
Current Management of Hemangiomas
*Corresponding Author(s):
Farooq ShahzadAnn And Robert H Lurie Childrens Hospital Of Chicago, Northwestern University Feinberg School Of Medicine, Chicago, United States
Tel:+1 3122276250,
Email:fshahzad@luriechildrens.org
INTRODUCTION
Vascular anomalies are the most common pediatric congenital malformations. The management of vascular anomalies has historically been plagued with inconsistencies in terminology, which has led to incorrect diagnosis and treatment. A major step in streamlining terminology was the classification of vascular anomalies into two groups: Tumors and malformations [1]. The International Society for Study of Vascular Anomalies (ISSV) has further refined this classification over the years as our understanding of vascular malformations has improved and new entities have been described [2].
Most vascular anomalies can be diagnosed on the basis of history and exam. If diagnosis is still in question, color Doppler and ultrasound can be performed. The gold standard imaging modality is MRI, which gives information about the extent and characteristics of the lesion. If the diagnosis is still unclear, biopsy should be performed to clarify the diagnosis and exclude a malignant process. Pathologic analysis should include immunohistochemical staining for Glucose Transporter 1 (GLUT1), which is positive in infantile hemangioma and negative in vascular malformations [3].
Vascular anomalies are best managed by multidisciplinary teams. These teams consist of experts from many specialties including plastic surgery, dermatology, interventional radiology, hematology, physiatry, physical therapy, psychology and nursing. Other specialties - e.g., otolaryngology, orthopedics, urology, neurosurgery, pediatric general surgery etc., - are called upon when their services are needed. This ensures accurate diagnosis and optimal treatment plans for these challenging problems. Hemangiomas are of two types: Infantile and congenital. This review will focus on the current management of hemangiomas.
INFANTILE HEMANGIOMAS
Incidence
Clinical features
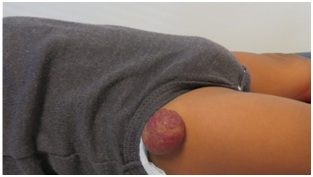 Figure 1: Untreated infantile hemangioma on right thigh in plateau phase.
Figure 1: Untreated infantile hemangioma on right thigh in plateau phase.| Clinical Appearance | Condition or Syndrome | Characteristics | Work Up |
| 5 or more hemangiomas | Diffuse neonatal hemangiomatosis [7] | Hepatic hemangiomas most common gastrointestinal tract, central nervous system and lungs less commonly involved hypothyroidism with diffuse hepatic hemangiomas |
Ultrasound liver |
| Segmental hemangiomas of the lower face and neck (beard distribution) | Upper airways or sub-glottic hemangioma [8,9] | Progressive airway obstruction manifesting clinically as stridor | Endoscopic airway examination |
| Large facial hemangiomas | PHACE(S) syndrome [10] |
Segmental facial hemangioma in trigeminal distribution, plus one other finding: |
MRI & MRA of head and neck ophthalmologic, cardiac and endocrine consults |
| Segmental lumbosacral or perineal hemangiomas |
PELVIS syndrome [11] SACRAL syndrome [12] LUMBAR syndrome [13] |
Perineal hemangioma |
Ultrasound of genitourinary system |
Management
Propranolol was introduced in 2008 for treatment of hemangiomas and is now considered the first line therapy in the proliferative phase [16]. It has a 98% response rate [17]. Propranolol is a non-selective beta-antagonist and thus affects beta-receptors in the cardiovascular system, lungs and pancreas. Patients with certain congenital cardiac conditions are not candidates for the drug. Patients require baseline EKG and monitoring of vitals when therapy is initiated. The most common adverse effect is sleep disturbances. Other rare but life-threatening side effects are bradycardia, bronchospasm, and hypoglycemia. Propranolol can cross the blood-brain barrier and therefore there are concerns about possible long-term effects. Nadolol (a non-selective beta-antagonist) and acebutolol (a selective beta-1 antagonist) do not cross the blood-brain barrier and have been shown to be effective in small non-controlled studies [18,19]. Atenolol, a selective beta-1 antagonist that does not cross the blood-brain barrier, has been shown to be as effective as propranolol but with less reactive airway adverse effects [20].Topical beta-blockers like timolol have been found to be effective in small superficial hemangiomas [21]. They may be more effective than topical steroids [22]. Topical imiquimod 5% has been found to be efficacious for superficial hemangiomas; however, timolol is more effective with less adverse effects [23].
Lasers are typically not used during the proliferative phase, as they can cause ulceration and pain. The 595 nm Pulsed Dye Laser (PDL) can improve the appearance and clearance of hemangiomas [24,25]. PDL can also be used in conjunction with topical or systemic beta-blockers, and the combination has been shown to improve the clearance of hemangiomas [26,27]. PDL has also been used with topical 5-aminolevulinic acid as a photosensitizer. This combination has been shown to be superior to PDL alone [28]. Surgical excision of IH during the proliferative phase can be performed for obstructive lesions of the visual or auditory axis, for ulcerated lesions causing pain or recurrent bleeding, or for large hemangiomas in cosmetically sensitive areas where the final deformity after involution will result in the same extent of surgery.
The most common complication during hemangioma proliferation is ulceration. This occurs most commonly in the anogenital and perianal regions. It can result in pain and bleeding, which is very distressing to the patients and the parents. The primary management of an ulcerated hemangioma is local wound care with an occlusive dressing to protect the wound from desiccation and friction. Topical lidocaine jelly can be used for pain control. Pulsed Dye Laser (PDL) therapy can promote healing of ulceration [29]. Typically, a few treatments are needed, spaced 3-4 weeks apart. Propranolol helps accelerate involution and thus promotes healing of ulceration. Therapy with both oral propranolol and prednisone can be effective for ulceration that does not respond to propranolol monotherapy [30]. Surgical excision can be performed for recalcitrant pain, recurrent bleeding or if parents are extremely anxious and want the problem to be resolved quickly.
Hemangiomas, especially of the head and neck, can result in functional impairment. Periorbital hemangiomas can cause deprivation amblyopia, astigmatism and strabismus. Preauricular hemangiomas can obstruct hearing. Large nasal hemangiomas can result in nasal obstruction. Hemangiomas of the lip can impair feeding. Large neck hemangiomas can cause torticollis. Urgent treatment is thus needed to prevent permanent dysfunction. Hemangiomas in a beard distribution can be concerning for airway involvement, and warrant airway evaluation by an otolaryngologist.
Hemangiomas can involve any organ system. Visceral hemangiomas are most commonly located in the liver. Hepatic hemangiomas are solitary, diffuse or multifocal. Solitary and diffuse hemangiomas can develop Arteriovenous (AV) shunts and cause high output cardiac failure. Pharmacotherapy with oral propranolol or prednisone is initiated. Vincristine is second line therapy and is used when steroids or beta-blockers are ineffective or contraindicated [31]. If there is worsening of heart failure, percutaneous embolization of the AV shunts can be performed [32]. Pharmacotherapy is continued till one year of age when natural involution of the hemangioma starts to occur. Multifocal and diffuse hepatic hemangiomas can cause severe hypothyroidism due to expression of type 3 deiodinase.
During the involutional phase, expectant management is usually undertaken to allow the hemangioma to naturally regress. However, surgery can be performed for an area of significant cosmetic deformity prior to school age i.e., around 4 to 5 years of age to normalize appearance and prevent teasing by peers (Figure 2) after involution, the residual superficial capillaries and telangiectasias can be treated with PDL therapy. PDL with a wavelength of 585 nm most closely matches the absorption spectrum of oxyhemoglobin, which is 577 nm. However, its depth of penetration is only 1 mm and thus it does not affect the deeper components. Redundant and damaged tissue can be surgically excised to improve appearance and contour.
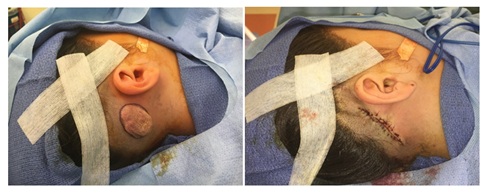 Figure 2: Intraoperative views of involuted postauricular hemangioma.
Figure 2: Intraoperative views of involuted postauricular hemangioma.Future therapies
CONGENITAL HEMANGIOMAS
Congenital hemangiomas are fully developed at birth. They are typically raised, red to purple, with superficial telangiectasias and a pale halo around them. They are GLUT-1 negative. There are 3 types of congenital hemangiomas: 1) Rapidly Involuting Congenital Hemangioma (RICH), 2) Non-Involuting Congenital Hemangioma (NICH), and 3) Partially Involuting Congenital Hemangioma (PICH). RICH undergo rapid involution after birth, which is usually completed by 14 months of age [35]. NICH (Figure 3) on the other hand do not undergo involution. PICH start as a RICH but fails to completely involute [36]. RICH typically leave behind hypoplastic tissue that can be surgically excised. NICH and PICH can also be treated surgically, once the clinical course and diagnosis are clear.
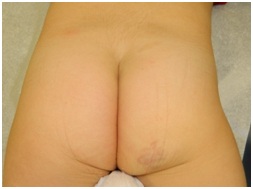 Figure 3: Untreated NICH on right buttock.
Figure 3: Untreated NICH on right buttock.
CONCLUSION
Hemangiomas are the most common pediatric vascular anomalies. They can be distinguished from other vascular anomalies by their characteristic life cycle, features on physical exam, and imaging characteristics. Biopsy is rarely performed and is usually to rule out a malignant process. Most infantile hemangiomas can be treated expectantly. Propranolol is considered the first line management in most centers for symptomatic infantile hemangiomas. Management of vascular anomalies should ideally be done in the setting of a multidisciplinary vascular anomalies team.
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
REFERENCES
- Mulliken JB, Glowacki J (1982) Hemangiomas and vascular malformations in infants and children: A classification based on endothelial characteristics. Plast Reconstr Surg 69: 412-422.
- Wassef M, Blei F, Adams D, Alomari A, Baselga E, et al. (2015) Vascular anomalies classification: Recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics 136: 203-214.
- van Vugt LJ, van der Vleuten CJM, Flucke U, Blokx WAM (2017) The utility of GLUT1 as a diagnostic marker in cutaneous vascular anomalies: A review of literature and recommendations for daily practice. Pathol Res Pract 213: 591-597.
- Munden A, Butschek R, Tom WL, Marshall JS, Poeltler DM, et al. (2014) Prospective study of infantile haemangiomas: Incidence, clinical characteristics and association with placental anomalies. Br J Dermatol 170: 907-913.
- Goelz R, Poets CF (2015) Incidence and treatment of infantile haemangioma in preterm infants. Arch Dis Child Fetal Neonatal Ed 100: 85-91.
- Bauland CG, Lüning TH, Smit JM, Zeebregts CJ, Spauwen PH (2011) Untreated hemangiomas: Growth pattern and residual lesions. Plast Reconstr Surg 127: 1643-1648.
- Glick ZR, Frieden IJ, Garzon MC, Mully TW, Drolet BA (2012) Diffuse neonatal hemangiomatosis: An evidence-based review of case reports in the literature. J Am Acad Dermatol 67: 898-903.
- Orlow SJ, Isakoff MS, Blei F (1997) Increased risk of symptomatic hemangiomas of the airway in association with cutaneous hemangiomas in a "beard" distribution. J Pediatr 131: 643-646.
- O-Lee TJ, Messner A (2008) Subglottic hemangioma. Otolaryngol Clin North Am 41: 903-911.
- Metry D, Heyer G, Hess C, Garzon M, Haggstrom A, et al. (2009) Consensus statement on diagnostic criteria for PHACE Syndrome. Pediatrics 124: 1447-1456.
- Girard C, Bigorre M, Guillot B, Bessis D (2006) PELVIS syndrome. Arch Dermatol 142: 884-888.
- Stockman A, Boralevi F, Taïeb A, Léauté-Labrèze C (2007) SACRAL syndrome: Spinal dysraphism, anogenital, cutaneous, renal and urologic anomalies, associated with an angioma of lumbosacral localization. Dermatology 214: 40-45.
- Iacobas I, Burrows PE, Frieden IJ, Liang MG, Mulliken JB, et al. (2010) LUMBAR: Association between cutaneous infantile hemangiomas of the lower body and regional congenital anomalies. J Pediatr 157: 795-801.
- Couto JA, Greene AK (2014) Management of problematic infantile hemangioma using intralesional triamcinolone: Efficacy and safety in 100 infants. J Plast Reconstr Aesthet Surg 67: 1469-1474.
- Garzon MC, Lucky AW, Hawrot A, Frieden IJ (2005) Ultrapotent topical corticosteroid treatment of hemangiomas of infancy. J Am Acad Dermatol 52: 281-286.
- Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, et al. (2008) Propranolol for severe hemangiomas of infancy. N Engl J Med 358: 2649-2651.
- Marqueling AL, Oza V, Frieden IJ, Puttgen KB (2013) Propranolol and infantile hemangiomas four years later: A systematic review. Pediatr Dermatol 30: 182-191.
- Pope E, Chakkittakandiyil A, Lara-Corrales I, Maki E, Weinstein M (2013) Expanding the therapeutic repertoire of infantile haemangiomas: Cohort-blinded study of oral nadolol compared with propranolol. Br J Dermatol 168: 222-224.
- Bigorre M, Van Kien AK, Valette H (2009) Beta-blocking agent for treatment of infantile hemangioma. Plast Reconstr Surg 123: 195-196.
- Bayart CB, Tamburro JE, Vidimos AT, Wang L, Golden AB (2017) Atenolol versus propranolol for treatment of infantile hemangiomas during the proliferative phase: A retrospective noninferiority study. Pediatr Dermatol 34: 413-421.
- Marey HM, Elmazar HF, Mandour SS, Khairy HA (2018) Combined oral and topical beta blockers for the treatment of early proliferative superficial periocular infantile capillary hemangioma. J Pediatr Ophthalmol Strabismus 55: 37-42.
- Danarti R, Ariwibowo L, Radiono S, Budiyanto A (2016) Topical timolol maleate 0.5% for infantile hemangioma: Its effectiveness compared to ultrapotent topical corticosteroids - A single-center experience of 278 cases. Dermatology 232: 566-571.
- Hu L, Huang HZ, Li X, Lin XX, Li W (2015) Open-label nonrandomized left-right comparison of imiquimod 5% ointment and timolol maleate 0.5% eye drops in the treatment of proliferating superficial infantile hemangioma. Dermatology 230: 150-155.
- Kessels JP, Hamers ET, Ostertag JU (2013) Superficial hemangioma: Pulsed dye laser versus wait-and-see. Dermat Surg 39: 414-421.
- Batta K, Goodyear HM, Moss C, Williams HC, Hiller L, et al. (2002) Randomised controlled study of early pulsed dye laser treatment of uncomplicated childhood haemangiomas: Results of a 1-year analysis. Lancet 360: 521-527.
- Reddy KK, Blei F, Brauer JA, Waner M, Anolik R, et al. (2013) Retrospective study of the treatment of infantile hemangiomas using a combination of propranolol and pulsed dye laser. Dermatol Surg. 39: 923-933.
- Park KH, Jang YH, Chung HY, Lee WJ, Kim DW, et al. (2015) Topical timolol maleate 0.5% for infantile hemangioma; it’s effectiveness and/or adjunctive pulsed dye laser - single center experience of 102 cases in Korea. J Dermatol Treat 26: 389-391.
- Zeng M, Shen S, Chen W, Yang C, Liu S (2017) Superficial hemangioma is better treated by topical 5-aminolevulinic followed by 595-nm pulsed dye laser therapy rather than 595-nm laser therapy alone. Lasers Med Sci 32: 1889-1893.
- David LR, Malek MM, Argenta LC (2003) Efficacy of pulse dye laser therapy for the treatment of ulcerated haemangiomas: A review of 78 patients. Br J Plast Surg 56: 317-327.
- Lie E, Püttgen KB (2017) Corticosteroids as an adjunct to propranolol for infantile haemangiomas complicated by recalcitrant ulceration. Br J Dermatol 176: 1064-1067.
- Wasserman JD, Mahant S, Carcao M, Perlman K, Pope E (2015) Vincristine for successful treatment of steroid-dependent infantile hemangiomas. Pediatrics 135: 1501-1505.
- Christison-Lagay ER, Burrows PE, Alomari A, Dubois J, Kozakewich HP, et al. (2007) Hepatic hemangiomas: Subtype classification and development of a clinical practice algorithm and registry. J Pediatr Surg 42: 62-67.
- Qiu MK, Wang SQ, Pan C, Wang Y, Quan ZW, et al. (2017) ROCK inhibition as a potential therapeutic target involved in apoptosis in hemangioma. Oncol Rep 37: 2987-2993.
- Li H, Teng Y, Sun J, Liu J (2017) Inhibition of hemangioma growth using polymer-lipid hybrid nanoparticles for delivery of rapamycin. Biomed Pharmacother 95: 875-884.
- Boon LM, Enjolras O, Mulliken JB (1996) Congenital hemangioma: Evidence of accelerated involution. J Pediatr 128: 329-335.
- Nasseri E, Piram M, McCuaig CC, Kokta V, Dubois J, et al. (2014) Partially involuting congenital hemangiomas: A report of 8 cases and review of the literature. J Am Acad Dermatol 70: 75-79.
Citation: Vascular anomalies are the most common congenital malformations. They are classified into two broad categories based on their endothelial characteristics: Vascular tumors and vascular malformations. Vascular tumors are characterized by endothelial proliferation. The most common vascular tumors are hemangiomas. Current management of infantile and congenital hemangiomas is presented in this review.
Copyright: © 2018 Farooq Shahzad, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

