
Dependent Head Posture Dizziness Syndrome: A Case Report
*Corresponding Author(s):
Saravanan ChockalingamClinical Instructor In Vestibular Rehabilitation And Clinical Mentor Orthopaedic Physical Therapy Re, Co-owner FYZICAL Therapy & Balance Centers, Waterford, Algonac, Michigan, United States
Email:institutemanualpt@yahoo.com
Abstract
Dizziness is a symptom frequently encountered in clinical settings and requires a comprehensive differential screening process. The dizziness types commonly referred to rehabilitation are peripheral and central vestibular disorders, vestibular hypofunction and cervicogenic dizziness. While cervicogenic dizziness continues to present as a diagnosis of exclusion, there is some agreement that cervical mobility is important to maintain vestibular integrity. This case report highlights the importance of cervical mobility in the maintenance of balance and equilibrium. Consequently, its lack thereof, in causing dizziness, is also described. While the relevance of cervical hypomobility to dizziness has been previously described, the possible correlation between cervical hypomobility and dependent postures of the semicircular canals is brough to light. Functional and postural compensations caused by cervical hypomobility which may in turn favor potential otoconia displacement, is described as a speculation. A case study relevant to this speculation is presented. Further research is needed to support and validate this speculation. The importance of restoring functional cervical mobility during routine vestibular rehabilitation is emphasized.
Keywords
BPPV; Cervical Spine; COR; Dizziness; Dependent postures; Hypomobility; Manual Therapy; OKR; Otoconia Dislodgment; VOR
Introduction
Dizziness is a symptom with a broad differential diagnosis. Clinicians evaluating dizziness primarily try to distinguish a benign cause from a serious one, to institute appropriate management. Multiple systems within the body can contribute to dizziness and the vestibular system has long been identified as one of the major contributors. Treatment options for the patient with dizziness originating from the vestibular system include medication, rehabilitation, surgery, and counseling [1]. Vestibular rehabilitation has gained popularity over the years and has offered benefit to individuals with central and peripheral vestibular disorders or hypo function [2]. While the inner ear and sensory organs are the primary focus of vestibular rehabilitation, the role of the cervical spine [3], especially with regards to mobility and alignment is not always stressed upon.
The literature suggests that the maintenance of steadiness, balance and equilibrium is brought about by the coordinated function of multiple systems. It has been suggested that incongruences between the vestibular, visual and neuromusculoskeletal systems are likely to be associated with dizziness and decreased postural stability [4]. The neurophysiological basis of the upper cervical region in maintaining gaze and balance via normal mobility, has been described in the literature [5]. The need for its concomitant management with regards to alignment and mobility during routine vestibular rehabilitation has also been suggested [3]. While this concept is being reviewed, the possible correlation between cervical hypomobility and dependent postures of the semicircular canals is hypothesized. Functional compensations caused by cervical hypomobility which may in turn favor potential otoconia displacement is described as a speculation. Thus, Benign Paroxysmal Positional Vertigo (BPPV) is described as a potential consequence of cervical hypomobility among the other possibilities described in literature. A case study relevant to this speculation is presented. The findings in this case study highlight the relevance to this speculation. It emphasizes the importance of addressing cervical mobility deficits as a precedent to optimize outcomes in two common types of dizziness encountered in rehabilitation settings, BPPV and cervicogenic dizziness.
Case Description
History and clinical findings
A 52-year-old male who experienced symptoms of disequilibrium followed by a bout of sudden onset dizziness when looking down and turning in bed, is presented. The disequilibrium was reported to have started gradually over a 3-month period, following a prior gradual onset of neck pain and stiffness. He reported a prior history of prolonged and excessive periods of physical activity and deskwork, followed by neck pain and stiffness and a combined episode of dehydration. He reported his symptoms to his primary care physician who also diagnosed him with associated vitamin D deficiency. He was treated with Vitamin D supplementation, lifestyle changes for hydration and physical rehabilitation including a regularly executed strengthening exercise program for neck and upper back stability, as a home program. The patient reported of being over enthusiastic with his neck strengthening exercise as well as protection by not moving his neck excessively. This had in turn led to decreased, but functional neck mobility, however in the absence of pain. He subsequently reported of gradually experiencing a feeling of disequilibrium when he performed excessive desk work and more obviously if he focused on any object with his head turned to the side. This included standing and talking to people with the head turned to the side. In addition, he reported feeling a sensation of disequilibrium when he was in crowded places and a shaky visual image if he jumped or jogged describing what may be termed oscillopsia [6]. He finally reported a spinning sensation one morning while turning in bed, like something he had experienced in the past if he had a few drinks. However, his concern grew when he looked down in the bathroom the next morning and felt the room spinning to a point where he had to sit down. The spinning sensation was brief for a few seconds and disappeared completely. He had two similar sensations that day when he lay on the ground that evening to perform abdominal oblique exercises that involved doing a sit up and turning to the side. The same evening he experienced a similar episode of dizziness when he turned his head back from the right to the left in a lying position, this time associated with a sensation of nausea. He had recently completed his annual physical and hence did not bother to contact his physician but called a friend who was a physical therapist in private practice specialized in treating dizziness. He was scheduled to come the next day for an assessment.
Testing
Testing began with subjective questions for medical causes of dizziness including metabolic syndrome and medications with dizziness as a side effect [7]. This was followed by looking for signs of Upper Motor neuron (UMN) involvement and possible signs of myelopathy. This was accomplished by assessing for hyperreflexia, Hoffman’s reflex, Babinski sign and the inverted supinator reflex [8]. There was no history of metabolic syndrome, and the medication history did not correlate to possible dizziness production. Additionally, UMN testing was negative. He did not report a history of trauma, however the integrity of the upper cervical ligaments namely alar and transverse and the vertebral artery was tested [9,10]. The testing resulted in a negative finding.
Tests for vestibular integrity
Testing proceeded to assessing the vestibular system [11], in the order of central vestibular disorders and balance testing first. The tests performed were smooth pursuit, saccade, VOR cancellation for central vestibular integrity and Romberg, sharpened Romberg, and single leg stance, for balance. The testing resulted in a negative finding. This was followed by testing the peripheral vestibular system utilizing head thrust, static and dynamic visual acuity, Dix Hallpike and the roll tests.
Tests For Central Vestibular Disorders
Smooth Pursuit and Saccade
The patient was in a seated position the patient was asked to focus on a target and the pupils were observed. Subsequently the target was moved while the focusing and moving (pursuit) pupils were observed (figure1). A smooth pursuit is a smooth eye movement that involve tracking a moving target Saccades are rapid eye jumps, bringing focus on to the object and may suggest a central vestibular challenge. There was no evidence of saccades on testing.
 Figure 1: Smooth Pursuit and Saccade.
Figure 1: Smooth Pursuit and Saccade.
VOR cancellation
In the seated position the patient was asked to raise both arms up to shoulder height with the thumbs pointed up and the patient was asked to look at the thumb nail. While the eyes maintained focus on the thumbs, the arm was made to rotate left and right, subsequently moving the eyes, head, and thumb all together (figure 2). Provocation of dizziness may suggest a cerebellar involvement. No dizziness was provoked on testing.
 Figure 2: VOR Cancellation.
Figure 2: VOR Cancellation.
Romberg Test
In the standing position the patient was asked to remove both shoes and stand with both feet together. The arms were held next to the body or crossed in front of the body. The patient was asked to first stand quietly with eyes open, and subsequently with eyes closed (figure 3). Loss of balance may suggest a proprioceptive deficit. There was no evidence of loss of balance.
 Figure 3: Romberg Test.
Figure 3: Romberg Test.
Single leg stance
The patient was made to stand unassisted on one leg, timed from the time the other foot left the ground till when the foot touched the ground again (figure 4). If unable to stand for 5 seconds or more it is considered the patient is at a greater risk of a fall. The patient was able to sustain this position for more than 5 seconds.
 Figure 4: Single leg stance.
Figure 4: Single leg stance.
Tests For Peripheral Disorders
Head thrust test
The patient’s head was tilted forward 30 degrees and the patient was asked to focus on the examiner’s nose. The head was then moved abruptly to one side and observed if the patient made a corrective saccade to achieve refixation on the target (figure 5). The direction of the head movement that caused the refixation saccade decides the involvement side. There were no saccades evident on testing.
 Figure 5: Head thrust test.
Figure 5: Head thrust test.
Static and dynamic visual acuity test
The patient was asked to read an eye chart in a static position and then he was asked to move the head side to side at a 2 Hz frequency (figure 6). More than a 3 line drop in identifying the letters and numbers with dynamic head movement suggest peripheral vestibular hypofunction. The patient exhibited a 1 line drop.
 Figure 6: Static and dynamic visual acuity test.
Figure 6: Static and dynamic visual acuity test.
Dix-Hallpike test
The patient’s head was turned 45 degrees and the patient was instructed to lie down quickly, allowing the patient’s head to be in a 30-degree extension position (figure 7a & 7b). This position was maintained for about 1 minute while the pupils were observed for nystagmus with regards to direction and duration. This test was done to identify the presence of anterior and posterior canal BPPV. The testing was negative.
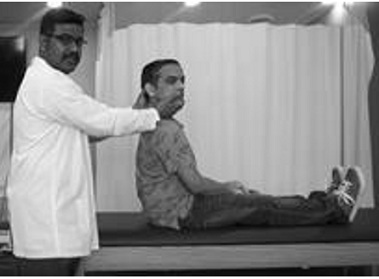 Figure 7a: Dix-Hallpike test.
Figure 7a: Dix-Hallpike test.
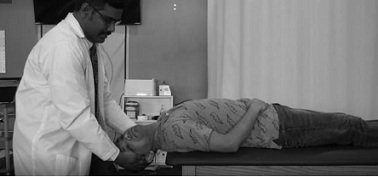 Figure 7b: Dix-Hallpike test.
Figure 7b: Dix-Hallpike test.
Roll test
This test was done to identify the presence of horizontal canal BPPV. Patient was laid supine with head flexed to 20 degrees. The head was then supported and rolled to one side. This position was maintained for about one minute while the pupils were observed for nystagmus with regards to direction and duration (figure 8). Slowly the head was moved back to midline and repeated on the other side to observe for duration and direction of nystagmus. The test was positive on the left side for a short latency of 10-15 seconds.
 Figure 8: Roll test.
Figure 8: Roll test.
All tests for the peripheral vestibular system were negative except the roll test. In supine, on cervical flexion and head rotation to the left, dizziness was provoked with a geotropic nystagmus to the left of a short latency. The test on the right side was negative. Testing concluded by assessing for a cervicogenic source [12], of dysfunction as the patient reported of disequilibrium when focusing gaze with the head turned. The tests performed were as follows.
Cervical Spine Screening For Dizziness And Disequilibrium
Test for Joint Position Error (JPE)
With the patient sitting 90 cm away from a target on the wall a head band with a laser pointer was placed on the head. The patient was asked to focus the laser on the target to get a feel of the natural resting head position. The eyes were then closed, and the head was rotated to the side and the patient was instructed and come back to the resting position (figure 9). The difference in the lengths between the starting and resting laser point positions were measured. A 7.1 cm error difference in the distance between the two laser points would translate to 4.5-degree error. More than 4.5 degree suggests impairment of relocation accuracy, also known as Joint Position Error (JPE). The patient exhibited a difference in distance of over 7.1 cm indicating joint position error.
 Figure 9: JPE testing.
Figure 9: JPE testing.
Smooth-Pursuit Neck Torsion (SPNT)
Smooth pursuit was tested in neutral and compared with neck torsion position. The patient was asked to fix his gaze on the moving pointer finger of the examiner (figure 10). Abnormal gaze pursuit in neck torsion position might suggest the examiner to consider involvement of neck immobility as cause of dizziness. Pt did not report of dizziness while the test was performed but reported that this position often caused a sensation of disequilibrium while at work.
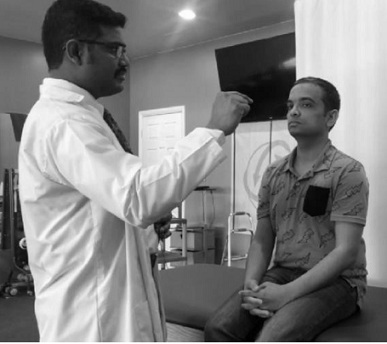 Figure 10: Smooth-Pursuit Neck Torsion (SPNT).
Figure 10: Smooth-Pursuit Neck Torsion (SPNT).
Head-Neck differentiation test
This test was done to differentiate the cause of the dizziness originating from a vestibular versus a cervicogenic source. For a cervicogenic source, the head was stabilized by the clinician and patients trunk was rotated. For a vestibular source, the head and trunk were rotated together (figure 11). Provocation of dizziness was noted. There was provocation of dizziness on combined head and trunk rotation.
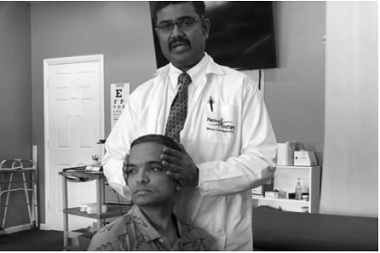 Figure 11: Head-Neck differentiation test.
Figure 11: Head-Neck differentiation test.
Cervical range of motion testing
The testing then proceeded to assessing cervical range of motion [3]. Which is typically not routinely done in a vestibular assessment. The testing proceeded to assessing cervical range of motion targeting the occiput (C0,C1) and upper cervical spine (C1,2). C0/C1 was assessed by performing occiput forward nodding (figure 12). C1C2 was assessed by performing cervical rotation with the occiput in flexion (figure 13). The normal range of occipital flexion is approximately 15-20 degrees and the normal range for upper cervical rotation is approximately 50 degrees on each side. There was obvious restriction of the C0, C1 joint in forward nodding and the C1C2 in rotation with the left side appearing more restricted than the right. This was followed by testing combined upper and lower cervical mobility. Normal range of motion at cervical spine is approximately 80-90-degrees of flexion, 70 degrees of extension, 20-45 degrees of side bending and 90 degrees of rotation. The ranges observed in the patient on testing were 60 degrees of flexion, 60 degrees of extension, 30 degrees of side bending and rotation of 60 degrees to the left and 75 degrees to the right.
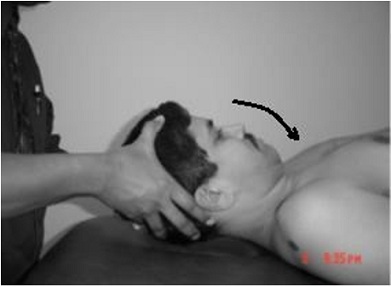 Figure 12: Assessing C0C1 mobility.
Figure 12: Assessing C0C1 mobility.
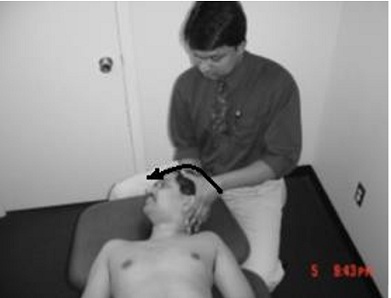 Figure 13: Assessing C1C2 mobility.
Figure 13: Assessing C1C2 mobility.
The functional index used during testing was the Dizziness Handicap Inventory (DHI) [13]. The DHI score was 20 suggesting a mild dizziness handicap. It is recommended that scores greater than 10 points be referred to a balance and vestibular specialist for further evaluation.
Evaluation, diagnosis and prognosis
Prior to seeking the services of a physical therapist, the patient had undergone a detailed medical evaluation, with no major medical concerns except for vitamin D deficiency. Given the above findings it was concluded that the patient presented with left horizontal canal BPPV and disequilibrium mediated from the cervical spine [11,12]. While the source of disequilibrium from the cervical region was obvious there was no conclusive explanation for the sudden onset of left horizontal canal BPPV. Based on the clinical findings, the patients age, activity level and general health, it was anticipated that the patient would respond well to treatment and regain normal physiological function and return to dizziness and disequilibrium free participation of his work and leisure activities.
Intervention and outcomes
The patient received 4 treatment sessions spread over 2 weeks at 2 times a week. On the first session following testing, the Barbecue maneuver was performed (figure 14 a-c) [14]. This procedure involved laying the patient supine and then having him turned around his longitudinal axis toward the affected side as in testing and waiting for the dizziness to stop. Then the patient was turned to the unaffected right side and maintained for 30 seconds. This was followed by the prone position and finally to the affected side and back to supine. Thus, a total of 360 degrees of rotation was accomplished. After every 90 degrees of rotation the patient was maintained in the new position for 30 seconds. Upon completion of the maneuver, re-testing was done with the Roll test. No apparent dizziness or nystagmus was evident.
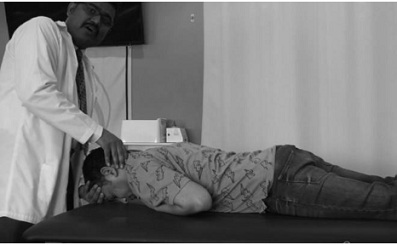 Figure 14a: Barbecue roll.
Figure 14a: Barbecue roll.
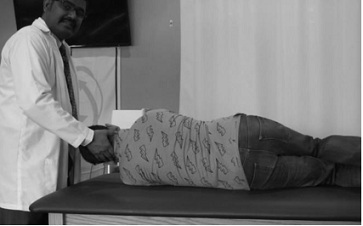 Figure 14b: Barbecue roll.
Figure 14b: Barbecue roll. 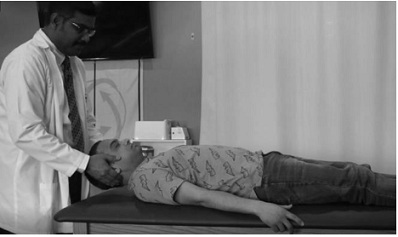
Figure 14c: Barbecue roll.
Treatment continued with manual therapy to the upper and mid cervical paraspinals and joint mobilization to improve OA forward nodding and AA rotation [3]. The first visit concluded with Brandt Daroff exercises [15], for home. This involved the patient seated in an upright position. He was then moved into a side lying position on to one side with the nose pointed up at about a 45-degree angle. He was made to remain in this position for about 30 seconds. He was then moved back to the seated position and the same maneuver was repeated on the other side (Figure 15).
 Figure 15: Brandt daroff exercise.
Figure 15: Brandt daroff exercise.
On the second visit the patient reported almost complete resolution of dizziness when looking down or turning in bed. He however continued to feel a sensation of disequilibrium, especially in wide open crowded spaces and when talking to someone with the head turned to the side. Procedures involved re-testing for horizontal canal BPPV which was completely negative. Treatment continued to address upper and mid cervical mobility with the addition of soft tissue mobilization to the upper thoracic region and exercises addressing cervical and upper thoracic stabilization [3]. In addition, he was taught gaze stabilization exercises [16], as follows. In the sitting position he was asked to put a post-it type sticker on a wall 3 feet or 1 meter away marked with a highlighted “X”. Alternately an object was held in front of the face with the arm fully extended. Throughout the exercise, he was asked to fix his gaze on the “X” mark on a wall, or the handheld object. He was then asked to rhythmically alternate head turns from the left to right and back again (figure 16). The turn from left to right was smooth and approximately 1 second in duration. This was done for approximately 1 to 2 minutes, twice a day.
 Figure 16: Gaze stabilization exercise.
Figure 16: Gaze stabilization exercise.
He was reassessed during the second week and exhibited almost full range of motion in the cervical region and a completely negative finding on the roll test. He also reported of a decreased intensity of disequilibrium, however still present. Manual mobilization and strengthening exercises were performed for the upper thoracic region [3], all previously instructed exercises were continued with a larger emphasis on gaze stabilization.
On the fourth visit in the second week, he continued to present with full range of motion in the cervical region and a completely negative finding on the roll test. He continued to have some sensation of disequilibrium, and a was fully compliant and independent with his home exercise program which included, head and cervical axial extension, flexion rotation, side bending and head rotation to the left and right with the head facing forward. This was concluded with strengthening exercises to the longus colli, multifidus and trapezius using a resistance band. During the las visit his DHI score was 8. He was discharged and advised to continue his home program with a larger emphasis on the gaze stabilization and cervical mobility. A three month follow up showed a substantial reduction in disequilibrium with negligible reminders on and off especially after extended computer work. He had resumed most of his activities with no major complaints of disequilibrium or dizziness.
Discussion
Anatomical and physiological basis of the vestibular apparatus, cervical spine, and neural networks
The main function of the cervical spine is to position the special sense organs, primarily the eyes, to perceive the world [17]. Additionally, the ability to fine tune functional activities like leaning forward to see and smell, extending the head back to yawn, position the ear to hear etc. is also accomplished by the cervical region directed by the central nervous system. Their cooperative roles in task execution by their neural networks and involuntary reflexes are well described in the literature [17-19]. This is especially true with the upper cervical spine. The connections between the cervical somatosensory system (proprioceptors in the facets) and the vestibular and visual nuclei are of primary interest. The consensus is that if the firing characteristics of the cervical somatosensory system (proprioceptors in the facets) change due to neck pain, stiffness, faulty alignment, or diminished neuromuscular coordination, a sensory mismatch can occur between vestibular and cervical inputs. The result is dizziness, unsteadiness, or dis equilibrium [16-20]. A relevant description of the anatomical and physiological basis of the vestibular apparatus, cervical spine and neural connections is as follows.
The human vestibular system is made up of the peripheral and central mechanisms. The peripheral system constitutes the semicircular canal’s and otoliths and are situated in the ear, behind the tympanum alongside the cochlea. They act as motion sensors and relay information to the central mechanism. The central mechanism constitutes the vestibular nuclei located in the pons and medulla and the cerebellum. The primary information the peripheral mechanism sends to the central mechanism is about head movement involving rotation (angular) and head movements in the upward and downward direction (linear). The central mechanism picks up these signals and deploys the appropriate team to maintain head position, spatial orientation, translated to normal balance and equilibrium. The cervical spine and cervical muscles, the eye with its ocular muscles and an intact spinal cord with the musculoskeletal system it commands with movement and reflexes constitute this team. When intact and functioning optimally, the coordinated action of these mechanisms contributes to steadiness and optimal balance. Challenges to the integrity and coordinated function of these structures may result in unsteadiness and altered balance [16-20].
The term head rotation or head movement is frequently encountered in vestibular literature. It is also described as integral to maintaining spatial orientation and balance. To reiterate, the head is not capable of mobility and the cervical spine is left to accomplish this. The head can move voluntarily as well as reflexively as seen in situations of threat or danger. So, the cervical spine must accommodate for this by musculature that are capable of fine movement, gross movement, and reflexive movement [16]. with central commands. As an example, the sternocleidomastoid (SCM) and trapezius muscle are strong head rotators and supplied by the accessory nerve, a cranial nerve with central supply. The vestibular nuclear complex [21], is the primary processor of vestibular input and needs to process direct and voluntary as well as fast and reflexive motion. The vestibular nuclear complex consists of four major nuclei (superior, medial, lateral, and descending). This large structure, located primarily within the pons, also extends caudally into the medulla. The superior and medial vestibular nuclei are relays for the VOR. The medial vestibular nucleus is also involved in VSR, and coordinates head and eye movements that occur together. The lateral vestibular nucleus is the principal nucleus for VSR. In the vestibular nuclear complex, processing of the vestibular sensory input occurs concurrently with the processing of extra-vestibular sensory information (proprioceptive, visual, tactile, and auditory). This is often referred to as sensorimotor integration.
The cerebellum [22], is considered one of three important brain areas contributing to coordination of movement in addition to the motor cortex and basal ganglia. The cerebellum monitors vestibular performance and readjusts central vestibular processing if necessary. The cerebellum receives afferent input from almost every sensory system, including the vestibular nuclear complex, consistent with its role as regulator of motor output. Although not required for vestibular reflexes, vestibular reflexes become uncalibrated and ineffective when the cerebellum is removed. Signs and symptoms of cerebellar dysfunction include muscle weakness, hypotonia, nystagmus (dancing eyes), intention tremors and ataxia.
The involuntary reflexes and relationship to cervical mobility in maintaining balance
The output of the central vestibular system goes to the ocular muscles, cervical and other skeletal muscles and spinal cord to serve various important involuntary reflexes, namely the Vestibulo-Ocular reflex (VOR), Vestibulocollic reflex (VCR) and Cervico-Ocular Reflex (COR). Their function is described below and the point to note is that the functional integrity of the cervical region is important for optimal functioning of these reflexes, Interestingly, the COR is a compensation seen in the presence of neck dysfunction, especially with stiffness, and is seen to increase when the VOR is challenged [19,23].
Vestibulo-ocular reflex (VOR)
Attempts to stabilize gaze by transforming sensory signals that direct head movements into motor commands. These in turn generate compensatory eye movements in the opposite direction of the head movement, ensuring stable vision.
Vestibulo-collic reflex (VCR)
Attempts to stabilize head position in space during movements of the trunk. The VOR and VCR operate synergistically to control gaze position and stabilize retinal images. Cervical-Ocular Reflex (COR) Stabilizes the eye in response to trunk-to- head movements People experiencing neck pain often present an altered timing in contraction of cervical muscles. This altered afferent information elicits the Cervico-Ocular Reflex (COR), which stabilizes the eye in response to trunk-to-head movements, in other words supplements the VOR.
Reflex and cervical mobility mismatch and dizziness production
The physiological importance of the above reflexes is to maintain a steady visual frame when the head is turned. These reflexes stabilize the visual frame and protect an individual from danger. This mechanism depends strongly on cervical mobility for functional integrity. What is interesting is that head and cervical turns are primarily done for this very purpose, to visually perceive the environment, assuming the visual frame is steady and not shaky (oscillopsia). To fine tune this situation, this perception head turn may be of two types, one that is slow, sustained, voluntary and planned. Contrastingly assume an otherwise non-suspecting individual hearing a loud explosion, turns rapidly and involuntarily to perceive possible imminent danger. Here the speed of the head turn activity is most certainly unplanned and rapid. Research shows the muscles performing these head vary with speed of torque, with the sub- occipital muscled working in the sustained and planned patterns and the SCM working in the rapid unplanned patterns. In other words, the sub occipital muscles especially the Obliquus Capitis Inferior (OCI) contributes to control of motion segments during rotation rather than provision of a strong rotatory torque [16]. The SCM was however more active when subjects directed their gaze to a certain direction as opposes to looking away and may be explained by the rapid reflex coordination of neck and eye muscle activity to coordinate movement of these two components [16]. It makes perfect sense that these muscles have a peripheral and central nerve supply, respectively. These varied speeds of head turns occur with the goals of visual perception while a steady visual frame is maintained by these reflexes. When there is a mismatch between these reflexes and cervical movement as in pain and hypomobility, dizziness or disequilibrium may result [16]. People experiencing neck dysfunction experience a decrease in the ability of cervical muscles to provide information to and receive information from the central nervous system. This leads to a worse joint position sense, indicating disturbed proprioception and improper afferent information from the cervical muscles sent to the vestibular nuclei. This affects the coordinated activity of the cervical, vestibular, and visual systems as a whole and are likely to be associated with dizziness and decreased postural stability [16].
The cervical musculature and afferents are not only important for controlling head movements but also involved in the cervico- ocular reflex (COR), which operates in conjunction with the Vestibulo-Ocular Reflex (VOR). While the VOR stabilizes the eye in response to vestibular input and head movement, the COR is elicited by proprioception of the cervical muscles facet joints. The strength of the COR can be modified by two factors, altered visual input and decreased cervical spine mobility. In people with neck strain, the strength of the COR is increased but there is no compensatory decline in VOR. The COR simply augments the VOR, and an increase is seen in the presence of neck hypomobility. The explanation for an increased COR in people with neck pain could be altered afferent information from the cervical spine [19]. Normally, the afferent information from the vestibular and cervical system cooperate to maintain a clear visual image during head and eye movements. Studies show that the VOR does not compensate for the increased COR in the presence of neck dysfunction. This mismatch between COR and VOR could lead to visual disturbances, dizziness, and postural control disturbances [16-20].
The dependent posture otoconia displacement theory
The displacement of otoconia into the semicircular canals causing positional dizziness has been described as Benign Paroxysmal Positional Vertigo (BPPV). While the displacement of utricular otoconia is the most widely accepted theory, the reason as to why they dislocate and displace is still being debated. Otoconia are composed of a core of glycosylated proteins and are formed as the result of complex interactions between certain proteins called otoconin 90, otopetrin, osteopontin, and several others. These proteins are critical for proper assembly and formation of otoconia, which exhibit significant degenerative changes due to age and other factors. It may be that age- related changes lead to fragmentation and degeneration of the otolithic membrane, and subsequent mobilization of sections of the otolithic membrane with attached otoconia. This in turn can lead to clinical manifestations of disease as this free-floating debris with negative buoyancy migrates into the dependent portions of semicircular canals under the influence of gravity. Thus the influence of gravity combined with vulnerable otoconia that exhibit degenerative changes and absence of interconnecting links [23], are key contributing factors to otoconia displacement. Risk factors (24), for the recurrence of BPPV included female gender, age (≥65years), hyperlipidemia, diabetes, hypertension, migraine, cervical spondylosis, osteopenia/osteoporosis, head trauma, otitis media, abnormal vestibular evoked myogenic potential, vitamin D deficiency and long use of computers. Cervical dysfunction, prolonged use of computers, head trauma and cervical whiplash area few of the risk factors that outline the path to speculating mechanical and postural causes for otoconia displacement. Head trauma and cervical whiplash are a justifiable correlation. Jerky or jolty motions in the presence of vulnerable otoconia that exhibit degenerative changes and absence of interconnecting links is a setting stage for dislodgement. Researchers suggest that this is where the cause and effect of the jerky trauma appears evident [24].
Other causes that are described in lines of mechanical reasons are vibratory stimuli (a surgical drill applied to the surface of the bony capsule), vibration from instruments used during dental procedures and loud music with nose canceling earphones both of which were able to detach the otoconia from the utricle. This is consistent with the view that mechanical insult could be a possible etiology of BPPV [25,26].
BPPV can develop in any of the three semicircular canals. The posterior canal variant is by far the most common (80-90%) because it is the most gravity-dependent part of the vestibular labyrinth. With regular head movements, free-floating endolymph debris can migrate into the posterior canal [27]. In contrast because of their orientation, the horizontal and anterior canal represent only 10% to 20% and 3% of cases, respectively [27].
Prolonged head postures and rapid whole-body head (combined head and trunk) turns
It is speculated that prolonged postures in a dependent gravity position can dislodge otoconia. Previous research suggests that gravity dependent prolonged postures can dislodge otoconia [28,29], was also used as a method of treating BPPV. Prolonged postures in front of a computer can lead to a postural dysfunction called forward head posture. Forward head posture is defined by hyperextension of the upper cervical vertebrae and forward translation of the cervical vertebrae [30]. Thoracic kyphosis is an additional feature that accompanies forward head posture, which is the combination of slouched-forward shoulders and rounded upper back. Collectively this leads to the cranium being in a relatively extended position, which is a dependent position for the posterior semicircular annals (figure 17). Thus, in the presence of prolonged forward head postures as in front of a computer, the posterior semicircular canals may be placed in a gravity dependent position of extension. This in turn can potentially increase their vulnerability to otoconia displacement. A similar predisposition has been described as a possible cause for otoconia displacement in individuals laying back on a Dentist’s chair with the head in extension [31]. In contrast the horizontal canal might be more vulnerable to rapid and jerky head turns (rotation) as opposed to head extension. Rotation arguably is the most functional movement of the head and as mentioned earlier, the purpose being to visually perceive the world around us. It makes sense that the visual frame needs to be stable while equilibrium is maintained in three- dimensional space. The vestibular reflexes and semicircular canals accomplish this amazing feat throughout life but the role of the cervical spine in being the vehicle to this important function is mostly downplayed. Cervical mobility is triplanar and complex from a mechanical perspective but this mechanical arrangement aligning with the orientation and functioning of the semicircular canals is worth discussing.
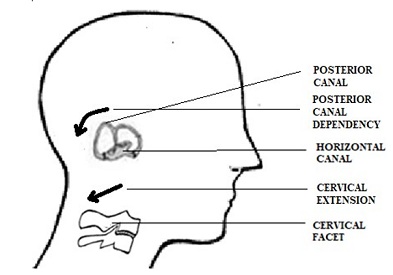 Figure 17: Extension of the cervical facet can create posterior canal dependency.
Figure 17: Extension of the cervical facet can create posterior canal dependency.
Note: Horizontal canal orientation aligns with mid cervical facet orientation.
Right head rotation causes right mid cervical downslide (extension and closing) and affects the right posterior canal. The atlas rotates right affecting the right horizontal canal (figure18). If the head looks forward and down (flexion) with right rotation (left opening), it will affect the right anterior canal. The ocular medial and lateral rectus muscles work in opposition to head rotation to stabilize gaze and synonymously with every degree of rotation in the mid cervical region there is an opposite movement of the upper cervical region to neutralize head position to bring the eyes level [32]. While there is agreement that mobility of the upper cervical spine is important for normal functioning of the reflexes that stabilize gaze providing a stable visual field, the above-described mechanics may support the speculation that functional cervical rotation also aligns with normal functioning of the semicircular canals. This may be especially important in maintaining the optimal velocity of the flow of endolymph within to appropriately stimulate the cupula which deflect the cilia to detect angular acceleration. Interestingly, researchers have noted that there is an increase in the pressure generated on the cupula with increase in rotational velocity of head [33]. They conducted harmonic analysis by importing the generated pressure of endolymph on the cupula and determining and reporting the response of cupula. The response of cupula was found to increase with increase in rotational velocity of head. Increased rotational velocity is seen during trauma as in whiplash injuries and there is evidence to support the whiplash mechanism to cause BPPV [34]. However, one may hypothesize that in the absence of adequate mobility in the cervical facets an individual may have to perform whole body or trunk rotation to position their gaze. This may occur repetitively during everyday activity potentially affecting endolymph velocity. To describe this mechanism in detail, rotation of the head is brought about by the cervical spine of which almost 50% occurs at C1C2, the atlanto-axial (AA) joint [33], the rest in the mid cervical articulations. The presence of AA or mid cervical tightness with restricted head rotation firstly challenges VOR [35,36]. This however causes disequilibrium more than BPPV [35,36]. The fact to consider is that quick motions like whole body trunk turns (as opposed to isolated cervical rotation) can occur in the presence of hypomobility of the cervical spine. This is especially possible while trying to turn reflexively as in hearing a loud noise or when honked in traffic. This rapid or jerky rotation [34], when done frequently may increase endolymph impact on the cupula and cilia secondary to increased endolymph velocity. This may in turn favor the dislodging of degenerated and vulnerable otoconia seated over the cilia being impacted (in the horizontal canal) causing horizontal BPPV [37-39].
Conclusion
The vulnerability of the patient described in this study might have been larger due to the presence of additional risk factors besides cervical hypomobility, as in possible degeneration of interconnecting links secondary to age, and vitamin D deficiency. The resolution of functional cervical mobility and mechanics in addition to vestibular rehabilitation may have improved VOR and decreased otoconia gravity dependence and vulnerability to mechanical challenges. Functional cervical mobility plays an integral role in the maintenance of balance and equilibrium. It is required for the normal relay of afferent information to the vestibular nuclei. The importance of segmental cervical mobility in maintaining ocular- cervical equilibrium has been highlighted with the need for a harmonious interplay between vestibular, visual, and neuromuscular systems. The importance of cervical mobility and alignment to prevent dislodgement of degenerating otoconia during dependent postures is outlined as a speculation. Further research is needed to support and validate this speculation. The concomitant management of cervical dysfunction alongside vestibular dysfunction and the importance of restoring functional cervical mobility during routine vestibular rehabilitation is emphasized.
References
- Luxon LM (2004) Evaluation and management of the dizzy patient. J Neurol Neurosurg Psychiatry 75: 45-52.
- Hall CD, Herdman SJ, Whitney SL, Cass SP, Clendaniel RA, et al. (2016) Vestibular Rehabilitation for Peripheral Vestibular Hypofunction: An Evidence-Based Clinical Practice Guideline: FROM THE AMERICAN PHYSICAL THERAPY ASSOCIATION NEUROLOGY SECTION. J Neurol Phys Ther 40: 124-155.
- Schenk RPT, Coons LB, Bennett SEPT, Huijbregts PA (2006) Cervicogenic dizziness. A case report illustrating orthopaedic manual andvestibularphysical therapy co-management. J Man Manip Ther 14: 56-68.
- Tjernstro F, Zur O, Jahn K (2016) Current concepts and future approaches to vestibular rehabilitation J Neurol 263: 65-70.
- Ischebeck BK, de Vries J, van Wingerden JP, Kleinrensink GJ, Frens MA, et al. (2018) The influence of cervical movement on eye stabilization reflexes: a randomized trial. Exp Brain Res 236: 297-304.
- Bonstein AM (2004) Vision and vertigo: some visual aspects of vestibular disorders. J Neurol 251: 381-387.
- Post RE, Dickerson LM (2010) Dizziness: A diagnostic approach. Am Fam Physician 82: 361-369.
- Cook C, Brown C, Isaacs R, Roman M, Davis S, et al. (2010) Clustered clinical findings for diagnosis of cervical spine myelopathy. J Man Manip Ther 18: 175-180.
- Sung YH (2020) Upper cervical spine dysfunction and dizziness J Exerc Rehabil 16: 385-391.
- Gottesman RF, Sharma P, Robinson KA, Arnan M, Tsui M, et al. (2012) Clinical Characteristics of Symptomatic Vertebral Artery Dissection: A Systematic Review. Neurologist 18: 245-254.
- Cohen HS (2019) A review on screening tests for vestibular J Neurophysiol 122: 81-92.
- Reiley AS, Vickory FM, Funderburg SE, Cesario RA, Clendaniel RA (2017) How to diagnose cervicogenic Arch Physiother 7: 1-12.
- Tamber AL, Wilhelmsen KT, Strand LI (2009) Measurement properties of the Dizziness Handicap Inventory by cross-sectional and longitudinal designs. Health Qual Life Outcomes 7: 101.
- Gold DR, Morris L, Kheradmand A, Schubert MC (2014) Repositioning maneuvers for benign paroxysmal positional vertigo. Curr Treat Options Neurol 16: 307.
- Cetin YS, Ozmen OA, Demir UL, Kasapoglu F, Basut O, et al. (2018) Comparison of the effectiveness of Brandt-Daroff Vestibular training and Epley Canalith repositioning maneuver in benign Paroxysmal positional vertigo long term result: A randomized prospective clinical trial Pak. J Med Sci 34: 558-563.
- Clendaniel RA (2010) The effects of habituation and gaze-stability exercises in the treatment of unilateral vestibular hypofunction-preliminary results. J Neurol Phys Ther 34: 111-116.
- Bexander CSM (2005) Effect of gaze direction on neck muscle activity during cervical rotation. Experimental Brain Research 167: 422-32.
- Pettorossi VE, Schieppati M (2014) Neck proprioception shapes body orientation and perception of motion. Front Hum Neurosci 4: 895.
- Brandt T, Bronstein A (2001) Cervical vertigo. J Neurol Neurosurg Psychiatry 71: 8-12.
- Montfoort I, Kelders WPA, Geest JNVD, Schippe IB, Feenstra L, et al. (2006) Interaction between ocular stabilization reflexes in patients with whiplash injury Invest Ophthalmol Vis Sci 47: 2881-4.
- Khan S, Chang R (2013) Anatomy of the vestibular system: Areview NeuroRehabilitation 32: 437-43.
- Barmack NH (2003) Central vestibular system: vestibular nuclei and posterior cerebellum. Brain Res Bull 60: 511-541.
- Britta KI, Vries DJ, Jan PWV, Gert JK, Maarten AF, et al. (2018) The influence of cervical movement on eye stabilization reflexes: a randomized trial Exp Brain Res 236: 297-304.
- Li S, Wang Z, Liu Y, Cao J, Zheng H, et al. (2022) Risk Factors for the Recurrence of Benign Paroxysmal Positional Vertigo: A Systematic Review and Meta-Analysis Ear, Nose & Throat Journal 10: 1-23.
- Dan-Goor E, Samra M (2012) Benign paroxysmal positional vertigo after use of noise-canceling headphones. Am J Otolaryngol 33: 364-6.
- Kao TK, Parnes LS, Chole RA (2017) Otoconia and Otolithic Membrane Fragments Within the Posterior Semicircular Canal in BPPV Laryngoscope 127: 709-714.
- Carlos RG, Levite R, Joffe V, Gadoth N (2004) Is Posttraumatic Benign Paroxysmal Positional vertigo different from the idiopathic form? Arch Neurol 61: 1590-3.
- You P, Instrum R, Parnes L (2019) Benign paroxysmal positional vertigo. Laryngoscope Investig Otolaryngol 4: 116-123.
- Vannucchi P, Giannoni B, Pagnini P (1997) Treatment of horizontal semicircular canal benign paroxysmal positional vertigo. J Vestib Res 7: 1-6.
- Crevits L (2004) Treatment of anterior canal benign paroxysmal positional vertigo by a prolonged forced position procedure. J Neurol Neurosurg Psychiatry 75: 779-781.
- Koseki T, Kakizaki F, Hayashi S, Nishida N, Itoh M (2019) Effect of forward head posture on thoracic shape and respiratory function. J Phys Ther Sci 31: 63-68.
- Chang TP, Lin YW, Sung PY, Chuang HY, Chung HY, et al. (2016) Benign Paroxysmal Positional Vertigo after Dental Procedures: A Population-Based Case-Control Study. PLoS One 11: 0153092.
- Shaikh AG, Wong AL, Zee DS, Jinnah HA (2013) Keeping your head on target. J Neurosci 33: 11281-11295.
- Joshi A, Babu D, Prakash AH, Chanrasekara CV (2020) Simulation of endolymph flow for response of cupula. Vibroengineering Procedia 31: 86-90.
- Li S, Wang Z, Liu Y, Cao J, Zheng H, et al. (2022) Risk Factors for the Recurrence of Benign Paroxysmal Positional Vertigo: A Systematic Review and Meta-Analysis. Ear Nose Throat J 2022 101: 112-134.
- Montfoort I, Kelders WP, van der Geest JN, Schipper IB, Feenstra L, et al. (2006) Interaction between ocular stabilization reflexes in patients with whiplash injury. Invest Ophthalmol Vis Sci 47: 2881-2884.
- Chin S (2018) Visual vertigo: Vertigo of oculomotor origin. Med Hypotheses 116: 84-95.
- Nuti D, Agus G, Barbieri MT, Passali D (1998) The management of horizontal-canal paroxysmal positional vertigo. Acta Otolaryngol 118: 455-460.
- Lee H, Kim HA (2020) The association of head shaking nystagmus with head-bending and lying-down nystagmus in horizontal canal benign paroxysmal positional vertigo. J Vestib Res 30: 95-100.
Citation: Sebastian D, Cockalingam S, Petel C (2022) Dependent Head Posture Dizziness Syndrome: A Case Report. J Phys Med Rehabil Disabil 8: 74.
Copyright: © 2022 Deepak Sebastian, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

