
Diode Laser Surgery for Early Stage Oral Cavity and Oropharyngeal Cancer - A Comparison Study with Conventional Electrosurgery
*Corresponding Author(s):
Lucas Ribeiro TenórioSanta Casa De São Paulo School Of Medical Science, Head And Neck Surgery Division, São Paulo, Brazil
Tel:+55 1121767272,
Email:tenoriolr@gmail.com
Abstract
Introduction: Any surgical resection of the oral cavity and pharynx could lead to serious impairment and compromise swallowing, chewing, breathing, and speaking. Early-stage tumors of the oral cavity and oropharynx are usually accessed by the transoral approach. The resections after using an electronic scalpel may create complex defects, with the raw area causing severe pain and bleeding in the postoperative period. Consequently, flap reconstruction (local flap or free flap) is usually indicated, which is also important to achieve better functional results. The diode laser is not a new tool for treating Head and Neck (H&N) tumors, and its usage for treating early glottic cancer is well established, but CO2 laser has been more broadly adopted worldwide.
Objective: Evaluate the hospital length of stay and postoperative complications in transoral surgical resection of oral cavity and oropharynx early-stage malignant neoplasms, comparing diode laser surgery with conventional electrosurgical resection.
Method: That study is a retrospective cohort that includes all patients with early-stage malignant neoplasm of oral cavity and oropharynx treated with transoral diode laser surgical resection compared to patients submitted to electrosurgery conventional resection between August 2019 and August 2022 in a Head and Neck Cancer Center. The evaluated outcomes were hospital length of stay, postoperative complications, enteral feeding tube, tracheostomy, and oncologic outcomes (recurrence rate and margins).
Results: The mean hospital stay was lower in the diode laser group (3.2 days vs. 4.8 days / p=0.01). Postoperative complication rates were similar in both groups. None of the patients presented local or regional recurrence during the follow-up. The mean follow-up time was 23 months.
Conclusion: The diode laser surgery is associated with a reduction in the hospital length of stay for early-stage oral cavity and oropharynx carcinomas.
Keywords
Diode laser; Head and neck cancer; Head and neck neoplasms; Laser; Laser surgery
Introduction
Any surgical resection of the oral cavity and pharynx could lead to serious impairment and compromise swallowing, chewing, breathing, and speaking. Great effort has been done to minimize collateral damage of surgery, which is a common treatment for head and neck tumors. Recently, much has been discussed about minimally invasive surgery, such as endoscopic and robotic approaches [1-9]. Overall, chemoradiation, Transoral Robotic Surgery (TORS), and Transoral Laser Surgery (TOLS), combined or not, are standards of care for pharynx and larynx cancer [10]; however, until now, there is no alternative treatment for oral cavity tumors besides conventional surgery, and chemoradiation protocols [11-13].
The diode laser is not a new tool for treating Head and Neck (H&N) tumors, and its usage for treating early glottic cancer is well established [14-19], but CO2 Laser has been more broadly adopted worldwide [20-22]. The Diode laser surgery for oral cavity and oropharyngeal cancer is part of the concept of TOLS [23,24]. This technique consists in a procedure performed through the mouth opening, to treat lesions of the upper aerodigestive tract, using a laser device, without needing to make skin incisions or mandibulotomy to access the tumor. It can be assisted by endoscopes and microscopes, but to some tumors, it can be done under direct vision [25]. It does not fit every case because the criteria for its indication depends on the patient profile, especially the mouth opening and the characteristics of the tumor, such as T stage and invasion of near structures [26,27]. Mainly, the laser works as a cutting instrument, with some important benefits such as the low marginal necrosis due to the low thermal spread, the enhancement of tissue recovery, and better aspect of the margins [28-30].
Some studies of laser resection in H&N cancer demonstrated the benefits in the management of glottic tumors or sites requiring associated procedures such as a cervical incision or mandibulotomy to access the tumor [20,22,31,32]. Early-stage tumors of the oral cavity and oropharynx are usually accessed by the transoral approach [31-35]. The resections after using an electronic scalpel may create complex defects, with the raw area causing severe pain and bleeding in the postoperative period. Consequently, flap reconstruction (local flap or free flap) is usually indicated, which is also important to achieve better functional results. Conversely, flap reconstruction may also be associated with delayed hospital discharge and postoperative complications. The role of diode laser in the surgical treatment of early-stage oral and oropharynx carcinoma is not well defined, particularly whether the diode laser can improve outcomes or impact the costs.
This study aimed to evaluate the hospital length of stay and postoperative complications in transoral surgical resection of oral cavity and oropharynx early-stage malignant neoplasms, comparing diode laser surgery with conventional electrosurgical resection.
Materials and Methods
This retrospective cohort study included all patients with early-stage malignant neoplasm of oral cavity and oropharynx treated with surgical resection between August 2019 and August 2022 in a tertiary referral Head and Neck Cancer Center.
Patients with T1 or T2 oral cavity or oropharynx malignant neoplasm (according to the AJCC 8ed.) without neck or distant metastasis and treated with surgery were included in the study. All cases lost to the follow-up were excluded. The exposure was defined as the transoral surgical resection with diode laser. The outcomes to be evaluated were: (1) hospital length of stay; (2) postoperative complications; (3) postoperative feeding tube; (4) postoperative tracheostomy; and (4) oncologic outcomes (recurrence rate and free margins). Demographic and clinical data were used as potential confounders. All the collected variables are described in table 1.
|
|
Group |
|
|
|
|
ESG (n=9) |
DLG (n=10) |
p |
|
Age (mean [SD], y) |
64y [8.1] |
52y [18] |
0.07 |
|
Hospital Length (mean [SD], d) |
4.8d [1.3] |
3.2d [0.9] |
0.01* |
|
Time with complaint (mean [SD], m) |
10m [10.6] |
8m [5.4] |
0.55 |
|
ECOG (n, %) |
|
|
|
|
0 |
9 (100%) |
10 (100%) |
|
|
Comorbidities (n, %) |
|
|
|
|
Yes |
5 (56%) |
3 (30%) |
0.26 |
|
No |
4 (44%) |
7 (70%) |
|
|
Gender (n, %) |
|
|
0.87 |
|
Female |
3 (33%) |
3 (30%) |
|
|
Male |
6 (67%) |
7 (70%) |
|
|
BMI (mean [SD]) |
26 [2.9] |
25 [4.9] |
0.57 |
|
Site (n, %) |
|
|
0.90 |
|
Oral Cavity |
7 (78%) |
8 (80%) |
|
|
Oropharyx |
2 (22%) |
2 (20%) |
|
|
Staging T - AJCC 8ed. (n, %) |
|
|
0.46 |
|
T1 |
2 (22%) |
1 (10%) |
|
|
T2 |
7 (78%) |
9 (90%) |
|
|
Reconstruction (n, %) |
|
|
0.00* |
|
Primary closure |
4 (44%) |
0 |
|
|
Flap |
5 (56%) |
0 |
|
|
No reconstruction |
0 |
10 (100%) |
|
|
Neck Dissection (n, %) |
|
|
0.27 |
|
Unilateral |
4 (45%) |
8 (80%) |
|
|
Bilateral |
3 (33%) |
1 (10%) |
|
|
None |
2 (22%) |
1 (10%) |
|
|
Overall rate of complication (n, %) |
3 (33%) |
4 (40%) |
0.76 |
|
Complication (n, %) |
|
|
0.03* |
|
None |
6 (67%) |
6 (60%) |
|
|
Yes, related with ND |
0 |
4 (40%) |
|
|
Yes, related with tumor resection |
2 (22%) |
0 |
|
|
Yes, related with both |
1 (11%) |
0 |
|
|
Post Operative Devices |
|
|
|
|
Feeding tube |
5 (56%) |
4 (40%) |
0.49 |
|
Time with feed tube (mean [SD], d) |
19d [10.9] |
11d [7] |
0.25 |
|
Trachostomy |
3 (33%) |
0 |
0.04 |
|
Recurrence rate |
0 |
0 |
— |
|
Follow-up time (mean [SD], m) |
23m [8.4] |
15m [12] |
0.13 |
Table 1: Sample characteristics.
Note: *adjusted for ND with logistic regression
**ND: Neck Dissection; ESG: Electronic Scalpel Group; SD: Standard Deviation; BMI: Body Mass Index;| DLG: Diode Laser Group
In September 2018, it was implemented an instrument based on the REDCap platform to standardly collect clinical, epidemiological, surgical, and follow-up data of all patients in order to provide a quality data repository for clinical research. The eligible patients were selected from this database and contacted by a phone call in which they were invited to participate in the study, and the consent form was applied. Demographic, clinical, surgical, and complication data were also collected from the same database. The study period was purposely chosen to include only patients registered in this database to reduce the risk of detection bias.
The follow-up data (survival and recurrence) were updated from electronic medical records and from a phone call interview to the patient or family. The REDCap platform was used to collect and manage the study data.
The patients were divided into two groups according to the device used for surgical resection of the primary tumor. The selection criteria for using each device was based on the disponibility of the laser as it was provided as a donation. In the Diode Laser Group (DLG), the patients were submitted to transoral resection with dual frequency diode laser 1470nm and 980nm (MediLaser® 980/1470, DMC Group, São Carlos, SP, Brasil), with both lasers delivered through the same optical fiber (ANVISA regulatory registration number 80030810166). The maximum output power levels of the laser equipment are 20W and 10W, respectively to 980nm and 1470nm wavelengths. The optical fiber used was 600mm silica core fiber (Introducer kit for Catheter FO 600, DMC, ANVISA register 80030810086). In the Electrosurgery Group (ESG), the tumor resections proceeded with conventional electronic scalpel (Valley Lab® - Force FX Covidien®). The electronic scalpel setup was: Pure mode, output power levels of 15 W to both coagulation and cut.
All the patients were operated by the same surgical team and with the same oncologic endpoints [36].
Statistical Analysis
The estimated sample size was 16 patients for an effect size of 50% difference between groups with alpha 0.05 and power 80%. The parameter used to calculate the sample size was the hospital length of stay (mean 4.79 days / standard deviation 1.5) of patients surgically treated with early-stage carcinoma of upper digestive tract (Stage I and II - AJCC 8ed.) in the same period of the study. That information was obtained from the REDCap database.
The numeric and continuous variables were described with central tendency measures and standard deviation. The categorical and ordinal variables were described with frequency and total count.
Univariate analyses were performed to evaluate unbalances between the groups in baseline characteristics. The normality distribution was tested with histogram, kurtosis, and skewness. The T-test was used for continuous variables, and Pearson’s chi-square was used for ordinal/nominal variables.
The relationship between the exposure (Diode Laser Surgery) and the outcomes was evaluated with multiple logistic regression. To adjust the results, the regression model included all the unbalanced variables, potential confounders, and effect modifiers.
Results
A total of 21 patients were initially selected using inclusion criteria. Two patients were excluded because they had T3 and T4 tumors after pathologic staging (AJCC 8ed). Finally, this study included 19 patients: 10 submitted to transoral Diode Laser Resection (DLG) and 9 to Electrosurgical Resection (ESG). The majority of the patients were male (13, 68%), the mean age was 58 years, the mean BMI was 25, and the mean hospital length was 4 days. All patients were ECOG 0, 42% had at least one comorbidity, and on average, patients had their main complaint for eight months. The main H&N site was the oral cavity (15, 79%), the main tumor stage was T2 (16, 84%), selective neck dissection (levels I-III for oral cavity tumors and II-IV for oropharyngeal tumors) was associated with tumor resection in most cases (16, 84%), and the mean follow-up time was 17 months. The groups were statistically comparable in these characteristics (Table 1).
Univariate analysis
All the numeric variables had normal distribution in the normality test. The baseline characteristics statistically differ only in the variable "Type of Reconstruction"(p=0.00). The univariate analysis of the outcomes found differences in "Hospital Length of Stay" (p=0.01) and postoperative complication type (p=0.03). These results are summarized in table 1.
In the DLG, none of the patients received reconstruction; the surgical bed was left raw for secondary healing. In the ESG, 4 patients (44%) had primary closure, and 5 (56%) had flap reconstruction to close the surgical defect (Table 1). The infrahyoid myocutaneous flap was used in two cases, and buccal mucosal and supraclavicular fasciocutaneous flaps were used in one case each (Table 2).
|
Gender |
Age |
Site |
Subsite |
Diagnosis |
T Stage (AJCC 8ed.) |
Neck Dissection |
Reconstruction |
Complication |
Tracheostomy |
Feeding tube |
Hospital Stay (days) |
|
Male |
67 |
Oral Cavity |
Buccal mucosa |
SCC |
T2 |
I-III unilateral |
Mucosal flap |
No |
No |
Yes |
4 |
|
Male |
71 |
Oral Cavity |
Lateral border of tongue |
SCC |
T1 |
No |
Primary closure |
No |
No |
No |
3 |
|
Female |
69 |
Oral Cavity |
Floor of mouth |
SCC |
T2 |
I-III bilateral |
Infrahyoid flap |
Dehiscence of flap |
Yes |
Yes |
6 |
|
Male |
50 |
Oral Cavity |
Ventral part of tongue |
SCC |
T2 |
I-III unilateral |
Infrahyoid flap |
Flap partial loss and pharyngocutaneous fistula |
No |
No |
5 |
|
Male |
69 |
Oropharyx |
Uvula |
SCC |
T2 |
II-IV unilateral |
Primary closure |
No |
No |
No |
5 |
|
Female |
68 |
Oropharyx |
Palatoglossal arch |
SCC |
T2 |
I-IV unilateral |
Primary closure |
Neck wound infection and pharyngocutaneous fistula |
Yes |
Yes |
5 |
|
Female |
68 |
Oral Cavity |
Retromolar area |
SCC |
T1 |
No |
Primary closure |
No |
No |
No |
3 |
|
Male |
51 |
Oral Cavity |
Floor of mouth |
SCC |
T2 |
I-III bilateral |
Supraclavicular flap |
No |
Yes |
Yes |
7 |
|
Male |
69 |
Oral Cavity |
Floor of mouth |
SCC |
T2 |
I-III bilateral |
Mucosal flap |
No |
No |
Yes |
5 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 2: Electrosugery group.
The overall rate of complication was similar in both groups (p=0.76). However, the sort of complication was different (p=0.03). The DLG had 40% of complications, and the ESG had 33%. All complications on DLG were related exclusively to the neck dissection procedure, while on the ESG, the presented complications were somehow associated with the reconstruction. The complications on the DLG were seroma, marginal mandibular nerve palsy, shoulder syndrome, and neck hematoma. On the ESG, the complications were flap dehiscence, partial loss of the flap with orocutaneous fistula, and neck wound infection with orocutaneous fistula (Tables 2 & 3).
|
Gender |
Age |
Site |
Subsite |
Diagnosis |
T Stage (AJCC 8ed.) |
Neck Dissection |
Reconstruction |
Complication |
Tracheostomy |
Feeding tube |
Hospital Stay (days) |
|
Female |
11 |
Oral Cavity |
Dorsal part of tongue |
Myxoid sarcoma |
T1 |
No |
Secondary wound healing |
No |
No |
No |
3 |
|
Male |
33 |
Oral Cavity |
Lateral border of tongue |
SCC |
T2 |
I-III unilateral |
Secondary wound healing |
No |
No |
No |
3 |
|
Male |
68 |
Oropharyx |
Palatoglossal arch |
SCC |
T2 |
II-IV unilateral |
Secondary wound healing |
No |
No |
Yes |
4 |
|
Male |
47 |
Oral Cavity |
Lateral border of tongue |
SCC |
T2 |
I-III unilateral |
Secondary wound healing |
No |
No |
Yes |
3 |
|
Male |
59 |
Oral Cavity |
Lateral border of tongue |
SCC |
T2 |
I-III unilateral |
Secondary wound healing |
No |
No |
Yes |
1 |
|
Female |
71 |
Oropharyx |
Valecula |
SCC |
T2 |
II-IV bilateral |
Secondary wound healing |
Seroma |
No |
No |
4 |
|
Male |
50 |
Oral Cavity |
Lateral border of tongue |
SCC |
T2 |
I-III unilateral |
Secondary wound healing |
Nerve palsy (marginal mandibular) |
No |
No |
4 |
|
Male |
64 |
Oral Cavity |
Lateral border of tongue |
SCC |
T2 |
I-III unilateral |
Secondary wound healing |
Shoulder sydrome |
No |
No |
3 |
|
Male |
45 |
Oral Cavity |
Lateral border of tongue |
SCC |
T2 |
I-III unilateral |
Secondary wound healing |
Neck Hematoma |
No |
No |
3 |
Table 3: Diode laser group.
Hospital Length of Stay
The mean hospital length of stay was lower on the DLG (3.2 days vs. 4.8 days / p=0.01). We considered the variables "Neck Dissection" and "Type of Reconstruction" as potential confounders and included them in the analysis to adjust the results (Figure 1).
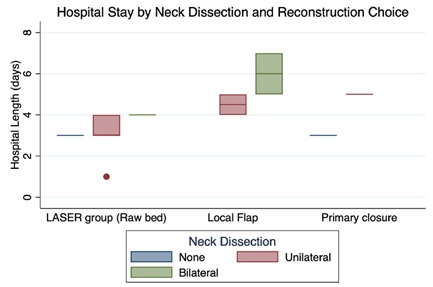 Figure 1: Hospital length of stay.
Figure 1: Hospital length of stay.
Note: *All the patients of Diode LASER group had the surgical bed left raw after tumor resection (No resection) and this only occurred in LASER group.
All patients on the DLG had the surgical bed left raw after tumor resection. This option was present only in this group; hence no reconstruction was considered equivalent to DLG in the regression model. Bilateral neck dissection was an isolated variable associated with an increased hospital stay in both groups (p=0.02). Graph 1 demonstrates these results. The adjusted r-squared of this model was 0.54, and the p was 0.005 (Table 4).
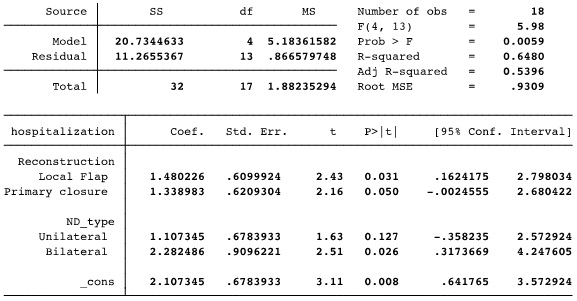 Table 4: Multiple linear regression model to evaluate the Hospital Length adjusted to “Reconstruction type” and “Neck Dissection (ND)”. The coefficients referred to the suppressed factors: “No reconstruction” in the Reconstruction variable and “without ND” in the ND variable. All the patients in the LASER group had surgical bed left raw -No reconstruction, which occurred only in the LASER group; therefore, “No reconstruction” was considered equivalent to the LASER group.
Table 4: Multiple linear regression model to evaluate the Hospital Length adjusted to “Reconstruction type” and “Neck Dissection (ND)”. The coefficients referred to the suppressed factors: “No reconstruction” in the Reconstruction variable and “without ND” in the ND variable. All the patients in the LASER group had surgical bed left raw -No reconstruction, which occurred only in the LASER group; therefore, “No reconstruction” was considered equivalent to the LASER group.
Post-Operative Devices and Recurrence Rate
Tracheostomy was required in 3(33%) patients of the ESG and none of the DLG (p=0.04). A nasoenteric feeding tube was used in 5(56%) patients in the ESG and 4(40%) patients in the DLG (p=0.49), and the average time with that device was 19 days in the ESG and 11 days in the DLG (p=0.25).
All patients had free margins and were N0 on the final pathology report. None patients presented local or regional recurrence during the follow-up. The mean follow-up time was 23 months in the ESG and 15 months in the DLG (p=0.13).
Discussion
Despite the well-described usage of the CO2 LASER for treating larynx and hypopharynx cancer in the last thirty years [20,22,32,33,35], little has been seen about LASER applicability for treating oral cavity and early-stage oropharyngeal tumors. Some studies have evaluated its effects on margins, especially regarding the extent of necrosis area and protein degradation [30,37], and others also compared the inflammatory reaction caused by the different cutting instruments used in the oral mucosa [3]. In this study, we evaluated clinical outcomes, showing the benefit of diode laser surgery on routine cases in a tertiary referral Head and Neck Cancer Center from a single hospital, and compared it with patients managed by electrosurgery conventional resection. Oral cavity and oropharyngeal tumor resections are usually followed by reconstruction. However, when using diode laser we can avoid flap reconstruction, leaving a raw area. Previous studies have shown that laser surgery presents a faster healing process, addressing less intense necrosis and inflammatory reaction [28-30,37].
Although usually confounded as a simple cutting instrument, the laser technology works differently from the electronic scalpel or any other device that uses the thermoelectric effect to achieve tissue incision and coagulation. The word laser is an acronym for "light amplification by stimulated emission of radiation” which means that this technology works using light radiation to make its effect on the tissues [24]. The interaction between the laser and the tissue is referred to as photo-thermal, and it varies according to the laser wavelength [38,39]. In turn, the wavelength determines how light is absorbed by objects [38,39]. The laser light energy is absorbed and transformed into heat inside the tissue leading to elevated temperatures that result in tissue ablation and coagulation [38,39]. Due to its different effects on the tissue, laser surgery is usually associated with many benefits such as more precision, reduced healing time, bleeding, swelling, and scarring [24,28,29,37]. Lasers of different wavelengths produce different effects on tissue, so not all lasers are efficient at cutting and coagulating. The carbon-dioxide laser was more adopted worldwide as it is referred to as an efficient tool to cut and coagulate, and the diode laser, in turn, was always described as a single wavelength device, only effective for coagulation [14,40-42]. However, in this study, we used a dual diode laser which has two different wavelengths working at the same time, delivered through the same optical fiber: 980nm, which is more absorbed by hemoglobin, and 1470nm, which is more absorbed by water. The surgeon can set up the potency of both wavelengths and even deactivate one of them according to the need of each case. Such characteristics make this laser device more effective and may be related to better outcomes. The CO2 laser has some limitations, such as increased cost, difficulty managing deep and curved areas, and short penetration in tissues [14,40]. In our experience, some benefits of the diode laser are the precision, once the surgeon uses a hand piece during the surgery; the usage of the device is intuitive because the handling is similar to an electronic scalpel; the easy displacement of the device since its generator is light and mobile; and the availability, because it is more common in our country.
The main contribution of this study is to show that using the dual wave-length diode laser for resecting oral cavity and oropharynx cancer probably dismisses the need for reconstruction, which is associated with better outcomes such as fewer complications, shorter hospital stay, and less usage of tracheostomy. One unobservant surgeon could fear having more complications leaving the raw area to heal by itself, but in fact we did not observe higher rates of complications in patients submitted to laser resection even though there was no kind of suture or reconstruction. Bleeding was not observed in any of these patients. The necessity of a feeding tube was less frequent (40% vs 56%, p=0,49) and for a shorter period (11 days vs 19 days, not significant) on patients submitted to LASER resection. Moreover, leaving the raw area open was not associated with pain or dysphagia as this would result in a longer period of a feeding tube. Although not addressed in this article, maintaining the feeding tube for a longer period may cause a negative impact on quality of life, especially if the patient uses a nasoenteral catheter [43,44].
In our Hospital, dismissing any kind of reconstruction after oral and oropharyngeal tumor resection was a paradigm shift, as it was a consensus that reconstruction was always needed in order to achieve excellent outcomes. The reconstructive step may vary according to the raw area left after resection, but it is technically complex and requires a great team effort, usually with the collaboration of multiple surgeons of different specialties [45,46]. Some disadvantages of reconstruction should be pointed out, such as voluminous and bulky flaps that commonly require multiple sequential procedures to reach their final aspect. When surgical reconstruction is needed, a longer operative time is expected, especially if a free flap is performed [47,48]. Moreover, most of them will need an ICU bed and a longer period with drains [49,50]. Sometimes we try to fit huge flaps in small defects in the oral cavity or in the oropharynx, making tracheostomy mandatory. Tongue fixation may occur in order to accommodate the flap on the surgical bed. In addition, reconstruction is associated with an increased risk of general surgical complications such as hypothermia, bleeding, thrombosis, and infections (surgical site, pneumonia, and urinary tract infection). Besides that, complications in the donor site, such as wound infection, dehiscence, and worse cosmetic outcomes, could happen [51,52].
All the complications on the ESG were directly related to the reconstruction procedure, and the great majority were associated with flaps. Patients who underwent any kind of reconstruction, including primary closure, had worse outcomes, with more complications such as dehiscence, wound infection, and fistula. Patients with this sort of complication obviate specialized care with daily dressings and antibiotics. Among the patients of DLG, no reconstruction was made, which may explain the reduced length of stay (3.2 days vs. 4.9 days; p=0.01). No patients require tracheostomy in the DLG, probably due to a lack of flap reconstruction and less edema. Using flap reconstruction for these tumors elevates the need for a tracheostomy to eliminate the risk of respiratory distress.
All the included patients had tumors in anatomic sites, which can be accessed transorally, so the usage or not of the diode laser did not change the surgical approach. In conventional TOLS the main advantage is to avoid mandibulectomy or other access that can aggregate morbidity.
This was a retrospective study that may include detection and selection bias. However, many efforts were made to reduce their impact; all the collected data were obtained exclusively from a databank in the REDCap platform implemented with the purpose of collecting quality clinical data for research; the statistical analysis was performed with multiple regression to include the potential confounders; the use of diode laser to proceed the resection was not a choice of the surgeon, our Hospital did not have this device available free, all the cases were done by donation, so the selection of patients that were submitted to resection with laser depended of the availability and when it was absent the patients were operated with conventional electronic scalpel. Despite not having robust power to prove causality between the usage of diode laser and improvement in surgical outcomes, the results of this study may be valuable to further clinical trials on that topic.
In this study, the diode laser surgery was associated with an improvement in surgical outcomes, such as length of hospital stay and surgical complications, probably because it allowed the surgical bed to remain raw, dismissing the reconstruction step. At the same time, it delivered the same rates of oncologic outcomes as the conventional electrosurgical approach.
Conclusion
The diode laser surgery is associated with a reduction in the hospital length of stay in transoral surgery for early-stage oral cavity and oropharyngeal carcinomas compared with conventional electronic scalpel surgery.
Declarations
Ethical Approval
This study has IRB approval from Santa Casa de São Paulo School of Medical Sciences (Number: 61705622700005479) in accordance with the ethical standards of the committee on human experimentation of the Helsinki Declaration of 1975 (revised in 1983). All patients enrolled in this study gave written informed consent to participate in this study.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflict of Interest
Prof. Dr. Marianne Nakai is a speaker for DMC Medical and Physiomed. Dr. Antonio Bertelli is a speaker for Johnson&Johnson and Cook Medical.
The other authors declare no conflict of interest.
Acknowledgment
We thank DMC for the donation of the disposable optical fibers and Medilaser Dual lending and for Instituto Nupen, São Carlos-SP, Brazil for preparatory laser lecturers and training
Availability of Data and Materials
All data were compiled using REDCap database where the study protocols were stored as well. In acordance to data protection policy these informations are not freely available, needing formal request to Santa Casa De São Paulo’s REDCap administrator to have access.
References
- Richmon JD, Pattani KM, Benhidjeb T, Tufano RP (2011) Transoral robotic-assisted thyroidectomy: A preclinical feasibility study in 2 cadavers. Head Neck 33: 330-333.
- Finegersh A, Holsinger FC, Gross ND, Orosco RK (2019) Robotic Head and Neck Surgery. Surg Oncol Clin N Am 28: 115-128.
- Hamilton D, Paleri V (2017) Role of transoral robotic surgery in current head & neck practice. The Surgeon 15: 147-154.
- Nakayama M, Holsinger FC, Chevalier D, Orosco RK (2019) The dawn of robotic surgery in otolaryngology-head and neck surgery. Jpn J Clin Oncol 49: 404-411.
- Holsinger FC, Ferris RL (2015) Transoral endoscopic head and neck surgery and its role within the multidisciplinary treatment paradigm of oropharynx cancer: Robotics, lasers, and clinical trials. J Clin Oncol 33: 3285-3292.
- Weinstein GS, O’Malley BW, Magnuson JS, Carroll WR, Olsen KD, et al. (2012) Transoral robotic surgery: A multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope 122: 1701-1707.
- Kim WS, Lee HS, Kang SM, Hong HJ, Koh YW, et al. (2012) Feasibility of robot-assisted neck dissections via a Transaxillary and Retroauricular (“TARA”) approach in head and neck cancer: Preliminary results. Ann Surg Oncol 19: 1009-1017.
- Nota CLMA, Smits FJ, Woo Y, Rinkes IHMB, Molenaar IQ, et al. (2019) Robotic Developments in Cancer Surgery. Surg Oncol Clin N Am 28: 89-100.
- Howard J, Dwivedi RC, Masterson L, Kothari P, Quon H, et al. (2018) De-intensified adjuvant (chemo) radiotherapy versus standard adjuvant chemoradiotherapy post transoral minimally invasive surgery for resectable HPV-positive oropharyngeal carcinoma. Cochrane Database Sys Rev 12:
- Tolman DE (1984) Oral cancer: The diagnosis, therapy, management and rehabilitation of the oral cancer patient. Mayo Clin Proc 59: 729-731.
- Shibahara T (2017) Oral cancer -diagnosis and therapy. Clin Calcium 27: 1427-1433.
- Casiglia J, Woo SB (2001) A comprehensive review of oral cancer. Gen Dent 49: 72-82.
- Arroyo H, Neri L, Fussuma C, Imamura R (2016) Diode laser for laryngeal surgery: A systematic review. Int Arch Otorhinolaryngol 20: 172-179.
- Karkos PD, Koskinas I, Stavrakas M, Triaridis S, Constantinidis J (2021) Diode Laser for Laryngeal Cancer: “980 nm” and Beyond the Classic CO2. Ear Nose Throat J 100: 19S-23S.
- Sencan Z, Cömert E, Tunçel Ü, Kiliç C (2020) Voice and quality-of-life outcomes of diode laser for Tis-T1a glottic cancer. Ear Nose Throat J 99: 229-234.
- Cömert E, Tunçel Ü, Dizman A, Güney YY (2014) Comparison of early oncological results of diode laser surgery with radiotherapy for early glottic carcinoma. Otolaryngol Head Neck Surg 150: 818-823.
- Tunçel U, Cömert E (2013) Preliminary results of diode laser surgery for early glottic cancer. Otolaryngol Head Neck Surg 149: 445-450.
- Ferri E, Armato E (2008) Diode laser microsurgery for treatment of Tis and T1 glottic carcinomas. Am J Otolaryngol 29: 101-105.
- Steiner W (1993) Results of curative laser microsurgery of laryngeal carcinomas. Am J Otolaryngol 14: 116-121.
- Harris A, Tanyi A, Hart R, Trites J, Rigby M, et al. (2018) Transoral laser surgery for laryngeal carcinoma: Has Steiner achieved a genuine paradigm shift in oncological surgery? Ann R Coll Surg Engl 100: 2-5.
- Steiner W (1994) Therapy of hypopharyngeal carcinoma. Part V: Discussion of long-term results of transoral laser microsurgery of hypopharyngeal carcinoma. HNO 42: 157-165.
- Kutter J, Lang F, Monnier P, Pasche P (2007) Transoral laser surgery for pharyngeal and pharyngolaryngeal carcinomas. Arch Otolaryngol Head Neck Surg 133: 139-144.
- Werner JA, Dünne AA, Folz BJ, Lippert BM (2002) Transoral laser microsurgery in carcinomas of the oral cavity, pharynx, and larynx. Cancer Control 9: 379-386.
- Remacle M, Arens C, Eldin MB, Campos G, Estomba CC, et al. (2017) Laser-assisted surgery of the upper aero-digestive tract: A clarification of nomenclature. A consensus statement of the European Laryngological Society. Eur Arch Otorhinolaryngol 274: 3723-3727.
- Cabanillas R, Rodrigo JP, Llorente JL, Suárez C (2008) Oncologic outcomes of transoral laser surgery of supraglottic carcinoma compared with a transcervical approach. Head Neck 30: 750-755.
- Piazza C, Mangili S, Bon FD, Paderno A, Grazioli P, et al. (2014) Preoperative clinical predictors of difficult laryngeal exposure for microlaryngoscopy: The laryngoscore. Laryngoscope 124: 2561-2567.
- Roodenburg JLN, Witjes MJH, de Veld DCG, Tan IB, Nauta JM (2002) Lasers in dentistry 8. Use of lasers in oral and maxillofacial surgery. Ned Tijdschr Tandheelkd 109: 470-474.
- Burkey MD BB, Garrett G (1996) Use of the laser in the oral cavity. Otolaryngol Clin North Am 29: 949-961.
- Jerjes W, Hamdoon Z, Hopper C (2012) CO2 lasers in the management of potentially malignant and malignant oral disorders. Head Neck Oncol 4: 17.
- Shah JP, Gil Z (2009) Current concepts in management of oral cancer - surgery. Oral Oncol 45: 394-401.
- Ambrosch P, Kron M, Steiner W (1998) Carbon dioxide laser microsurgery for early supraglottic carcinoma. Ann Otol Rhinol Laryngol 107: 680-688.
- Meyers EN, Steiner W, Ambrosch P, Hess CF, Kron M (2001) Organ preservation by transoral laser microsurgery in piriform sinus carcinoma. Otolaryngol Head and Neck Surg 124: 58-67.
- Steiner W, Fierek O, Ambrosch P, Hommerich CP, Kron M (2003) Transoral laser microsurgery for squamous cell carcinoma of the base of the tongue. Arch Otolaryngol Head Neck Surg 129: 36-43.
- Steiner W, Vogt P, Ambrosch P, Kron M (2004) Transoral carbon dioxide laser microsurgery for recurrent glottic carcinoma after radiotherapy. Head Neck 26: 477-484.
- Pfister DG, Spencer S, Adelstein D, Adkins D, Anzai Y, et al. (2020) Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 18: 873-898.
- Tuncer I, Özçakir-Tomruk C, Sencift K, Çöloglu S (2010 ) Comparison of conventional surgery and CO2 laser on intraoral soft tissue pathologies and evaluation of the collateral thermal damage. Photomed Laser Surg 28: 75-79.
- Hillegersberg RV (1997) Fundamentals of laser surgery. Eur J Surg 163: 3-12.
- Haina D, Landthaler M (1988) Fundamentals of laser light interaction with human tissue, especially in the cardiovascular system. Thorac Cardiovasc Surg 36: 118-125.
- Vilaseca I, Bernal-Sprekelsen M, Blanch JL (2009) Transoral laser microsurgery for T3 laryngeal tumors: Prognostic factors. Head Neck 32: 929-938.
- Ferri E, Armato E (2008) Diode laser microsurgery for treatment of Tis and T1 glottic carcinomas. Am J Otolaryngol 29: 101-105.
- Sullins KE (2002) Diode laser and endoscopic laser surgery. Vet Clin North Am Small Anim Prac 32: 639-648.
- Bruning PF, Halling A, Hilgers FJM, Kappner G, Klein Poelhuis EK, et al. (1988) Postoperative nasogastric tube feeding in patients with head and neck cancer: A prospective assessment of nutritional status and well-being. Eur J Cancer Clin Oncol 24: 181-188.
- Brotherton AM, Judd PA (2007) Quality of life in adult enteral tube feeding patients. Journal of Human Nutrition and Dietetics 20: 513-522.
- Torabi SJ, Chouairi F, Dinis J, Alperovich M (2020) Head and neck reconstructive surgery: Characterization of the one-team and two-team approaches. J Oral Maxillofac Surg 78: 295-304.
- Chim H, Salgado C, Seselgyte R, Wei FC, Mardini S (2010) Principles of head and neck reconstruction: An algorithm to guide flap selection. Semin Plast Surg 24: 148-154.
- Ong M, Parray A, Le BD, Choi CB, Paskhover B (2021) An analysis of prolonged operation time on outcomes of free flap procedures. J Am Coll Surg 233: 138.
- Lindeborg MM, Puram SV, Sethi RKV, Abt N, Emerick KS, et al. (2020) Predictive factors for prolonged operative time in head and neck patients undergoing free flap reconstruction. Am J Otolaryngol 41: 102392.
- Forner D, Phillips T, Rigby M, Hart R, Taylor M, et al. (2016) Submental island flap reconstruction reduces cost in oral cancer reconstruction compared to radial forearm free flap reconstruction: A case series and cost analysis. J Otolaryngol Head Neck Surg 45: 11.
- Irawati N, Every J, Dawson R, Leinkram D, Elliott M, et al. (2023) Effect of operative time on complications associated with free flap reconstruction of the head and neck. Clin Otolaryngol 48: 175-181.
- Bianchi B, Copelli C, Ferrari S, Ferri A, Sesenna E (2009) Free flaps: Outcomes and complications in head and neck reconstructions. J Craniomaxillof Surg 37: 438-442.
- Kucur C, Durmus K, Uysal IO, Old M, Agrawal A, et al. (2016) Management of complications and compromised free flaps following major head and neck surgery. Eur Arch Otorhinolaryngol 273: 209-213.
- Suh JD, Sercarz JA, Abemayor E, Calcaterra TC, Rawnsley JD, et al. (2004) Analysis of outcome and complications in 400 cases of microvascular head and neck reconstruction. Arch Otolaryngol Head Neck Surg 130: 962-966.
Citation: Nakai MY, Tenório LR, Menezes MB, Lima LRC, Gusmão RV (2024) Diode Laser Surgery for Early Stage Oral Cavity and Oropharyngeal Cancer - A Comparison Study with Conventional Electrosurgery. J Otolaryng Head Neck Surg 10: 088.
Copyright: © 2024 Marianne Yumi Nakai, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

