2) showed scattered areas of ground-glass opacities, severe bronchiectasis and fibrotic changes in the upper lobes. Sputum smear and Quantiferon test were negative for tuberculosis. COVID PCR was positive and patient was treated with tofacitinumab (Anakinra). Patient refused plasma infusion therapy. Hydroxychloroquine and azithromycin was also given to the patient. Guaifenesin and chest physiotherapy were started. Patient stayed for 2 weeks and was discharged as the symptoms got better. The patient was followed after 4 weeks. He still had the cough and sputum production but no fever. Patient refused repeat follow-up CT.
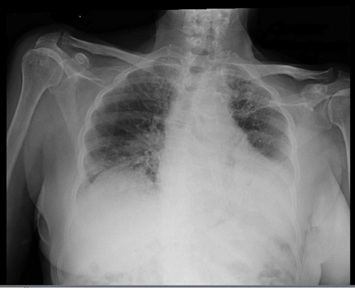 Figure 1: Chest x ray showing bilateral opacities.
Figure 1: Chest x ray showing bilateral opacities.
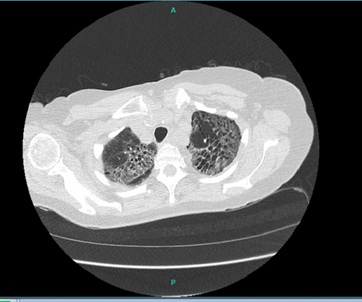 Figure 2: Computed Tomography showing bilateral bronchiectasis.
Figure 2: Computed Tomography showing bilateral bronchiectasis.
DISCUSSION
Bronchiectasis is a progressive, irreversible bronchial dilatation that has been shown to be resistant to long-term follow-up. Inflammation and infectious damage to the bronchi and bronchial walls result in a vicious cycle and ultimately resulting in bronchiectasis. The pathophysiology of bronchiectasis is limited. There is a so called “vicious cycle hypothesis” first proposed in 1986 by Cole [7]. The key components of the disease are chronic inflammation, impaired mucociliary clearance, chronic bronchial infection and structural lung damage. Chronic airways infection, most frequently with Haemophilus influenzae and Pseudomonas Aeruginosa, stimulates and sustains inflammation and is associated with a higher frequency of exacerbations, and increased mortality [8]. This is particularly the case with Pseudomonas Aeruginosa infection where chronic infection is associated with a three-fold increase in mortality and seven-fold increase in hospitalization [9]. Recognised etiologies include post-infection, COPD, Primary Ciliary Dyskinesia (PCD), Allergic Bronchopulmonary Aspergillosis (ABPA), immune deficiencies and connective tissue diseases [10]. However, despite extensive testing, up to 53% of patients may have no identifiable cause labelling it as idiopathic bronchiectasis [11].
The recent European Respiratory Society (ERS) guidelines suggest the following minimum bundle of etiological tests to perform in adults with a new diagnosis of bronchiectasis: measurement of differential blood count, immunoglobulins (IgA, IgM and IgG) and screening for ABPA (total IgE, specific IgE to Aspergillus, IgG to Aspergillus and eosinophil count). Additional tests may be appropriate in specific clinical features or in patients with severe or rapidly progressive disease. Sputum culture is recommended for monitoring bacterial infections [12]. Standardised tests are important to seek causes of underlying bronchiectasis because they lead to a change in treatment in 7-37% of cases [10,11,13,14].
Chest High-Resolution Computed Tomography (HRCT) features could be useful to detect the underlying causes. HRCT is now the accepted standard to establish the diagnosis of bronchiectasis [15]. The prerequisite is the identification of dilation of the airways, seen as an increased ratio between the internal lumen of a bronchus and its immediately adjacent pulmonary artery. The lack of normal tapering, mucus plugging, nodules, bronchial wall thickening, “tree-in-bud” pattern, lung volume loss and mosaicism pattern are all additional features useful to support a diagnosis of bronchiectasis. Furthermore, all these signs can be associated with particular distributions of bronchiectasis and can guide us to a specific cause [16]. As the ERS guidelines recommend only a small number of tests are performed routinely, it is important that clinicians know how to recognize other treatable causes and “phenotypes. Although COVID-19 affects various systems of the body, but the lungs are the primary organs affected from where it likely distributes to the other parts of the body. Various literature reviews have shown COVID causing ground glass opacities, bilateral infiltrates, but none of the case reports has shown bronchiectasis sequelae.
CONCLUSION
COVID-19 virus is an evolving virus with a wide array of presentations. Many cases of COVID-19 causing pneumonia and ARDS have been reported but COVID causing severe bronchiectasis in a previously healthy individual have not so far been documented. Therefore, the aim of this case report was to highlight the importance of COVID-19 infection causing unusual lung changes such as bronchiectasis that has so far not been reported yet.
REFERENCES
- Reid LM (1950) Reduction in bronchial subdivision in bronchiectasis. Thorax 3: 233-247.
- Morechi MA, Fiel SB (1995) An update on bronchiectasis. Curr Opin Pulm Med 1: 119-124.
- Kang EY, Miller RR, Müller NL (1995) Bronchiectasis: comparison of preoperative thin-section CT and pathologic findings in resected specimens. Radiology 195: 649-654.
- Barker AF (2002) Bronchiectasis. N Engl J Med 346: 1383-1393.
- Müller NL, Fraser RS, Lee KS, Johkoh T (2003) Diseases of the Lung: Radiologic and Pathologic Correlations, (1st edn). Lippincott Williams and Wilkins, Philadelphia, USA.
- King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW (2006) Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir Med 100: 2183-2189.
- Cole PJ (1986) Inflammation: a two-edged sword--the model of bronchiectasis. Eur J Respir Dis Suppl 147: 6-15.
- McDonnell MJ, Aliberti S, Goeminne PC, Dimakou K, Zucchetti SC, et al. (2016) Multidimensional severity assessment in bronchiectasis: an analysis of seven European cohorts. Thorax 71: 1110-1118.
- Finch S, McDonnell MJ, Abo-Leyah H, Aliberti A, Chalmers D (2015) A comprehensive analysis of the impact of Pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc 12: 1602-1611.
- Shoemark A, Ozerovitch L, Wilson R (2007) Aetiology in adult patients with bronchiectasis. Respir Med 101: 1163-1170.
- Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, et al. (2000) An investigation into causative factors in patients with bronchiectasis. Am J RespirCrit Care Med 162: 1277-1284.
- Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, et al. (2017) European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 50: 1700629.
- Anwar GA, McDonnell MJ, Worthy SA, Bourke SC, Afolabi G, et al. (2013) Phenotyping adults with non-cystic fibrosis bronchiectasis: a prospective observational cohort study. Respir Med 107: 1001-1007.
- Lonni S, Chalmers JD, Goeminne PC, McDonnell MJ, Dimakou K, et al. (2015) Etiology of non-cystic fibrosis bronchiectasis in adults and its correlation to disease severity. Ann Am Thorac Soc 12: 1764-1770.
- Pasteur MC, Bilton D, Hill AT, British Thoracic Society Bronchiectasis non-CF Guideline Group (2010) British Thoracic Society guideline for non-CF bronchiectasis. Thorax 1: 1-58.
- Cartier Y, Kavanagh PV, Johkoh T, Mason AC, Müller NL (1999) Bronchiectasis: accuracy of high-resolution CT in the differentiation of specific diseases. AJR Am J Roentgenol 173: 47-52.
Citation: Malik ZR, Razaq Z, Anwar MS, Bansod S, Umair M (2020) COVID Causing Severe Bronchiectasis. J Surg Curr Trend Innov 4: 040.
Copyright: © 2020 Zohra R Malik, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

