
Discuss the Use of Oncolytic Viruses as Cancer Therapeutics
*Corresponding Author(s):
Rachita Radhakrishna PaiDivision Of Biomedical Science School Of Life Sciences, University Of Bradford, England, United Kingdom
Tel:+65 90267186,
Email:rachita16pai@gmail.com
Abstract
Cell death by oncolytic viruses occurs through direct or indirect mechanisms such as amplification of anti-cancer immune response, tumor blood vessels destruction or through transgene-encoded proteins manifested from modified viruses. Despite the different mode of action, the ultimate function of the oncolytic viruses is to disrupt the cancer cells’ transcriptional or translational mechanism and cause apoptosis without causing harm to normal healthy cells.
Oncolytic viruses are versatile and work via several different mechanisms depending on their clinical indication as well as their platform. With a closer understanding between the host and virus communication, a tailored therapeutic strategy could be developed.
Oncolytic viruses have been given consent for a limited number of cases because of the varied testing stages. The very first oncoytic viral therapy came in use for the treatment of upper nasal cancers in China in the year 2006, in combination with conventional therapies like chemotherapy, after which there has been an extensive amount of research on.
This dissertation concentrates on the interferences oncolytic virotherapy encounter and the current advancements taking place to overcome them, specifically in emphasizing Adenoviruses.
INTRODUCTION
Some other reasons that contribute to the death by cancer are; cancer causing viruses like epstein barr virus that are also known to cause burkitt's lymphoma and cervical cancer caused as a result of human papillomavirus.
Although chemotherapy and radiotherapy are directed towards the prevention and cure, they have a low impact comparatively due to their low therapeutic index and numerous side effects. Thereby, calling for a high necessity for greater efficacious modalities.
What are oncolytic viruses?
A typical oncolytic virus
General mechanism of action
Cancer cells destruction takes place via several distinct mechanisms inclusive of virus mediated cytotoxicity through their cytotoxic immune effector, by lysing the cell directly, toxic protein expression, protein synthesis inhibition, induction of antitumoral immunity as well as cell receptor targeting, apoptosis and virus replication [4,5].
Viruses, after a deeper understanding of the pathways has shown improvements in the treatment of many human diseases, and oncolytic viral therapy has shown improvements towards different varieties of cancers, hence it is a novel approach for treating cancer.
ONCOLYTIC VIRUS IMMUNOTHERAPY
A vaccination against viruses has been an essential discovery, and viral pathogenicity has been the major driver in this evolution. Activation and re-directing innate and adaptive immune response function is a main objective of oncolytic virus mediated immunotherapy. There often is an involvement of the interactions between the immune cells and signaling factors such as chemokines, in viral infections as they are important for successful immunotherapies.
The figure 1 below represents the effects of oncolytic viruses on tumor cells. In phase 1, the oncolytic viruses delivered systemically disrupts the tumor cells by infecting it. Phase 2, represents recruitment of dendritic cells by inflammation. Thereby inducing an adaptive immune response, which then targets tumors via TAA?
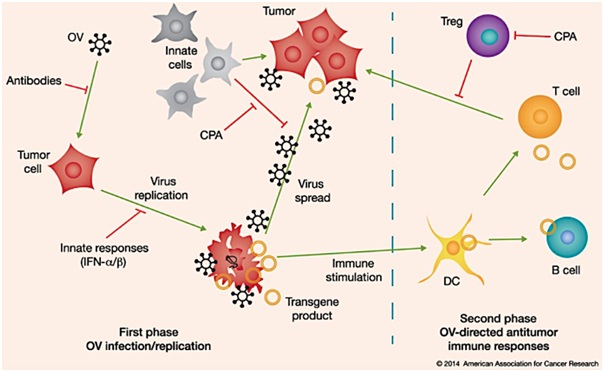
Figure 1: Showing oncolytic virus mediated effects in tumor cells.
1st phase: Intramural or systemically delivery of oncolytic viruses causes infection in the tumor cells, which can be obstructed by the antibodies, humoraldefence pathway. Post infection, oncolytic viruses undergo replication, blocked by the innate response (IFNα/β, to destroy the cells via ICD as well as diffuse throughout the tumor (can be obstructed by macrophages or NK cells which posses the anti tumor activities). There is a presence of an expression of a transgene product, immunomodulatory transgene, which enhances the antitumor response once an armed oncolytic virus is used.
2nd Phase: there is a recruitment of dendritic cells to the site of tumor by inflammation and ICD. There is a take up of TAA’s by these cells, which then targets the tumor, which can in turn be blocked by Tregs and MDSC’s.
The immune system initially, by the oncolytic viral therapy was seen as a negative factor due to an inflammatory response that leads to an antiviral immunity. Some studies performed displayed that human xenograft tumor models in the mice lacked adaptive immune responses (SCID mice) due to some viruses that replicated well in human cells comparatively. A syngeneic tumor model in immunocompetent mice showed the complexity of the immune system as well as proving the efficacy of antitumor immunity. Particularly, distinct oncolytic viruses induce adaptive anti-tumor responses (CD8+ T cell-mediated), which are long term. Additionally, adaptive anti-viral immunity enhanced the antiviral immunity for herpes simplex virus but not for vesicular stomatitis virus [6].
A deeper understanding of chemotherapy and radiation therapy for the treatment of cancer shows a classification of cell death via apoptosis. This lead to a new concept of apoptosis being classified into, ICD, an abbreviation for ‘Immunogenic cell death’ and NICD, abbreviated for ‘Non Immunogenic cell death’.
The immunogenic cell death, which is promoted by tumors of the oncolytic viral infection, makes oncolytic viruses a potent inducer of antitumoral immunity.
Together with some different types of cell death like immunogenic apoptosis and necrosis that are caused by oncolytic viruses, released a characteristic danger associated molecular pattern or DAMP’s like calreticulin, High Mobility Group Protein B1 (HMGB1) as well as a release of Tumor Associated Antigen (TAA) had been observed [7].
Pyroptosis, is also responsible for the danger signal release known as DAMP’s, which is a favorably inflammatory type of an organized cell death. Unlike a non-immunogenic and non-inflammatory cell death by apoptosis, this is commonly activated via pathogens.
Some studies show that apoptosis could be an immunogenic cell death [8]. At the initial stages of immunogenic apoptosis, the calreticulin, that is exposed on the surface before apoptosis takes place, which dictates the immunogenicity of the cancerous cell death. An important protein known as ERP57 maintains this exposure of the calreticulin [9].
Endoplasmic reticulum stress has the ability to maintain the danger mechanism. This occurs in response to Immunogenic cell death promotion, by activating the endoplasmic reticulum stress. Another method of self destruction followed by some cells called autophagy, possesses the ability to promote danger signals thereby also promoting the antitumor immune response after which there is a release of DAMP’s and HMGB1 from the gradually ceasing cancer cells [10].
Stromal cells along with tumor cells exhibit varied proteins that play a role as antigens or mutated proteins or as TAA (Tumor Associated Antigens). These tumor-selective over-expressed proteins (TAA), target active immunization. Interactions between cells of the stroma, cancer and immune cells in the tumor microenvironment dominate its properties. Hence the microenvironment of the tumor can be changed to cause activation of the anti tumor immunity in a treatment. Thus a number of immuno therapeutic mechanisms are directed towards the disruption of immune regulation and at the same time are necessary for the maintenance of tumor tolerance [11].
The interaction between TAA expression and oncolytic virus dependent cell death, enhances T-cell migration in the tumor cells in comparison to an oncolytic virus infected tumor cells that express for TAA. This mechanism can be integrated to an adenovirus that has a limited replication ability that expresses for TAA with an oncolytic vaccinia virus that exhibits for the same TAA which shows that the secondary response is dominant over the primary anti oncolytic viral response [12].
A drug known as cyclophosphamide helps to increase oncolytic viral replication and also interferes with the innate immune cell as it is used to suppress cancerous growth by attaching to one of the cancer cells DNA strands [13]. Identification of immunosuppressive mechanisms that can weaken the innate cells from obstructing viral replication and its advancement and at the same time enabling the inflammation towards anti tumor immunity is still under research as it is challenging. A potential aspect could be by incorporating oncolytic viruses with conventional therapies like chemotherapy, which may promote immunogenic cell death and enhance tumor cell antigenicity [14]. Therefore, an extensive research in the area of virotherapy demonstrates its efficacy against anti cancer mechanism in tumor cells with the help of oncolytic viruses.
TUMOR SELECTIVITY MECHANISM
The oncolytic viruses mediate destroying uninfected cancer cells. Production and replication is increased due to the evolvement of viruses and their ability to utilize molecular factors in an infected cell. Viruses and cancer cells work towards attaining similar outcomes, of DNA replication. They achieve this via interference with the signal transduction pathways, which promotes G1-S continuance [15].
The pathways for the control of pathogenic viral particles detection and deamination are activated viathe local Interferon (IFN) discharge or Toll Like Receptors (TLR) that are intracellularreceptors that possess an affinity for intracellular pattern and cell surface and are activated once they recognize a repeated sequence known as PAMP’s (Pathogen Associated Molecular Patterns) which involve viral capsids, DNA, RNA and viral proteins. These sequences are most frequently found on pathogenic bacteria’s as well as viruses.
TNF Receptor Associated Factor 3 (TRAF3), Retinoic acid Inducible Gene 1 (RIG-1), (IFN) Interferon Regulated Factor 3 (IRF3) and IRF7 are associated with the oncolytic virus demolition. JAK-STAT, which is a Janus Kinase Signal-Transducer and Activator of Transcription, is responsible for the anti-viral mechanism amongst impaired cells. This mechanism supports IFN (Iterferon) discharge that activates a Protein Kinase (PKR). A defect in the IFN pathway, which regulates cellular functions, leads to cancer. IFN are cytokines that activate gene transcription and have products that are antiviral or anti-proliferative [16]. A type 1 cytokine IFNβ, is produced due to viral infection, as first line of defense to protect normal neighboring cells [17].
Oncolytic viruses are classified into
The alteration in the signal mechanism fosters favorable microenvironment specific to oncolytic viruses to replicate effectively in endothelial cells or cancer cells, leading tooncolysis of the aberrant cells [19].
As there is a continuous improvement directed towards oncolytic viruses, there are some emerging novel viruses that demand exploration. In vitro testing and Xenograft SCID mice, the SIN (Sindbis) receptors are highly expressed on tumor cells and bind to SIN upregulating the progress of different tumor cell lines [20]. Positive results have been observed in cervical as well as ovarian cancers whereas the healthy cells had no observed results. An RNA virus known as Semliki Forest Virus (SFV), favors oncolytic viral agents, as they are consistent in the blood and increase the transgene expression. Once injected, they were not found to have an immune response. These are types of togaviruses, which are also found to play a role in oncolytic therapy due to their high replication rate. Although these viruses possess distinctive properties when differentiated against molecular drugs, they have the ability to proliferate as well as transport antitumoral therapeutic genes. The human body, however, overcomes this and obstructs this mechanism towards attaining the oncolytic viral therapeutic efficacy (Figure 2) [21].
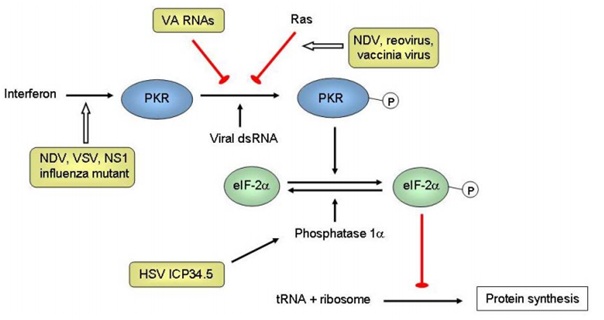
Figure 2: Tumor selectivity mechanism of oncolytic viruses [5].
Some viruses used in therapy have the capability of being used as vaccine vectors as they can be genetically modified, these viruses include, Poliovirus (PV- Picornavirus), Measles Virus (MV- paramyxovirus) as well as Vaccinia Virus (VV- Poxvirus). Oncolytic viruses that have been genetically modified with transmutations or gene deletions necessary for cell multiplication in the healthy and not in cancerous cells include, Adenovirus (Ad), Herpes Simplex Virus (HSV), Vaccinia Virus and Vesicular Stomatitis Virus (VSV- Rhabdovirus). For example, thymidine kinase is required for the metabolism of nucleic acid, a deletion of a gene encoding this enzyme results in viruses like HSV and VV to be dependentupon the expression of thymidine, which thereby is increased in the case of cancer cells and is absent in normal healthy cells. Vaccinia virus causes the production of Vaccinia Growth Factor (VGF) which causes activation of Epidermal Growth Factor Receptor (EGFR), thus causing a microenvironment for replication.
Herpes simplex virus protein ICP34.5 dephosphorylates eIF-2 via the interaction with cellular phosphatase 1 resulting in protein synthesis. Selective replication is the cause of a gene deletion of ICP34.5 (RL1) in defective IFN PKR pathway. This mechanism is also seen in the deleted influenza virus NS1 mutant [22].
Figure 3, (healthy Cells) represents post-viral infection, most normal healthy cells cause an activation of an antiviral mechanism, whereas, B represents cancer cells down-regulate key signaling components within the innate signaling pathway [4].

Figure 3: Oncolytic viruses exploit cancer immune evasion pathways [4].
USING OV FOR TREATMENT
Viral tropism is the ability of viruses to display specificity towards tissue, species or cell types. Cytokines like IFN’s and TNF (Tumor Necrosis Factors) are essential for controlling the viral tropism.
Poxvirus has the ability to effectively evade the immune system attack and travel to the site of the tumor systematically. Vaccinia Virus (VV) destroys the localized tissue and is the most recurrently used poxvirus for targeting cancer as it has a short-life and has an advantage of no genomic amalgamation [23]. There is a deletion of genes that code for Thymidine Kinase (TK) as well as Vaccinia Growth-Factor (VGF), increasing VV’s sensitivity towards tumor-selection. Thus causing an overexpression of E2F. Vaccinia Virus undergoes several mechanisms resulting in apoptosis for the destruction of cancer cells, for instance, administration of JX-594, a Poxvirus strain with a gene deletion of thymidine kinase, is a replicative competent vector that shows oncolysis of tumors and metastases.
There has been a presence of infected organs, which suggests the need to optimize the vaccinia vectors, even though the replicative competent vectors elevate the survival susceptibility of immuno-competent and immuno-deficient mouse models affected by various tumors [24]. Rapamycin, an immuno-suppressant, proved its competence when it was used in collaboration. Another type of poxvirus is Myxoma Virus (MYXV), that was found to be efficacious towards human glioma cancer cells, and is not known to be a cause of any diseases affecting humans thus proving its use as an oncolytic therapeutic [25,26]. Myxoma virus, earlier thought to be a rabbit specific possesses the ability to actively replicate in different types of human tumor cells. Another type of a species specific oncolytic virus is bovine herpesvirus type 1, which has the ability to infect and kill varied immortalized and modified human cell types but at the same time, does not successfully promote cytopathic effects in the normal human cells [27].
The earliest genetically modified Oncolytic virus was the HSV-1 (Herpes Simplex Virus-1) in 1991, in which includes a thymidine kinase UL23 gene deletion due to its ability of being replication selective.
The absence of this gene causes a loss in the ability of cell division in the normal cells. Due to this reason, there is a deletion of neurovirulence gene, γ34.5 for its therapeutic use in nearly all HSV vectors, in turn, restricting the replicative potential in the CNS and formation of latency [28].
It was proved that γ34.5 deletion declined the replicative potential by the deletion of HSV in multiple genes so as to evade the wild type generation.
One of the earliest genetically modified oncolytic virus, polyomavirus, consists of ds-DNA viruses with a circular genome and was originated was SV-40 (Simian Vacuolating Virus) as well as mouse polyomavirus.
The mouse polyomavirus induces several distinct tumors once it has been inoculated into a pup. An in depth knowledge of SV-40 and mouse polyomavirus has contributed by providing us a model for understanding DNA replication and other essential pathways taking place in a eukaryotic cell. It was found that SV-40 enhances the formation of tumors this was shown by an enhanced formation of sarcoma after it was subcutaneously injected into pups [29].
Cordelier et al., in 2007 demonstrated that there was an existence of tumor specificity in vitro along with an inhibition in a mouse model affected with pancreatic cancer growth. This inhibition was performed with promoters specific for the tumor.
The capability of a recombinant polyomavirus of avoiding the immune recognition makes them advantageous vectors.
Through gene deletion, which is essential for replication in non carcinogenic cells, the modified viruses can be utilized to target cancer cells. The oncolytic viruses can target specific tumor cells once the promoters and regulators are placed into the viruses from the tumor genes. This is a direct treatment, which is conducted with the help of oncolytic viruses by either with or without conventional methods.
In an indirect treatment, immunity is caused in anti-tumor cells with the help of oncolytic viruses by the destruction of cancer cells.
HMGB1 (High Mobility Group Box 1), acts as a DNA chaperone internally and acts with growth factors externally and is overexpressed in human cancer. It has been proved that HMGB1 functions as an oncogene, thus contributing to the pathogenesis of several cancers and can also play a role in the progression of certain carcinomas, due to its potential of being an independent prognostic indicator [30,31]. There is cell death and survival promotion by HMGB1, which is released by the tumors in the process of oncolytic viral treatment.
Adenoviruses have the capability of causing the symptoms of the disease that are isolated from tissues experiencing regression at the adenoid.
Lysis of an infected cell occurs once the adenovirus enters and binds to Coxsackievirus and Adenovirus Receptor (CAR), a cell adhesion particle. This binding via clathrin coated pits causes an internalization of the virus [32].
Engineering of the selective replication mechanism of Oncolytic Adenoviruses (Ads) via the deletion of the E1A, E1B 19K or E1B 55K gene, to create tumor-specific viruses, causes the products of these genes to aid the host cells to enter into S phase. Deletion of EIA blocks the G1 to S-phase by rendering the virus responsive to anti viral pathways of RB (Retinoblastoma) protein.
An Adenovirus with E1B 55K gene deletion was first approved by the Chinese Government in 2005, which often is used in clinical and experimental gene therapy, to be used together with radiation for the head and neck cancer treatment [33].
E1B deletion enables apoptosis of the damaged cells, terminating cellular division and virus proliferation, originated by p53. Only cells lacking in Rp and p53 undergo division of adenoviral E1-deletion mutants, by which nearly all gliomas become targets of for the process of oncolysis [34].
Viruses like adenovirus, Vaccinia Virus (VV) and Herpes Simplex Virus (HSV) that are genetically modified have been in use extensively as an anti-tumoral agent. Intra-tumoral replication selectively may cause enhanced efficacy due to its sustainability of the treatment in the presence of viral replication, lysis of affected tumor and the development of neighboring cells. reduced oncolytic potency could be achieved by deletion of genes, which also result in tumor selectivity.
In a study conducted, Onyx-15, a dl1520 Oncolytic Ad2/Ad5 hybrid was found to infect as well as induce apoptosis [35]. In this modified virus, there is a deletion of E1B 55K as well as E3B genes, which are proteins, essential for the inhibition of p53, protein synthesis of the host cells as well as transportation of viral mRNA. In 2009 it was found by Thomas et al., that Onyx-015, known as H101 in China, was not as efficient in causing lysis of the cells that are damaged in the G1 state of the cell-cycle mechanism as a result of the adenoviral gene product [36]. As the first generation of the selectively replicating adenovirus was used under clinical trials, progress has been made in order to enhance the potency by researching on various types of Ad genes.
P53 is a transcription factor essential for cell cycle checkpoints and the maintenance of the genome. Due to the defect in the apoptotic mechanism, there is an increase in cell proliferation, which is a hallmark of all cancer cells [37]. Despite this defect, the mechanism can be restored. In a study conducted, tumor cells were observed to have an increase in the levels of a proto-oncogene and a known as Mdm2. Mdm2 is found to negatively regulate the function of p53, hence degrading it to allow proliferation of tumor cells as well as evasion of apoptosis [38].
Figure 4, Shows ongoing oncolysis in the nodule to the left, whereas tumor destruction is almost complete in the nodules to the right. There is a formation of new nodules at the top, which consists of viral resistant cells that appear under the pressure of selective mechanism of oncolysis. Tumor cells possess the ability to obscure inside the connective tissue so as to evade viral destruction [4].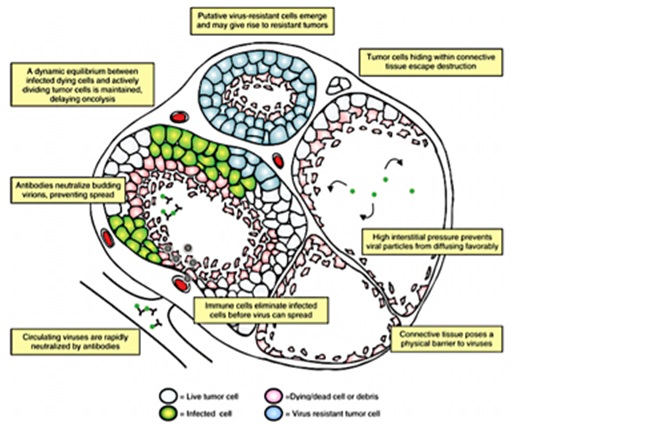
Figure 4: Shows clinical trial replication-competent HSV-1 vectors [41].
CLINICAL TRIALS
Phase I: Clinical trials are directed towards a few healthy volunteers with but kept under close observation. The aim of this is to verify if the drug under trial is risk-free for human usage.
A phase II: Clinical trial is to assess the efficacy of new drugs. These patient volunteers are targeted with the intention of being completely treated.
Phase III: Drugs are tested for their efficacy and dose levels in the treatment of the disease. This stage of the trial also assesses the effectiveness of drugs on different levels of disease. A phase IV trial takes place to determine the adverse and long term effects of a new drug on a larger patient population.
Clinical trials on oncolytic viruses for the treatment of cancer have been extensively researched a very few for phase II and maximum for phase I. Herpes simplex virus 1 that expresses for melanoma (T-Vec, Talimogenelaherparepvec) is currently under a phase III trial. T-vec is an oncolytic immunotherapy, designed in a way so that it selectively replicates in tumors to produce CSF-GM. Results prove that it can serve as a novel therapeutic drug towards the treatment of metastatic melanomas as it is well tolerated and has high durability and overall response (Table 1) [39,40].
|
Generation |
Recombinant of |
Deletion |
|
First generation 1716 |
HSV-1 |
γ34.5 |
|
Second generation, NV1020 |
HSV1/2 |
UL24 and UL56 One copy of γ34.5 |
|
Third generation, G207 |
with multiple mutations |
ICP6 and both copies of γ34.5 |
|
OncoVEXTM (GM-CSF) |
clinical isolate of HSV-1 |
γ34.5, engineered to express higher levels of US11 which compensates for the reduced replication efficiency |
|
HF10 |
HF strain |
UL56 |
Another phase II clinical trial was conducted against metastatic melanoma where reolysin, a commercial product name for a strain of reovirus by oncolytics biotech, was delivered intravenously, showed a considerable decrease in the size of the tumors [44]. Even though there was not much positive outcome, some patients showed reoviral copy in tumors. Advancements in clinical trials provide evidence of the anti-cancer mechanism for successful virotherapy, however, a deeper understating is still in demand.
Enhancing virus delivery
Maximum of the oncolytic viruses have been delivered intratumorally and have been successful in treating easily accessible solid tumors. A death resulting from cancer is most of the time due to metastasis [47]. Hence stating that intratumor delivery depends on viral replication at the site of the tumor, whereas, the latter to the distant sites. This strategy is not always effective due to the immune response.
Therefore by the help of these clinical trials, we understand that oncolytic viruses can be systemically delivered with minimal latency and toxicity. These agents could be effective towards the treatment of either primary or even metastatic tumors. Hence this mechanism of delivery is appealing towards the treatment of metastasized or inaccessible diseases such as pancreatic cancer patients, but if for the transportation intravenously there is a necessity of deeper understanding.
The blood-brain barrier is an example of an obstruction that various oncolytic viruses can pass, thus implying its use towards the treatment of brain tumors.
A disadvantage of systemic delivery could be pre-existing immunity, which could be a result of previous oncolytic therapy or prior vaccination. The use of VV against small pox has caused a pre existing immunity in developing cancer patients. Another example is of reovirs causing immunity since it is present freely in the environment [48].
A study in 2008 by Flanagan et al., stated the effectiveness of systemic delivery of Vaccinia Virus since the Extracellular-Enveloped Matrix (EEM) can be used to neutralize antibodies that are, modifying the virus such that it produces an increased amount of immune evasion [49]. However, a form of the vaccinia virus known as the Intra-cellular Mature Virion (IMV) could be potentially systemically delivered as this modified virus reaches the target site first. Therefore proving its efficacy as IMV is comparatively more immunogenic as compared to EEV.
CONCLUSION
Due to a scarcity of animal models mimicking human cancer, tumor recurrence is a taxing virotherapy process regardless of the increase in the number of growing oncolytic virus and advancement. This difficulty possibly could be avoided by the use of sophisticated models.
Another concern faced, is of cancer cells developing insensitivity towards virotherapy and the conventional treatments, which are optimal therapeutic regimens, and avoidance of this mechanism is a necessity. Even though many efficacious treatments for a single host have dire consequences, it introduces other variables in a complex treatment.
An in depth understanding of the immunological pathways and its communication betwixt viruses and other varied types of treatments available is a necessity because of the complications in estimating the degree of immune response in oncolysis, specifically for viruses with high dosage. Another concern is of the use of viral replicative-competent in individuals with weakened immunity, like post radiation therapy.
These cancer trials clearly illustrate anti-cancer activity against several types of cancer. Oncolytic viral therapy in combination with other therapies will serve as potentially powerful adjuncts. However, certain challenges, which will upgrade the treatment, will remain, like identification of the virus and delivery method. These must be resolved before being a clinical strategy against cancer. The war on cancer has led to sophisticated treatments, however, cancer related deaths still occur. In depth understanding of oncolytic viruses could increase survival rates.
A blend methodology and an ideal application, to improve the systemic conveyance of oncolytic viruses could be possibly employed, which could be attained via targeting varied factors.
This approach might offer an efficacious therapeutic strategy towards the primary tumor and associated treatment, which is not readily available due to their physiological barriers for conventional therapeutic agents.
The BBB (Blood Brain Barrier) is an example of an obstacle, where oncolytic viruses passes through, and hence could be a potential strategy in the treatment of brain tumors. In this way of targeting every factor, there could be a major advancement of many different types of cancers.
Genetic material can be injected by the virus into the enzyme, which causes activation of proteins that decelerate translation of RNA and cause apoptosis.
Consequences of radiation increase proliferation by administering a transporter code [50].
Major advances are made to improve efficacy and selectivity of oncolytic viruses and to overcome hurdles towards efficacious virotherapeutics [51].
A possible approach towards the treatment of cancer could be by, integrating oncolytic viruses with other types of conventional cancer therapies or it could be aimed towards immunologically sensitizing the tumors.
ACKNOWLEDGEMENT
I would like to thank Dr Jim Boyne, Lecturer in Molecular and Cellular Biology, University of Bradford for his supervision and guidance in writing this dissertation.
REFERENCES
- Dela Cruz CS, Tanoue LT, Matthay RA (2011) Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med 32: 605-644.
- Wakimoto H, Ikeda K, Abe T, Ichikawa T, Hochberg FH, et al. (2002) The Complement Response Against an Oncolytic Virus Is Species-Specific in Its Activation Pathways. Mol Ther 5: 275-282.
- Carson J, Haddad D, Bressman M, Fong Y (2010)Oncolytic Herpes Simplex Virus 1 (HSV-1) vectors: Increasing treatment efficacy and range through strategic virus design. Drugs Future 35: 183-195.
- Kaufman HL, Kohlhapp FJ, Zloza A (2015) Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 14: 642-662.
- Wong HH, Lemoine NR, Wang Y (2010) Oncolytic viruses for cancer therapy: Overcoming the obstacles. Viruses 2: 78-106.
- Melcher A, Parato K, Rooney CM, Bell JC (2011) Thunder and Lightning: Immunotherapy and Oncolytic Viruses Collide. Mol Ther 19: 1008-1016.
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, et al. (2007) Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 13: 54-61.
- Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, et al. (2008) Immunogenic cancer cell death: a key-lock paradigm. Curr Opin Immunol 20: 504-511.
- Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, et al. (2008) The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ 15: 1499-1509.
- Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT (2012) PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol Rev 249: 158-175.
- Topalian SL, Drake CG, Pardoll DM (2012) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24: 207-212.
- Diaz RM, Galivo F, Kottke T, Wongthida P, Qiao J (2007)Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res 67: 2840-2848.
- Fulci G, Breymann L, Gianni D, Kurozomi K, Rhee SS (2006).Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses. Proc Natl Acad Sci USA 103: 12873-12878.
- Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, et al. (2006) Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 203: 1259-1271.
- Ries S, Korn WM (2002) ONYX-015: mechanisms of action and clinical potential of a replication-selective adenovirus. Br J Cancer 86: 5-11.
- Elde NC, Child SJ, Geballe AP, Malik HS (2009) Protein kinase R reveals an evolutionary model for defeating viral mimicry. Nature 457: 485-489.
- Li Q, Tainsky MA (2011) Epigenetic silencing of IRF7 and/or IRF5 in lung cancer cells leads to increased sensitivity to oncolytic viruses. PLoS One 6: 28683.
- Krüger L, Eskerski H, Dinsart C, Cornelis J, Rommelaere J, et al. (2008) Augmented transgene expression in transformed cells using a parvoviral hybrid vector. Cancer Gene Ther 15: 252-267.
- McFadden G, Mohamed MR, Rahman MM, Bartee E (2009) Cytokine determinants of viral tropism. Nat Rev Immunol. 9: 645-655.
- Tseng JC, Levin B, Hirano T, Yee H, Pampeno C, et al. (2002) In vivo antitumor activity of Sindbis viral vectors. J Natl Cancer Inst 94: 1790-1802.
- Rodriguez-Madoz JR, Liu KH, Quetglas JI, Ruiz-Guillen M, Otano I, et al. (2009) Semliki Forest Virus Expressing Interleukin-12 Induces Antiviral and Antitumoral Responses in Woodchucks with Chronic Viral Hepatitis and Hepatocellular Carcinoma. J Virol 83: 12266-12278.
- Muster T, Rajtarova J, Sachet M, Unger H, Fleischhacker R, et al. (2004) Interferon resistance promotes oncolysis by influenza virus NS1-deletion mutants. Int J Cancer 110: 15-21.
- McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, et al. (2001) Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res 61: 8751-8757.
- Guo ZS, Naik A, O’Malley ME, Popovic P, Demarco R, et al. (2005) The enhanced tumor selectivity of an oncolytic vaccinia lacking the host range and antiapoptosis genes SPI-1 and SPI-2. Cancer Res 65: 9991-9998.
- Lun X, Yang W, Alain T, Shi ZQ, Muzik H, et al. (2005) Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res 65: 9982-9990.
- Chan WM, McFadden G (2014) Oncolytic Poxviruses. Annu Rev Virol 1: 119-141.
- Tai CK, Wang WJ, Chen TC, Kasahara N (2005) Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol Ther 12: 842-851.
- Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, et al. (2000) Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci USA 97: 2208-2213.
- White MK, Khalili K (2004) Polyomaviruses and human cancer: Molecular mechanisms underlying patterns of tumorigenesis. Virology 324: 1-16.
- Xu Y, Sumter TF, Bhattacharya R, Tesfaye A, Fuchs EJ, et al. (2004) The HMG-I oncogene causes highly penetrant, aggressive lymphoid malignancy in transgenic mice and is overexpressed in human leukemia. Cancer Res 64: 3371-3375.
- Wu D, Ding Y, Wang S, Zhang Q, Liu L (2008) Increased expression of high mobility group box 1 (HMGB1) is associated with progression and poor prognosis in human nasopharyngeal carcinoma. J Pathol 216: 167-175.
- Li C, Bowles DE, van Dyke T, Samulski RJ (2005) Adeno-associated virus vectors: potential applications for cancer gene therapy. Cancer Gene Ther 12: 913-925.
- Garber K (2006) China approves world’s first oncolytic virus therapy for cancer treatment. J Natl Cancer Inst 98: 298-300.
- Jiang H, Gomez-Manzano C, Lang FF, Alemany R, Fueyo J (2009) Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas. Curr Gene Ther 9: 422-427.
- Ganly I, Kirn D, Eckhardt G, Rodriguez GI, Soutar DS, et al. (2000) A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer . Clin Cancer Res 6: 798-806.
- Thomas MA, Broughton RS, Goodrum FD, Ornelles DA (2009) E4orf1 Limits the Oncolytic Potential of the E1B-55K Deletion Mutant Adenovirus. J Virol 83: 2406-2416.
- Rao B, van Leeuwen IM, Higgins M, Campbel J, Thompson AM, et al. (2010) Evaluation of an Actinomycin D/VX-680 aurora kinase inhibitor combination in p53-based cyclotherapy. Oncotarget 1: 639-650.
- Koo T, Choi IK, Kim M, Lee JS, Oh E (2010) Negative Regulation-Resistant p53 Variant Enhances Oncolytic Adenoviral Gene Therapy. Hum Gene Ther 23: 609-622.
- Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, et al. (2015) Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol 33: 2780-2788.
- Manservigi R, Argnani R, Marconi P (2010) HSV Recombinant Vectors for Gene Therapy. Open Virol J 4: 123-156.
- Everts B, van der Poel HG (2005) Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Ther 12: 141-161.
- Parato KA, Breitbach CJ, Le Boeuf F, Wang J, Storbeck C, et al. (2012) The Oncolytic Poxvirus JX-594 Selectively Replicates in and Destroys Cancer Cells Driven by Genetic Pathways Commonly Activated in Cancers. Mol Ther 20: 749-758.
- Hasson CJ, Caldwell GE, Emmerik REA Van (2009) NIH Public Access 27: 590-609.
- Galanis E, Markovic SN, Suman VJ, Nuovo GJ, Vile RG (2012) Phase II trial of intravenous administration of Reolysin(®) (Reovirus Serotype-3-dearing Strain) in patients with metastatic melanoma. Mol Ther 20:1998-2003.
- McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, et al. (2006) Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res 66: 2509-2513.
- Jain RK, Stylianopoulos T (2010) Delivering nanomedicine to solid tumors. Nat Rev ClinOncol 11: 223-235.
- Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, et al. (2013) A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res 9: 555-561.
- White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, et al. (2008) Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther 15: 911-920.
- Flanagan K, Glover RT, Hörig H, Yang W, Kaufman HL (2004). Local delivery of recombinant vaccinia virus expressing secondary lymphoid chemokine (SLC) results in a CD4 T-cell dependent antitumor response. Vaccine 22: 2894-2903.
- Pol JG, Rességuier J, Lichty BD (2011).Oncolytic viruses: A step into cancer immunotherapy. Virus Adaptation and Treatment 4: 1-21.
- Liu TC, Kirn D (2008) Gene therapy progress and prospects cancer: oncolytic viruses. Gene Ther 15: 877-884.
Citation: Pai RR (2019) Discuss the Use of Oncolytic Viruses as Cancer Therapeutics. J Cell Biol Cell Metab 6: 016.
Copyright: © 2019 Rachita Radhakrishna Pai, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

