
Cytokines:Their Relation with Mineral Dust Induced Diseases
*Corresponding Author(s):
Ilker AtesDepartment Of Toxicology, Ankara University, Tandogan, Ankara,, Turkey
Tel: +9031 22033121,
Fax:+9031 22131081
Email:iates@pharmacy.ankara.edu.tr
Abstract
Mineral dusts naturally can be generated via wind erosion or via human activities such as agricultural land use or mining. Some types of mineral dusts for instance asbestos, coal and silica can evoke several respiratory diseases. Inhalation of these dusts can lead to asbestosis, Coal Workers’ Pneumoconiosis (CWP), silicosis and lung cancer. Inflammatory cell activation, fibroblast cell proliferation and the increased synthesis and/or disruption of extracellular matrix components are the underlying facts of the fibrotic lung diseases pathogenesis. Several mediators such as cytokines, chemokines and growth factors play a major role in the onset, progression and termination of these reactions. Cytokines have major role in inflammation and immune response that are important mediators in humans related with mineral dusts exposure and toxicity. Presence of permanent stimulus and chronic release of cytokines may end in some autoimmune and inflammatory diseases such as silicosis and CWP. Cytokine genes polymorphisms have been reported to assist in the inflammatory diseases. Epidemiological studies have indicated that Single Nucleotide Polymorphisms (SNPs) arose in cytokine genes are related with chronic inflammatory or autoimmune illnesses. Due to the data of recent studies it’s obviously shown that the inflammatory cytokines TNF-? and IL-1 are related with the occurrence and development of the CWP, silicosis and asbestosis. In this article, the toxic potentials of most common mineral dusts, the relationship and the roles of cytokines and their possible genetic variations in the development of these dust-induced diseases were highlighted.
Keywords
INTRODUCTION
The surface of Earth is coated by approximately 29% land area and most of this surface area comprised of soils whose composition is changing over time by several biological, chemical and physical factors [1]. Soil is a mixture of organic and inorganic materials and thus can contain lots of mineral particles including feldspars, quartz, phyllosilicates in various crystalline forms, carbonates, sulfates, phosphates, salts, heavy minerals like pyroxenes and also special ones with regard to human health like asbestos and erionite. However, the world-wide main constituents of mineral dust are clay minerals and quartz [2].
Mineral dusts naturally can be generated via wind erosion or via human activities such as agricultural land use or mining. The naturally originated ones from the land surface is the major fragment. Atmospheric dust’s mineral particles can be of prominent size range. 500-1000 μm sized particles get dislodged from the soil surface, but the ones with a diameter less than 75 μm can get suspended in the atmosphere and follow air currents [1]. The median size of the far travelled dust is even smaller, around 2 μm [3]. Therefore soil dust also includes nanoparticles. The term nanoparticle is used for particles in the size range of 1-100nm which makes their possible reaction potential unclear and unexpected reactions may be caused [4]. The atmospheric dust loading has been enhancing over the last years depending on global warming, increasing desertification and especially human activities [5]. As a result, air pollution is a big threat for people living in mega cities. It is an important health problem causing several diseases including respiratory diseases, cardiovascular disorders, conjunctivitis and skin irritations [6].
Several minerals in the composition of atmospheric dust culminate in health problems for humans. Silicates represent the soil minerals with the highest health risks [1]. Atmospheric soil dust of crystalline silica, coal, asbestos and erionite (a fibrous sodium-rich zeolite) can induce adverse respiratory health effects. Silica, coal and asbestos have unique toxic features and almost no other mineral can be compared to them [7-9]. Other minerals like metal oxides, talc, kaolinite, smectites and mica can also give harm, but only if the exposure is for a definite time period and certain intensity [10].
Some aerosol particles with large sizes can be formed in the atmosphere under special atmospheric conditions. Pinkish mineral microspherulites defined as iberulites are spherical mineral aggregates with a large size (50-300 µm) that can be found at the highest levels of solid additions in summer. Diaz-Hernandez and Parraga (2008) collected samples of iberulites in Southern Spain [11]. Iberulites are formed and structured in the troposphere after transport of the dust from far places like Sahara Desert and composed of complex mineral associations whose phases have diverse hygroscopic properties including mainly silicates, carbonates, sulphates, halides, oxides and phosphates [12]. It is clearly seen that particles > 10 µm have attracted little attention but they can be transported over long distances directly from their sources and may play major roles in regional circulation of materials [13]. Long-range transport of giant particles have been reported in Saharan dust across the Atlantic Ocean and the Mediterranean Sea [11,14].
Common and iberulite-rich aerosols from the Sahara include mainly quartz, feldspars, carbonates and clays (illite, smectite and kaolinite). The texture of iberulites consists of large mineral particles embedded in a matrix of clay minerals also surrounding the entire spheroidal aggregate. Clays play a major role in their formation and providing a mechanical strength to the aggregates. The clay mineralogy has been addressed in several occasions and pointed out to depend on sources and sampling location [14,15]. Due to the data from the recent studies clay minerals are essential for their formation of these large mineral aggregates within water droplets in the atmosphere. They also influence the fabric and porosity of aggregates [11,16,17].
The best known minerals due to their human health effects are silica, coal, asbestos and erionite. Mineral dusts can affect humans by several ways of action. Dust particles penetrate the human body especially by inhalation or ingestion and through the skin. While some mineral dust is toxic by itself, others can carry toxic substances entering the human body together [1]. Exposure risk can be increased at places mostly related with the origin of the mineral dust. People living or working closer to dust sources are at higher risk of health problems of mineral dust. Therefore, mostly affected individuals are agriculture workers, construction workers and miners. The health risk of inhaled mineral dust depends on the exposure level the duration and the frequency of the exposure the chemical and mineralogical composition of the particle [1,5]. If the aerodynamic diameters of the inhaled mineral dusts are bigger than 10 μm, they are stuck in the upper respiratory tract where they get trapped in the mucous lining of the nasopharyngeal tract. If their aerodynamic diameters are smaller than 10 μm (PM10), they can easily penetrate more deeply into the lung passages to the tracheobronchial regions, where they also get trapped in a layer of mucus [10]. Due to WHO limits and EPA standards, particles ≤ 2.5 μm (PM2,5) are accepted as unhealthy to humans and defined as respirable dust and those particles can easily reach the alveoli region of the lung where gas-exchange is performed [18]. According to WHO (accessed 29th June, 2013) the acceptable annual mean value of PM10 is 20 μg/m3 and the acceptable 24 h mean value is 50 μg/m3 [19]. The EU (accessed 29th June 2013) has 1 year mean PM2.5 values of 25 μg/m3, 1 year PM10 mean values of 40 and 24 h mean PM10 values of 50 [20]. On Dec. 14, 2012, the US Environmental Protection Agency (EPA) strengthened the nation’s air quality standards for fine particle pollution to improve public health protection by revising the primary annual PM2.5 standard to 12 micrograms per cubic meter (μg/m3). The EPA also has a 24 h PM2.5 standard of 35 μg/m3 and a 24 h standard of 150 g/m3 for PM10 (accessed 29th June, 2013) [21]. The dust deposition adds exogenous mineral and organic materials to terrestrial surfaces, having a significant impact on ecosystems, biogeochemical cycles [22,23] and also on health [24-26].
Subject to shape, size, chemical composition, surface state of the particle, length of exposure and certain lung functions, different responses can be triggered [5,9]. Mineral dust inhalation can lead to severe diseases such as silicosis, asbestosis, coal workers’ pneumoconiosis (termed as pneumoconiosis) lung and pleura cancer [18].
Figure 1 demonsatrates the fate of inhaled mineral dust particles in the human body. Xenobiotics cause the activation of macrophages [1]. The activated macrophages ingest the particles and due to the acidic pH and digestive enzymes found in their lysosomes they can degrade and clear the particles [2]. They also release chemicals [3] to activate other macrophages [4]. Depending on their death [5], they release their contents, to recruit new macrophages [6]. This cycle of cell death and newly recruited cells in the alveoli can give rise to enhanced inflammation [27].
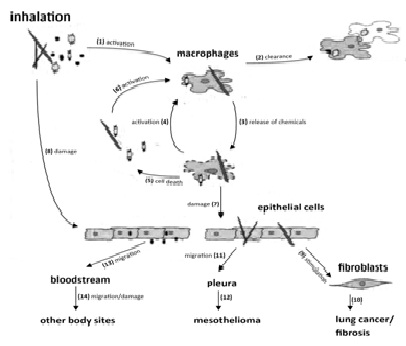
The cytokines, growth factors and Reactive Oxygen Species (ROS) released following the death of macrophages [7], can directly harm the alveoli cells. Not all of the particles in the alveoli get degraded by the macrophages or dissolved but some remain as free. While some of the remaining ones give no harm others can damage the epithelial cells [8] and stimulate fibroblastic cells relatively [9]. Fibroblastic cells can make a way for the deposition of a protein called collagen. If those processes become permanent, it may end in the development of lung cancer or fibrosis [10].
The mineral fibers can give harm to the surrounding cells and macrophages and they are even able to cause mesothelioma [12] a fatal neoplasia of pleural mesothelial cells (membrane that covers the lung) because of their possible migration to the pleura [9,11,29]. The nanoparticles are capable of migrating through the alveolar membrane to enter the interstitial lung tissue. They can abide there or furthermore migrate to the lymphatic system. Generally, most of those filtered particles in the lymph nodes stay there. Nevertheless some entered into the bloodstream via the lymph [13]. By this way, they can easily arrive other organs and tissues [14] to give harm at other regions of the body [9,29].
Silicosis, asbestosis and Coal Workers Pneumoconiosis (CWP) are the well known mineral dust induced diseases.
SILICOSIS
Silicosis, the most ancient recognized occupational disease, exclusively occurs by crystalline silica exposure [30]. At the same time, it appears frequently even in developed countries, particularly in certain occupations such as mining, sandblasting, surface drilling, stone cutting, construction, pottery making and silica flour mill operations [31]. Due to environmental silica and mixed dust exposures, lung fibrosis and pulmonary alterations have been observed in the lungs of humans and farm animals. Exposure to crystalline silica can culminate in adverse pulmonary responses such as acute, accelerated, chronic and conglomerate silicosis [32]. In addition, silica exposure can also be associated with systemic and autoimmune diseases such as Rheumatoid Arthritis (RA), Systemic Lupus Erythematosis (SLE), nephropathy, proliferative glomerulonephritis [33], tuberculosis and lung cancer [34,35].
ASBESTOSIS
Asbestos is the common industrial term covering six different natural fibrous silicates. Amosite, crocidolite, tremolite, anthophyllite and actinolite all pertain to the amphibole mineral group, chrysotile is a serpentine exceptionally. These were exploited largely for industrial processes in the past century because of their unique and versatile properties. Nowadays, asbestos is taken into account because of its potency to develop asbestosis (a debilitating and often fatal lung disease) and malignancies such as lung cancer and pleural mesothelioma, occurring many time later after exposure.
Asbestos refinement and its usage have been restricted progressively or banned by several countries. The European Union banned asbestos in 2005. On the contrary, asbestos is yet widely produced and used in developing countries [36]. Chronic inhalation of asbestos can lead to asbestosis a degenerative fibrosis of the lung, mesothelioma a cancerous tumor of the lung lining or pleural cavity or lung cancer [5]. All asbestos minerals contain iron ions, as a result the fibers can also release substances like reactive iron, triggering free-radical production. Those radicals give harm to the DNA [9,10].
COAL WORKERS’ PNEUMOCONIOSIS (CWP)
Coal Workers’ Pneumoconiosis (CWP) is an occupational lung disease characterized by fibrotic nodular lesions following inhalation of coal dusts. The severity of the disease is associated with the total dose and exposure intensity. Coal is a fossil fuel that have been mining throughout the world. The formation of coal mine dust during underground mining is the most important source of exposure. Surface mining and underground mining are the two basic types of coal mining processes. Underground miners are at higher risk of developing CWP than strip or surface miners because of the higher ambient dust levels. CWP is defined as the accumulation of the coal dust in the lungs and the tissue’s reaction to its presence [37] and divided into two stages: Simple Pneumoconiosis (SP) and Progressive Massive Fibrosis (PMF) according to the size and profusion of the lesions [38]. Cytokines play a major role in a wide spectrum of biological processes such as inflammation and immune response and are important mediators of the toxic and pathogenic effects observed in exposed individuals [39]. Inhalation of coal dust can also cause bronchitis, emphysema, caplan syndrome and silicosis [33].
For many years, it has been thought that within the coal components quartz was the active agent leading the development of CWP but due to the data of recent studies, it has not an important role in the prevalence of CWP as thought [40]. Following Heppleston’s report [40], Ghio and Quigley [41], addressed the role of iron in CWP. They indicated certain types of transition metals including iron tend to be concentrated in the lungs of miners with CWP. They suggested that humic-like substances in coal dust with iron clad-ions catalyze the oxidation generation and the accumulation of iron in tissues of CWP. The level of iron in coal is termed as Bio Available Iron (BAI) is related with the development of the disease. Several studies have been done to see whether there is a relationship between BAI in coal account for regional differences in both the prevalence and severity of CWP or not and according to their results, they found positive relationship [42-47]. Interstitial lung disease caused by silica and/or coal dust exposure is the outcome of lung cells damage and lung scarring related with fibrotic process activation. The under mentioned mechanisms have been proposed to characterize this damage and scars [31,48]; Direct cytotoxicity: Chemical features of silica or coal dust reacted with lung cells, causes damage to cell membranes following membrane lipid peroxidation. Damaged cells may release intracellular enzymes, able to provoke further tissue damage, resulting in scarring or alveolar septa destruction.
Activation of oxidant formation by alveolar macrophages: Silica or coal dust stimulates the formation of ROS from alveolar macrophages, destructing the antioxidant lung defense leading to lipid peroxidation and cell injury. This kind of injury may result in scarring or alveolar septa destruction.
Stimulation of the inflammatory cytokine and chemokine secretion from alveolar macrophages and/or alveolar epithelial cells: These inflammatory mediators act as chemoattractants in order to recruit Polymorphonuclear leukocytes (PMNs) and macrophages from pulmonary capillaries to the air gaps. These cytokines also activate pulmonary phagocytic formation of oxidant species, ending up with tissue injury and scarring.
Stimulation of fibrogenic factor secretion from alveolar macrophages and/or alveolar epithelial cells: Fibrogenic factor release leads to induction of fibroblast proliferation and/or the stimulation of collagen synthesis, resulting in fibrosis.
GENETIC FACTORS
Multifactorial diseases include complex interactions among multiple genes and environmental factors. Susceptibility attaches to both intrinsic features of the host and the influence of environmental factors [49]. Genetic factors like polymorphisms are not usually sufficient for most diseases by themselves but important for modifying the extent or severity of the disease after initiation. Counter to mutations, common allelic variants are present in high frequencies (>1%) in the general population. Among these, the most represented type of variations is single nucleotide substitutions, defined as Single Nucleotide Polymorphisms (SNPs). Even though genetic association studies assist to reveal the contribution of genetic background in disease susceptibility and severity complex interactions between genetic and environmental factors create a challenge in understanding the aetiology of complicated diseases. Environmental epidemiology using genetic information has focused primarily on investigating hypothesis-driven relations between specific polymorphisms and environmental occupational diseases such as silicosis and CWP. The pathogenesis of fibrotic lung diseases contain activation of inflammatory cells, fibroblast cell proliferation and the increased synthesis and/or breakdown of extracellular matrix components [50]. Cytokines, chemokines and growth factors play a major role in the onset, progression and termination of these reactions so that the appeared SNPs will affect all the processes of the diseases.
CYTOKINES
Cytokines are small cell-signaling protein molecules excreted from the glial cells of the nervous system and numerous cells of the immune system. They are a set of molecules used as signalling extensively in intercellular communication and can be classified into six groups: Interleukins (IL), colony-stimulating factors, interferons, Tumor Necrosis Factor (TNF), Growth Factors (GF), and chemokines. They are playing important role in a wide spectrum of biological procedures such as inflammation and immune response and they are crucial mediators of the toxic and pathogenic effects observed in human mineral dust exposure. Macrophage-derived cytokines such as TNF-α and IL-1 are involved in coal dust-induced inflammation as proinflammatory cytokines. Presence of permanent stimulus and chronic release of cytokines may result in autoimmune and inflammatory diseases such as silicosis and CWP.
As cytokines are key regulators of homeostatic processes, possible variations in their levels or their structures may be associated with the disease development [51]. Polymorphisms in cytokine genes have been demonstrated to contribute to the recognized stable inter-individual variation in the level of cytokine production rates [52-54]. Inter-individual differences in spontaneous as well as stimulated production of IL-1 and TNF-α encourage the possibility that silicosis and pneumoconiosis severity are associated with the genetic propensity of the host to produce these proteins. At the IL-1 and TNF loci, several allelic variants have been found to be significantly over-represented in inflammatory diseases. These variations affect the level of TNF-α expression in response to various stimuli. Epidemiological studies have indicated that cytokine SNPs occurring in both pro- and anti-inflammatory cytokine genes are related with chronic inflammatory or immune-mediated diseases [55-66].
We also carried out two studies in our laboratory aimed to evaluate possible association of some TNF-?, IL-1, TGF-? and IL-6 cytokines gene polymorphisms in CWP and its severity in Turkish coal workers [67,68]. According to the results we found that TNF-? (-238) variant may be a risk factor in both development and the severity of CWP, while TNF-? (-308) variant seems to be important only in disease severity. On the contrary, IL-6 variant may have a protective effect on the development and disease severity [67] and the secretion of TNF-? from the blood monocytes of the coal workers having variant allele is significantly higher than those of the controls [68]. According to the data of recent studies, it’s obviously shown that the inflammatory cytokines TNF-? and IL-1 are related with the occurrence and development of the CWP, silicosis and asbestosis [69-74].
ACKNOWLEDGMENTS
The studies done in our laboratory mentioned in the manuscript referenced with 67 and 68 are supported by Research Fund of Ankara University with a grant number of 20030803036. The author of this manuscript declares that there is no conflict of interest. The author is alone responsible for the content and writing of the paper.
REFERENCES
- Smith JL, Lee K (2003) Soil as a source of dust and implications for human health. Adv Argon 80: 1-32.
- Claquin T, Schulz M, Balkanski YJ (1999) Modelling the mineralogy of atmospheric dust sources. J Geophys Res 104: 22243-22256.
- Tegen I (2006) Effects of atmospheric dust In: Encyclopaedia of Quaternary Science. Elsevier, Netherlands.
- Nowack B, Bucheli TD (2007) Occurrence, behaviour and effects of nanoparticles in the environment. Environ Pollut 150: 5-22.
- Derbyshire E (2007) Natural minerogenic dust and human health. Ambio 36: 73-77.
- Goudie AS (2014) Desert dust and human health disorders. Environ Int 63: 101-113.
- Une H, Esaki H, Osajima K, Ikui H, Kodama K, et al. (1995) A prospective study on mortality among Japanese coal miners. Ind Health 33: 67-76.
- Peng JH, Zhu CD, Shen YL, Xu WS, Zhang TK, et al. (2003) Study on the expression of C-erbB-2 gene in coal miners with pneumoconiosis complicated by pulmonary cancer. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 21: 169-171.
- Dziegiel MH, Nielsen LK, Berkowicz A (2006) Detecting fetomaternal hemorrhage by flow cytometry. Curr Opin Hematol 13: 490-495.
- Fubini B, Fenoglio I (2007) Toxic potential of mineral dusts. Elements 3: 407-414.
- Plumlee GS, Ziegler TL (2006) “The Medical Geochemistry of Dusts, Soils, and Other Earth Materials”. US Geological Survey, Denver, CO, USA.
- Diaz-Hernandez JL, Parraga J (2008) The nature and tropospheric formation of iberulites: Pinkish mineral microspherulites. Geochimica et Cosmochimica Acta 72: 3883-3906.
- Diaz-Hernandez JL, Sanchez-Soto PJ, Serrano-Delgado A (2012) Biological nanostructures associated to iberulites: A SEM study. In: Mendez-Vilas A (ed.). Current Microscopy Contributions to Advances in Science and Technology. Formatex, Spain 1: 154-161.
- Jeong GY, Kim JY, Seo J, Kim GM, Jin HC, et al. (2014) Long-range transport of giant particles in Asian dust identified by physical, mineralogical, and meteorological analysis. Atmos Chem Phys 14: 505-521.
- Goudie AS, Middleton NJ (2001) Saharan dust storms: nature and consequences. Earth Sci Rev 56: 179-204.
- Bout-Roumazeilles V, Nebout NC, Peyron O, Cortijo E, Landais A, et al. (2007) Connection between South Mediterranean climate and North African atmospheric circulation during the last 50, 000 yr BP North Atlantic cold events. Quat Sci Rev 26: 3197-3215.
- Cuadros J, Diaz-Hernandez JL, Sanchez-Navas A, Garcia-Casco A (2015) Role of clay minerals in the formation of atmospheric aggregates of Saharan dust. Atmos Environ 120: 160-172.
- Diaz-Hernandez JL, Martin-Ramos JD, Lopez-Galindo A (2011) Quantitative analysis of mineral phases in atmospheric dust deposited in the south-eastern Iberian Peninsula. Atmos Environ 45: 3015-3024.
- Clausnitzer H, Singer MJ (1999) Mineralogy of agricultural source soil and respirable dust in California. Environ Qual 28: 1619-1629.
- http://www.who.int/mediacentre/factsheets/fs313/en/
- http://ec.europa.eu/environment/air/quality/standards.htm
- http://www.epa.gov./airquality/particlepollution/2012/decfsstandards.pdf
- Jickells TD, An ZS, Andersen KK, Baker AR, Bergametti G, et al. (2005) Global iron connections between desert dust, ocean biochemistry and climate. Science 308: 67-71.
- Lawrence CR, Neff JC (2009) The contemporary physical and chemical flux of Aeolian dust: a synthesis of direct measurements of dust deposition. Chem Geol 267: 46-63.
- Rashki A, Eriksson PG, Rautenbach CJ, Kaskaoutis DG, Grote W, et al. (2013) Assessment of chemical and mineralogical characteristics of airborne dust in the Sistan region, Iran. Chemosphere 90: 227-236.
- Larney FJ, Leys JF, Muller JF, McTainsh GH (1999) Dust and endosulfan deposition in cotton-growing area of Northern New South Wales, Australia. J Environ Qual 28: 692-701.
- Nastos PT, Paliatsos AG, Anthracopoulos MB, Roma ES, Priftis KN (2010) Outdoor particulate matter and childhood asthma admissions in Athens, Greece: a time-series study. Environ Health 9: 45.
- Hoet PHM, Geys J, Nemmar A, Nemery B (2007) Inhalation of nanomaterials: Short overview of the local and systemic effects. In: Nanotechnology - Toxicological Issues and Environmental Safety, Springer, Netherlands.
- Fubini B, Fenoglio I, Martra G, Ceschino R, Tomatis M, et al. (2006) An overview on the toxicity of inhaled nanoparticles. In: Surface Chemistry in Biomedical and Environmental Science, Springer, Netherlands.
- Fubini B, Otero Areàn C (1999) Chemical aspects of the toxicity of inhaled mineral dust. Chem Soc Rev 28: 373-381.
- Harley RA, Vallyathan V (1996) History of silicosis. In: V Castranova, V Vallyathan, WE Wallace (eds.). Silica and Silica-Induced Lung Diseases, CRC Press, USA. Pg: 7-15.
- Castranova V (1998) Particulates and the airways: basic biological mechanisms of pulmonary pathogenicity. Appl Occup Environ Hyg 13: 613-616.
- Craighead JE, Kleinerman J, Abraham JL, Gibbs AR, Gren FHY, et al. (1988) Diseases associated with exposure to silica and nonfibrous silicate minerals. Arch Pathol Lab Med 112: 673-720.
- Green FH, Vallyathan V (1996) Pathologic responses to inhaled silica. In: Castranova V, Vallyathan V, Wallace WE (eds.). Silica and Silica-induced Lung Diseases. Boca Raton, FL, CRC Press, USA. Pg no: 39-59.
- Heppleston AG (1988) Prevalence and pathogenesis of pneumoconiosis in coal workers. Environ Health Perspect 78: 159-170.
- Ghio AJ, Quigley DR (1994) Complexation of iron by humic-like substances in lung tissue: role in CWP. Am J Physiol 267: 173-179.
- Zhang Q, Huang X (2003) Induction of interleukin-6 by coal containing bioavailable iron is through both hydroxyl radical and ferryl species. J Bio Sci 28: 95-100.
- Huang X, Li W, Attfield M, Nádas A, Frenkel K, et al. (2005) Mapping and prediction of coal workers’ pneumoconiosis with bioavailable iron content in the bituminous coals. Environ Health Perspect 113: 964-968.
- Huang C, Li J, Zhang Q, Huang X (2002) Role of bioavailable iron in coal dust-induced activation of activator protein-1 and nuclear factor of activated T cells: difference between Pennsylvania and Utah coal dusts. Am J Respir Cell Mol Biol 27: 568-574.
- Zhang Q, Huang X (2002) Induction of ferritin and lipid peroxidation by coal samples with different prevalence of coal workers’ pneumoconiosis: Role of iron in the coals. Am J Ind Med 42: 171-179.
- Harrington AD, Tsirka SE, Schoonen MA (2013) Inflammatory stress response in A549 cells as a result of exposure to coal: evidence for the role of pyrite in coal workers’ pneumoconiosis pathogenesis. Chemosphere 93: 1216-1221.
- Aladdin M, Jian J, Yang Q, Chen L, Finkelmann RB, et al. (2013) Laboratory studies of the impact of calcite on in vitro and in vivo effects of coal dust: a potential preventive agent for coal workers’ pneumoconiosis? Am J Ind Med 56: 292-299.
- McDonald JC (1996) Silica and cancer. In: Castranova V, Vallyathan V, WE Wallace (eds.). Silica and Silica-induced Lung Diseases. CRC Press, USA. Pg no: 383-396.
- IARC (1997) Silica, IARC Monographs on the Evaluation of the carcinogenic risk of chemicals to humans (Vol 68). IARC, France.
- Vogel L (2005) Asbestos in the World. Special Report, Health and Safety Department Newsletters, ETUI-REHS 27. Pg no: 7-21.
- Morgan WK, Seaton A (1975) Occupational lung diseases. WB Saunders Co, USA, Pg no: 149-210.
- Lynn TT (2002) Coal workers’ lung disease and silicosis. In: Grippi MA, Elias JA, Fishman JA, Kotloff RM, Pack AI, et al. (eds.). Pulmonary Diseases and Disorders (5thedn). Mc Graw Hill, USA. Pg no: 238-249.
- Schins RPF, Borm PJA (1999) Mechanisms and mediators in coal dust induced toxicity: a review. Ann Occup Hyg 43: 7-33.
- Lapp NL, Castranova V (1993) How silicosis and coal workers' pneumoconiosis develop - a cellular assessment. Occup Med: State Art Rev 8: 35-56.
- Katsnelson BA, Polzik EV, Privalova LI (1986) Some aspects of the problem of individual predisposition to silicosis. Environ Health Perspect 68: 175-185.
- Borm PJA, Schins RPF (2001) Genotype and phenotype in susceptibility to coal workers’ pneumoconiosis. The use of cytokines in perspective. Eur Respir J 18: 127-133.
- Ollier WE (2004) Cytokine genes and disease susceptibility. Cytokine 28: 174-178.
- Pociot F, Molvig J, Wogensen L, Worsaae H, Nerup J (1992) A TaqI polymorphism in the human interleukin-1 beta (IL-1 beta) gene correlates with IL-1 beta secretion in vvitro. Eur J Clin Invest 22: 396-402.
- Danis VA, Millington M, Hyland VJ, Grenan D (1995) Cytokine production by normal monocytes: inter-subject variation and relationship to an IL-1 receptor antagonist (IL-1Ra) gene polymorphism. Clin Exp Immunol 99: 303-310.
- Perrey C, Pravica V, Sinnott PJ, Hutchinson IV (1998) Genotyping for polymorphisms in interferon-gamma, interleukin-10, transforming growth factor-beta 1 and tumour necrosis factor-alpha genes: a technical report. Transpl Immunol 6: 193-197.
- Mc Dowell TL, Symons JA, Ploski R, Førre O, Duff GW (1995) A genetic association between juvenile rheumatoid arthritis and a novel interleukin-1 alpha polymorphism. Arthritis Rheum 38: 221-228.
- Sullivan KE, Wooten C, Schmeckpeper BJ, Goldman D, Petri M (1997) A promoter polymorphism of tumor necrosis factor alpha associated with systemic lupus erythematosus in African-Americans. Arthritis Rheum 40: 2207-2211.
- Fishman D, Faulds G, Jeffery R, Mohamed-Ali V, Yudkin JS, et al. (1998) The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic onset juvenile chronic arthritis. J Clin Invest 102: 1369-1376.
- Zhai R, Jetten M, Schins RPF, Franssen H, Borm PJA (1998) Polymorphisms in the promoter of the tumor necrosis factor-alpha gene in coal miners. Am J Ind Med 34: 318-324.
- Francis SE, Camp NJ, Dewberry RM, Gunn J, Syrris P, et al. (1999) Interleukin-1 receptor antagonist gene polymorphism and coronary artery disease. Circulation 23: 861-866.
- Tountas NA, Casini-Reggi V, Jang H, Di Giovinne FS, Vecchi M, et al. (1999) Functional and ethnic association of allele 2 of the interleukin-1 receptor antagonist gene in ulcerative colitis. Gastroenterology 117: 806-813.
- Franceschi C, Valensin S, Lescai F, Olivieri F, Licastro F, et al. (2001) Neuroinflammation and the genetics of Alzheimer’s disease: The search for a pro-inflammatory phenotype. Aging (Milano) 13:163-170.
- Yucesoy B, Vallyathan V, Landsittel DP, Sharp DS, Weston A, et al. (2001) Association of tumor necrosis factor-alpha and interleukin-1 gene polymorphisms with silicosis. Toxicol Appl Pharmacol 172: 75-82.
- Kim KA, Cho YY, Cho JS, Yang KH, Lee WK, et al. (2002) Tumor necrosis factor-α gene promoter polymorphism in coal workers’ pneumoconiosis. Mol Cell Biol 234-235: 205-209.
- Ohtsuka T, Yamakage A, Yamazaki S (2002) The polymorphism of transforming growth factor-β1 gene in Japanese patients with systemic sclerosis. Br J Dermatol 147: 458-463.
- Yucesoy B, Peila R, White LR, Wu KM, Johnson VJ, et al. (2005) Association of interleukin-1 gene polymorphisms with dementia in a community-based sample: The Honolulu-Asia Aging Study. Neurobiol Aging 2: 211-217.
- Jonth AC, Silveira L, Fingerlin TE, Sato, H, Luby JC, et al. (2007) TGF-beta 1 variants in chronic beryllium disease and sarcoidosis. J Immunol 179: 4255-4262.
- Ates I, Suzen SH., Yucesoy B, Tekin, IO, Karakaya A. (2008) Association of cytokine gene polymorphisms in CWP and its severity in Turkish coal workers. Am J Ind Med 51: 741-747.
- Ates I, Yucesoy B, Yucel A, Suzen SH, Karakas A (2011) Possible effect of gene polymorphism on the release of TNFα and IL1 cytokines in coal workers' pneumoconiosis. Exp Toxicol Pathol 63: 175-179.
- Fan HM, Wang Z, Feng FM, Zhang KL, Yuan JX, et al. (2010) Association of TNF-alpha-238 G/A and 308 G/A gene polymorphisms with pulmonary tuberculosis among patients with coal worker’s pneumoconiosis. Biomed Environ Sci 23: 137-145.
- Li J, Hao JF, Yao W, Yun YX, Gao B, et al. (2011) A meta-analysis of susceptibility to pneumoconiosis and polymorphism of tumor necrosis factor-α 308 and 238 locus. Zhonghua Yu Fang Yi Xue Za Zhi 45: 547-552.
- Li Z, Xue J, Yan S, Chen P, Chen L (2013) Association between tumor necrosis factor-α 308G/A gene polymorphism and silicosis susceptibility: A meta-analysis. PLoS One 8: 76614.
- Wang YW, Lan YJ, Wang De J, Kuang J (2012) TNF-α and IL-1RA polymorphisms and silicosis susceptibility in Chinese workers exposed to silica particles: a case-control study. Biomed Environ Sci 25: 517-525.
- Liu Q, Su WZ, Shan YL, Zhang ZH, Xu G, et al. (2012) Meta-analysis of association of tumor necrosis factor alpha and transforming growth factor beta gene polymorphisms with pneumoconiosis. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 30: 587-592.
- Helmig S, Aliahmadi N, Schnedier J (2010) Tumour necrosis factor-alpha gene polymorphisms in asbestos-induced diseases. Biomarkers 15: 400-409.
Citation: ?lker Ate? (2017) Cytokines: Their Relation with Mineral Dust Induced Diseases. J Pharmacol Pharmaceut Pharmacovigil 1: 002.
Copyright: © 2017 ilker Ates, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

