
Do the Oncologists Systematically follow Treatment Recommendations and Protocol Adaptations Suggested by prior Comprehensive Geriatric Assessment in Older Patients with Colorectal Cancer?
*Corresponding Author(s):
Frédérique RetornazInternal Medicine Research And Care Unit, European Hospital, Marseille, France
Tel:+33 628325242,
Fax:+33 413427712
Email:frederique.retornaz@gmail.com
Abstract
Introduction: In order to assess the ability of older patient with Colorectal Cancer (CRC) to receive chemotherapy, a Comprehensive Geriatric Assessment (CGA) is recommended before the final therapeutic decision. The primary objective was to determine whether the presence of geriatric factors and/or frailty markers was associated with a dose reduction of prescribed chemotherapy in older patient with CRC. Secondary objectives were to determine which parameters were associated with early chemotherapy toxicity and to evaluate the correlation of dose reductions with geriatric oncologist treatment recommendations.
Materials and methods: This retrospective, monocentric study included patients ≥70 years, with a CRC requiring chemotherapy, for whom CGA was performed at the request of oncologists prior to the decision. Frailty markers (nutrition, physical activity, energy, mobility and strength), comprehensive geriatric assessment (functional status, comorbidities, falls, nutrition, cognition and depression) were collected.
Results: Out of the 30 patients (mean age 79.9 years) 50% had early toxicities and 46.4% had an immediate dose reduction. One in five patients had at least 3 markers of frailty and 46.7% had at least 3 abnormal CGA parameters. Immediate dose reduction was not associated with any oncological parameters, geriatric domains or frailty markers. Only 50% of the patients with CGA-suggested adjustment of cancer treatment were actually prescribed a chemotherapy dose reduction. On the contrary, 44.4% of patients with CGA-recommended standard treatment were prescribed reduced-dose chemotherapy. Factors significantly associated with early grade 3-4 toxicities were delayed treatment and unscheduled hospitalizations (respectively, p<0.001 and p = 0.003). Factors associated with early hematotoxicity was delayed treatment (p = 0.003) and with non-hematological toxicities were dose adjustments after onset of toxicity (p = 0.035), unscheduled hospitalizations at 3 months (p<0.001) and hearing impairment (p = 0.043).
Discussion: Our study shows that oncologists do not systematically follow the treatment recommendations and protocol adaptations suggested by the results of CGA of patients with CRC.
Keywords
Chemotoxicity; Colon cancer; Decision making; Frailty markers; Geriatric assessment
INTRODUCTION
As life expectancy and cancer incidence increase with age, more and more older patient with cancer are diagnosed [1]. The median age of diagnosis of Colorectal Cancer (CRC) is 70 years in men and 73 years in women, and colorectal cancer is the second leading cause of death from all cancers combined [2]. Older patients appear to benefit from chemotherapy, but advanced age is a risk factor for chemotoxicity [3,4]. The oncogeriatric population represents a category of potentially vulnerable patients for whom a number of age-related factors need to be assessed and taken into consideration when choosing treatment. Older patient with cancer frequently present undernutrition [5-7], sarcopenia [8,9] and frailty markers [10,11] which may increase the risk of chemotherapy-related toxicity. In order to assess the suitability of these patients to receive chemotherapy, a standardized geriatric assessment (comprehensive geriatric assessment: CGA) is recommended prior to the therapeutic decision [12]. During the CGA, certain geriatric factors or frailty criteria are highlighted and then play a role in the risk-benefit balance of the choice of treatment to be carried out. Indeed, the presence of co-morbidities has been shown to be associated with increased severe toxicity of chemotherapy and hospitalizations [13-15], and early discontinuation of cancer treatment [16]. Chemotoxicity has been associated with some domains of CGA such as depression, cognitive impairment, loss of autonomy in Instrumental Activities of Daily Living (IADL) or poor social support [17,18]. In older patients with advanced colorectal cancer, a study by the Fédération Française de Cancérologie Digestive showed that low Mini Mental State Examination (MMSE) and IADL scores were independently associated with unexpected hospitalization, and a low MMSE score was also significantly associated with increased toxicity from grade 3 to 5 [19].
At the end of the CGA, recommendations on the type of care are sent to oncologists. Nevertheless, the link between the findings of the geriatric assessment and the oncologic decision-making process is not well studied. A comparative study showed that CGA with interventions was associated with better completion of planned treatment, fewer treatment changes and reduced grade 3 and higher chemotoxicity in the intervention group versus the control group [20]. The study of Chaibietal [21], analyzed the impact of CGA on treatment in 161 older patients with cancer. Cancer treatment was modified in half of the patients, one third of whom had their treatment intensified. In the study of Girre V et al., [22] only body mass index and absence of depressive symptoms were significantly associated with a change in treatment plan.
Other prognostic markers have recently been studied in addition to CGA domains such as frailty phenotype markers. According to Fried et al., [23], the frailty phenotype is identified using five markers: nutrition, mobility, strength, energy and physical activity. The presence of at least three markers allows to define a patient as fragile and one or two markers as pre-fragile. In the general population, Fried showed that “frail” and “pre-frail” people were at greater risk of death within 3 years or of adverse outcomes (development of disabilities, mobility disorders, falls, and hospitalization). The study of Retornaz et al., [24] showed that, of older patient with cancer and without disability, 42% of patients had at least one marker of frailty. Some frailty markers are predictive of chemotoxicity [25,26] and early death [27,28]. Thus, the use of frailty markers appears to be complementary to CGA to detect patients with underlying vulnerability that may interfere with cancer treatment.
The main objective of this study was to determine whether the presence of geriatric factors and/or frailty markers, assessed during the pre-chemotherapy geriatric oncology evaluation, was associated with a dose reduction of prescribed chemotherapy in older patients with CRC. Secondary objectives were 1. to determine whether geriatric factors and markers of frailty were associated with early chemotherapy toxicity and 2. Whether changes in chemotherapy doses prescribed by oncologists were associated with treatment recommendations for geriatric oncologist.
PATIENTS AND METHODS
Study design
This is a retrospective, descriptive, monocentric study conducted from October 2012 to June 2019 at the European Hospital in Marseille. All patients aged 70 years and older with CRC requiring chemotherapy for whom a geriatric oncology assessment was performed at the request of oncologists prior to the treatment decision were included (Figure 1). Patient records were analyzed using the Chemotherapy Prescribing Software Chimio and the DPI Qc are computerized patient record software.
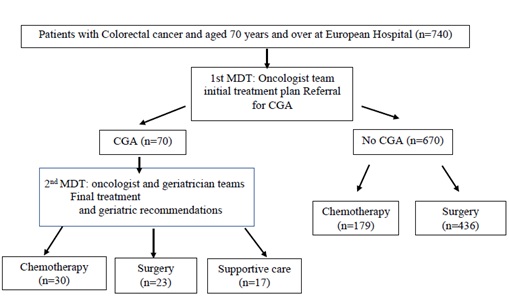 Figure 1: Flow chart of patients selection.
Figure 1: Flow chart of patients selection.
Abbreviations: MDT: Multidisciplinary Team Meeting; CGA: Comprehensive Geriatric Assessment.
Data collection
Demographic data (age, gender) were collected by the geriatric oncologist. CGA data and frailty markers were collected by the geriatric oncologist, nurse practitioner and dietician. The CGA included the following 9 domains: social status, autonomy, depression, cognition, neurosensory deficits, falls, nutrition, co-morbidities and medications. Disability for domestic Activities of Daily Living (ADL) was assessed using six tasks from the Katz index [29]. Abnormality of IADL was assessed using the seven elements of Older American Resources and Services [30]. The denominator has been adjusted to take into account patients who were not normally engaged in activities such as cooking or laundry. Loss of autonomy in ADL or IADL has been defined as the need for assistance to complete at least one activity. The 4 item Geriatric Depression Scale (mini GDS) was used to screen for depression. A score of 1 or more indicated depression [31]. Cognition was assessed by the following tests: Mini Cog [32], or Clock test [33]. Patients with hearing disorders and/or requiring hearing aids, and/or patients with vision disorders (despite the use of glasses) were considered to have a neurosensory deficit. Patients who had experienced one or more falls in the previous six months were considered to have a positive history of falls. Nutritional status was assessed according to Body Mass Index (BMI). Patients with a BMI of less than 22 were considered underweighted and malnourished [34]. Co-morbid conditions have been codified according to the International Classification of Diseases (ICD-10th Revision, French version). Ten co-morbidity groups were selected: cardiovascular disease, hypertension, diabetes, depression, dementia, other neurological diseases, respiratory diseases, gastrointestinal diseases, osteoarticular diseases and renal failure [35]. In each group, patients were positive if they had one or more co-morbidities. Co-morbidity was defined as the presence of three or more co-morbidities [36]. The number of drugs (excluding those for cancer treatment) was counted for each patient. A number of 5 drugs were considered as polypharmacy.
The 5 frailty markers adapted from Fried’s phenotype were also collected: physical activity, grip strength, mobility, energy and nutrition [23]. Reduced physical activity analyzed by the Canadian Study of Health and Aging Risk Factors Questionnaire [37] which assesses physical activity: no exercise or low exercise level was considered a positive marker for the frailty of physical activity. The strength was assessed by measuring the grip strength (in kilograms) in the dominant hand using a dynamometer; grip strength was adjusted for gender and BMI as described by Fried et al., [23]. Mobility was assessed using the Timed Up and Go (TUG) test [38] or the unipodal support test [39]. A TUG time of less than ten seconds or a patient’s inability to balance on one leg for more than five seconds was considered a positive marker of frailty for mobility. Energy was assessed using a visual scale ranging from 0 (no energy) to 10 (full of energy). A score
Chemotherapy-related toxicities were assessed at 3 months using version 4.0 of the common terminology Criteria for Adverse Events (CTCAE) and collected by a research coordinator. Toxicities were rated on a scale of 0 to 4. Grade 3 or 4 toxicities, as well as any unscheduled hospitalization within the first three months of cancer treatment, were collected.
Data analysis
The events studied were:
- • Which geriatric assessment parameters were associated with the dose reduction of chemotherapy (presence of frailty markers or geriatric factors)?
- • Which geriatric assessment parameters were associated with toxicity (grade 3-4 and hospitalization) of chemotherapy in the first three months of treatment
- • Was the dose reduction imputed from the outset associated with the geriatric oncologist's treatment recommendations: standard treatment or modified treatment (dose reduction)?
- • Was the presence of more than 2 or 3 markers of frailty, and/or at least one geriatric factor, or at least 2 geriatric factors, or at least 3 factors associated with an immediate dose reduction or grade 3-4 toxicity within the first three months of treatment?
Statistical analysis
Data are expressed as n (%) or mean ± SD. Statistical analysis was performed with the SPSS software (V.23) using the Fisher’s exact test or the χ2 test or the Mann-Whitney U test, as appropriate. A value of p< 0.05 was considered significant.
RESULTS
Thirty patients (mean age 79.9 ± 4.9 years), 63.3% of men were included (Table 1). Almost all patients (29/30, 93.3%) lived at home. The majority of patients were treated with multidrug therapy (60%). The 2 main protocols used were Capecitabin (26.7%) for monotherapy and Folfox (20%) for multidrug therapy. The initial dose reduction of chemotherapy protocols involved 13 patients (46.4%). Among the reduced molecules, 55% of the 5FU boluses were reduced with an average percentage reduction of 54.1 and 57.1% of the oxaliplatin prescriptions were reduced to an average percentage of 56.3%. Two drugs were systematically reduced from prescriptions: irinotecan was reduced in 100% of prescriptions to an average percentage reduction of 31.7%; bevacizumab was reduced for 2 patients (33.3%) with a percentage reduction of 100%.
|
|
n (%)/Mean ± SD |
|
Sex |
|
|
Female |
11 (36,7) |
|
Male |
19 (63,3) |
|
Age (years) |
79,9 ± 4,9 |
|
Weight (kg) |
69,2 ± 13,2 |
|
Size (m) |
1,63 ± 0,09 |
|
Living place |
29 (96,7) |
|
Oncological treatments Adjuvant chemotherapy Metastatic chemotherapy |
14 (48,3) 12 (41,4) |
|
Monochemotherapy: Capecitabin |
12 (40,0) 8 (26,7) |
|
LV5FU |
4 (13,3) |
|
Polychemotherapy: |
18 (60,0) |
|
Folfox |
6 (20,0) |
|
LV5FU + Bevacizumab |
4 (13,3) |
|
LV5FU + Aflibercept |
2 (6,7) |
|
Folfiri |
2 (6,7) |
|
Folfox +Bevacizumab |
1 (3,3) |
|
Folfiri + Bevacizumab |
1 (3,3) |
|
Other |
2 (6,7) |
|
Immediate dose reduction |
13 (46,4) |
|
Reduced drugs |
|
|
Oxaliplatin |
4 (57,1) |
|
5FU bolus |
11 (55,0) |
|
5FU infusion |
5 (25,0) |
|
Capecitabin |
2 (33,3) |
|
Irinotecan |
3 (100,0) |
|
Bevacizumab |
2 (33,3) |
|
Percentage reduction |
|
|
Oxaliplatin |
56,3 ± 31,5 |
|
5FU bolus |
54,1 ± 25,2 |
|
5FU infusion |
34,0 ± 14,8 |
|
Capecitabin |
25,0 ± 7,1 |
|
Irinotecan |
31,7 ± 16,1 |
|
Bevacizumab |
100,0 ± 0,0 |
|
Toxicity grade 3-4 |
15 (57,7) |
|
Hematotoxicity |
8 (30,8) |
|
Other stoxicities |
11 (42,3) |
|
Dose delay |
8 (34,8) |
|
Number of cures with dose delay |
|
|
1 |
4 (57,1) |
|
2 |
3 (42,9) |
|
Dosage adjustment after toxicity |
8 (44,4) |
|
Stop after toxicity |
8 (44,4) |
|
Hospitalizations |
9 (30,0) |
|
Death |
4 (13,3) |
|
CGA |
|
|
Presence of social support |
28 (93,3) |
|
Abnormal ADL |
3 (10,0) |
|
Abnormal IADL |
17 (56,7) |
|
Depression |
8 (26,7) |
|
Cognitive impairment |
7 (24,1) |
|
Visual deficit |
4 (13,3) |
|
Hearing deficit |
10 (33,3) |
|
Falls |
5 (18,5) |
|
BMI (kg/m2) |
26,1 ± 4,1 |
|
Comorbidities |
3,14 ± 1,89 |
|
Hypertension |
14 (48,3) |
|
Cardiovascular |
12 (42,9) |
|
Osteoarticular |
12 (41,4) |
|
Diabetes |
10 (34,5) |
|
Previous cancer |
8 (27,6) |
|
Neurological |
3 (10,3) |
|
Digestive |
3 (10,3) |
|
Respiratory |
3 (10,3) |
|
Depression |
1 (3,4) |
|
Dementia |
0 |
|
Chronic renal failure |
0 |
|
Number of no-cancer drugs |
4,28 ± 2,57 |
|
Score CGA |
|
|
0 |
3 (10,0) |
|
≥2 |
20 (66,7) |
|
≥3 |
14 (46,7) |
Frailty markers |
|
|
Impaired physical activity |
11 (37,9) |
|
Abnormal grip strength |
9 (32,1) |
|
Mobility |
17(56,7) |
|
Abnormal Time up and go |
7 (33,3) |
|
Abnormal Unipodal support |
10 (40,0) |
|
Abnormal energy |
0 |
|
Impaired nutrition |
18 (60,0) |
|
Loss of appetite |
11 (39,3) |
|
Undernutrition |
21 (84,0) |
|
Number of kg lost |
4,9 ± 4,5 |
|
Frailty markers |
|
|
0 |
8 (26,7) |
|
1-2 |
16 (53,3) |
|
≥3 |
6 (20,0) |
|
Treatment recommendations after Geriatric oncology evaluation: |
|
|
Identical treatment |
10 (34,5) |
|
Modification |
19 (65,5) |
|
Dose reduction performed |
13 (46,4) |
|
No dose reduction |
15 (53,6) |
Table 1: Characteristics of patients.
Abbreviations: kg: Kilograms; m: meters; LV5FU: 5-Fluorouracil + Calcium folinate; 5FU: 5-Fluorouracil; CGA: Comprehensive Geriatric Assesment; ADL: Activities of daily Living; IADL: Instrumental Activities of daily Living; BMI: Body Mass Index
Out of the CGA parameters, 53.3% had more than 3 co-morbidities, 10.0% had abnormal ADL score and 56.7% had abnormal IADL score, 30% had more than 5 drugs (excluding cancer-related drugs), 26.7% had depressive symptoms and 24.1% had cognitive disorders. Fourteen patients (46.7%) had at least 3 abnormal CGA parameters. The most frequent markers of frailty were impaired nutrition (60.0%), mobility (56.7%) and physical activity (37.9%). One in five patients had at least 3 markers of frailty.
Out of the 30 patients, 15 had grade 3-4 toxicity (50%), of whom 8 had hematotoxicity (30.8%) and 11 had non-hematological toxicity (42.3%). After the appearance of toxicity, 8 treatments were stopped (44.4%), 8 dosage adjustments were made (44.4%), 8 patients had a postponement of treatment (44.4%), and 9 patients were hospitalized (30%). A patient died during this hospitalization. Regarding the recommendations resulting from the geriatric oncology evaluation, 34.5% of the treatment strategies recommended standard treatment and 65.5% treatment adaptation. Only 50%of the patients with CGA-suggested adjustment of cancer treatment were actually prescribed a chemotherapy dose reduction. On the contrary, 44.4% of patients with CGA-recommended standard treatment were prescribed reduced-dose chemotherapy.
Dose reduction from the outset was not associated with any oncological parameters, geriatric domains or frailty markers (Table 2). Factors significantly associated with early grade 3-4 toxicity (onset in the first three months of treatment) were delayed treatment (p<0.001), and unscheduled hospitalizations within 3 months of the start of the chemotherapy protocol (p = 0.003, Table 3). The only factor associated with early hematotoxicity was delayed treatment (p = 0.003, Table 3). Factors associated with non-hematological toxicities were dose adjustments after onset of toxicity (p = 0.035), unscheduled hospitalizations at 3 months (p<0.001) and hearing impairment (p = 0.043, Table 3).
|
|
n (%)/Mean ± SD |
p |
||
|
|
|
Immediate dose reduction |
|
|
|
|
|
No |
Yes |
|
|
Sex |
Female |
5 (55,6) |
4 (44,4) |
|
|
|
Male |
10 (52,6) |
9 (47,4) |
1,000 |
|
Age (years) |
|
78,5 ± 5,6 |
81,5 ± 4,0 |
0,380 |
|
Weight (kg) |
|
71,3 ± 13,7 |
69,6 ± 11,1 |
0,733 |
|
Size (m) |
|
1,64 ± 0,08 |
1,63 ± 0,10 |
0,702 |
|
Report |
No |
7 (46,7) |
8 (53,3) |
|
|
|
Yes |
5 (62,5) |
3 (37,5) |
0,667 |
|
Number of cures reported |
1 |
2 (50,0) |
2 (50,0) |
|
|
|
2 |
2 (66,7) |
1 (33,3) |
1,000 |
|
Dosage adjustment after toxicity |
No |
4 (44,4) |
5 (55,6) |
|
|
|
Yes |
6 (75,0) |
2 (25,0) |
0,335 |
|
Stop after toxicity |
No |
6 (60,0) |
4 (40,0) |
|
|
|
Yes |
4 (57,1) |
3 (42,9) |
1,000 |
|
Hospitalization |
No |
10 (52,6) |
9 (47,4) |
|
|
|
Yes |
5 (55,6) |
4 (44,4) |
1,000 |
|
Death |
No |
14 (58,3) |
10 (41,7) |
|
|
|
Yes |
1 (25,0) |
3 (75,0) |
0,311 |
|
|
|
n (%)/Moyenne ± DS |
p |
|
|
|
|
Immediate dose reduction |
|
|
|
|
|
No |
Yes |
|
|
CGA |
||||
|
Presence of social support |
No |
1 (50,0) |
1 (50,0) |
|
|
|
Yes |
14 (53,8) |
12 (46,2) |
1,000 |
|
Abnormal ADL |
No |
14 (56,0) |
11 (44,0) |
|
|
|
Yes |
1 (33,3) |
2 (66,7) |
0,583 |
|
Abnormal IADL |
No |
7 (58,3) |
5 (41,7) |
|
|
|
Yes |
8 (50,0) |
8 (50,0) |
0,718 |
|
Depression |
No |
11 (55,0) |
9 (45,0) |
|
|
|
Yes |
4 (50,0) |
4 (50,0) |
1,000 |
|
Cognitive impairment |
No |
12 (60,0) |
8 (40,0) |
|
|
|
Yes |
2 (28,6) |
5 (71,4) |
0,209 |
|
Visual deficit |
No |
14 (56,0) |
11 (44,0) |
|
|
|
Yes |
1 (33,3) |
2 (66,7) |
0,583 |
|
Hearingdeficit |
No |
10 (52,6) |
9 (47,4) |
|
|
|
Yes |
5 (55,6) |
4 (44,4) |
1,000 |
|
Falls |
No |
12 (57,1) |
9 (42,9) |
|
|
|
Yes |
1 (25,0) |
3 (75,0) |
0,322 |
|
BMI (kg/m2) |
|
27,4 ± 4,8 |
25,7 ± 2,8 |
0,525 |
|
Comorbidities |
|
3,57 ± 2,0 |
2,67 ± 1,72 |
0,231 |
|
Hypertension |
No |
5 (35,7) |
9 (64,3) |
|
|
|
Yes |
9 (69,2) |
4 (30,8) |
0,128 |
|
Cardiovascular |
No |
9 (56,3) |
7 (43,8) |
|
|
|
Yes |
5 (45,5) |
6 (54,5) |
0,704 |
|
Osteoarticular |
No |
7 (46,7) |
8 (53,3) |
|
|
|
Yes |
7 (58,3) |
5 (41,7) |
0,704 |
|
Diabetes |
No |
7 (41,2) |
10 (58,8) |
|
|
|
Yes |
7 (70,0) |
3 (30,0) |
0,237 |
|
Previouscancer |
No |
12 (60,0) |
8 (40,0) |
|
|
|
Yes |
2 (28,6) |
5 (71,4) |
0,209 |
|
Neurological |
No |
12 (50,0) |
12 (50,0) |
|
|
|
Yes |
2 (66,7) |
1 (33,3) |
1,000 |
|
Digestive |
No |
12 (50,0) |
12 (50,0) |
|
|
|
Yes |
2 (66,7) |
1 (33,3) |
1,000 |
|
Respiratory |
No |
12 (50,0) |
12 (50,0) |
|
|
|
Yes |
2 (66,7) |
1 (33,3) |
1,000 |
|
Depression |
No |
14 (53,8) |
12 (46,2) |
|
|
|
Yes |
0 |
1 (100,0) |
0,482 |
|
Number of no-cancer drugs |
|
4,1 ± 2,6 |
4,5 ± 2,9 |
0,690 |
|
CGA |
|
|
|
|
|
0 |
|
3 (100,0) |
0 |
|
|
≥2 |
|
9 (50,0) |
9 (50,0) |
0,229 |
|
≥3 |
|
6 (46,2) |
7 (53,8) |
0,213 |
|
Frailty markers |
||||
|
Impaired physical activity |
No |
11 (61,1) |
7 (38,9) |
|
|
|
Yes |
3 (33,3) |
6 (66,7) |
0,237 |
|
Abnormal grip strength |
No |
9 (50,0) |
9 (50,0) |
|
|
|
Yes |
4 (50,0) |
4 (50,0) |
1,000 |
|
Mobility |
||||
|
Abnormal Time up and go |
No |
10 (76,9) |
3 (23,1) |
|
|
|
Yes |
2 (28,6) |
5 (71,4) |
0,062 |
|
Abnormal Unipodal support |
No |
9 (64,3) |
5 (35,7) |
|
|
|
Yes |
3 (33,3) |
6 (66,7) |
0,214 |
|
Impaired nutrition |
No |
8 (66,7) |
4 (33,3) |
|
|
|
Yes |
7 (43,8) |
9 (56,3) |
0,276 |
|
Loss of appetite |
No |
11 (68,8) |
5 (31,2) |
|
|
|
Yes |
3 (30,0) |
7 (70,0) |
0,105 |
|
Undernutrition |
No |
3 (100,0) |
0 |
|
|
|
Yes |
9 (45,0) |
11 (55,0) |
0,217 |
|
Number of kg lost |
|
4,14 ± 4,66 |
5,25 ± 4,57 |
0,432 |
|
Frailty markers |
||||
|
0 |
|
5 (62,5) |
3 (37,5) |
|
|
1-2 |
|
9 (60,0) |
6 (40,0) |
1,000 |
|
≥3 |
|
1 (20,0) |
4 (80,0) |
0,266 |
|
Treatment recommendations |
||||
|
after geriatric oncology evaluation |
||||
|
Identical treatment |
|
5 (55,6) |
4 (44,4) |
|
|
Modification |
|
9 (50,0) |
9 (50,0) |
1,000 |
Table 2: Dose reduction predictive data.
Abbreviations: kg: Kilograms; m: meters; LV5FU: 5-Fluorouracil + Calcium folinate; 5FU: 5-Fluorouracil; CGA: Comprehensive Geriatric Assesment; ADL: Activities of daily Living; IADL: Instrumental Activities of daily Living; BMI: Body Mass Index
|
|
|
n (%)/Mean ± SD |
p |
|
|
p |
|
|
p |
|||||||||||||||
|
|
|
Hématotoxicity |
|
Othertoxicity |
|
Toxicity grade 3-4 |
|
|||||||||||||||||
|
|
|
No |
Yes |
|
No |
Yes |
|
No |
Yes |
|
||||||||||||||
|
Sex |
Female |
5 (62,5) |
3 (37,5) |
|
3 (37,5) |
5 (62,5) |
|
1 (12,5) |
7 (87,5) |
|
||||||||||||||
|
|
Male |
13 (72,2) |
5 (27,8) |
0,667 |
12 (66,7) |
6 (33,3) |
0,218 |
10 (55,6) |
8 (44,4) |
0,084 |
||||||||||||||
|
Age (years) |
|
80,7 ± 3,8 |
79,0 ± 2,0 |
0,131 |
79,5 ± 4,1 |
81,0 ± 2,0 |
0,167 |
80,0 ± 4,6 |
80,3 ± 2,3 |
0,794 |
||||||||||||||
|
Weight (kg) |
|
70,2 ± 15,1 |
68,6 ± 9,9 |
0,792 |
72,4 ± 10,9 |
66,0 ± 16,3 |
0,244 |
75,2 ± 10,2 |
65,7 ± 14,6 |
0,079 |
||||||||||||||
|
Size (m) |
|
1,64 ± 0,10 |
1,64 ± 0,08 |
0,982 |
1,65 ± 0,07 |
1,62 ± 0,12 |
0,445 |
1,67 ± 0,07 |
1,61 ± 0,10 |
0,176 |
||||||||||||||
|
Reduceddrugs |
||||||||||||||||||||||||
|
5FU bolus |
No |
6 (66,7) |
3 (33,3) |
|
6 (66,7) |
3 (33,3) |
|
4 (44,4) |
5 (55,6) |
|
||||||||||||||
|
|
Yes |
7 (77,8) |
2 (22,2) |
1,000 |
6 (66,7) |
3 (33,3) |
1,000 |
5 (55,6) |
4 (44,4) |
0,637 |
||||||||||||||
|
5FU infusion |
No |
10 (71,4) |
4 (28,6) |
|
10 (71,4) |
4 (28,6) |
|
8 (57,1) |
6 (42,9) |
|
||||||||||||||
|
|
Yes |
3 (75,0) |
1 (25,0) |
1,000 |
2 (50,0) |
2 (50,0) |
0,569 |
1 (25,0) |
3 (75,0) |
0,257 |
||||||||||||||
|
Oxaliplatin |
No |
2 (66,7) |
1 (33,3) |
|
2 (66,7) |
1 (33,3) |
|
1 (33,3) |
2 (66,7) |
|
||||||||||||||
|
|
Yes |
1 (50,0) |
1 (50,0) |
1,000 |
2 (100,0) |
0 |
1,000 |
1 (50,0) |
1 (50,0) |
0,709 |
||||||||||||||
|
Capecitabin |
No |
2 (66,7) |
1 (33,3) |
|
1 (33,3) |
2 (66,7) |
|
1 (33,3) |
2 (66,7) |
|
||||||||||||||
|
|
Yes |
1 (50,0) |
1 (50,0) |
1,000 |
0 |
2 (100,0) |
1,000 |
0 |
2 (100,0) |
0,361 |
||||||||||||||
|
Irinotecan |
No |
|
|
|
|
|
|
|
|
|
||||||||||||||
|
|
Yes |
2 (66,7) |
1 (33,3) |
|
|
3 (100,0) |
|
|
3 (100,0) |
|
||||||||||||||
|
Bevacizumab |
No |
4 (100,0) |
|
|
4 (100,0) |
0 |
|
4 (100,0) |
0 |
|
||||||||||||||
|
|
Yes |
2 (100,0) |
|
|
1 (50,0) |
1 (50,0) |
0,333 |
1 (50,0) |
1 (50,0) |
0,121 |
||||||||||||||
|
Percentage reduction |
||||||||||||||||||||||||
|
5FU bolus |
|
52,9 ± 23,6 |
37,5 ± 17,7 |
0,384 |
54,2 ± 24,6 |
40,0 ± 17,3 |
0,357 |
60,0 ± 22,4 |
36,3 ± 16,0 |
0,081 |
||||||||||||||
|
5FU infusion |
|
31,7 ± 16,1 |
25,0 |
1,000 |
37,5 ± 17,7 |
22,5 ± 3,5 |
0,221 |
50,0 |
23,3 ± 2,9 |
0,157 |
||||||||||||||
|
Oxaliplatin |
|
50,0 |
25,0 |
0,317 |
37,5 ± 17,7 |
|
|
50,0 |
25,0 |
0,317 |
||||||||||||||
|
Capecitabin |
|
30,0 |
20,0 |
0,317 |
|
25,0 ± 7,1 |
|
|
25,0 ± 7,1 |
|
||||||||||||||
|
Irinotecan |
|
22,5 ± 3,5 |
50,0 |
0,221 |
|
31,7 ± 16,1 |
|
|
31,7 ± 16,1 |
|
||||||||||||||
|
Bevacizumab |
|
100,0 ± 0,0 |
|
|
100,0 |
100,0 |
1,000 |
100,0 |
100,0 |
1,000 |
||||||||||||||
|
Report |
No |
13 (100,0) |
0 |
|
11 (84,6) |
2 (15,4) |
|
11 (84,6) |
2 (15,4) |
|
||||||||||||||
|
|
Yes |
3 (37,5) |
5 (62,5) |
0,003 |
3 (37,5) |
5 (62,5) |
0,056 |
0 |
8 (100,0) |
< 0,001* |
||||||||||||||
|
Number of cures reported |
1 |
2 (50,0) |
2 (50,0) |
|
2 (50,0) |
2 (50,0) |
|
|
4 (100,0) |
|
||||||||||||||
|
|
2 |
0 |
3 (100,0) |
0,429 |
1 (33,3) |
2 (66,7) |
1,000 |
|
3 (100,0) |
|
||||||||||||||
|
Dosage adjustement |
No |
5 (55,6) |
4 (44,4) |
|
1 (11,1) |
8 (88,9) |
|
0 |
9 (100,0) |
|
||||||||||||||
|
aftertoxicity |
Yes |
3 (42,9) |
4 (57,1) |
1,000 |
5 (71,4) |
2 (28,6) |
0,035 |
2 (28,6) |
5 (71,4) |
0,175 |
||||||||||||||
|
Stop aftertoxicity |
No |
4 (50,0) |
4 (50,0) |
|
5 (62,5) |
3 (37,5) |
|
2 (25,0) |
6 (75,0) |
|
||||||||||||||
|
|
Yes |
4 (50,0) |
4 (50,0) |
1,000 |
1 (12,5) |
7 (87,5) |
0,119 |
0 |
8 (100,0) |
0,467 |
||||||||||||||
|
Hospitalization |
No |
13 (76,5) |
4 (23,5) |
|
14 (82,4) |
3 (17,6) |
|
11 (64,7) |
6 (35,3) |
|
||||||||||||||
|
|
Yes |
5 (55,6) |
4 (44,4) |
0,382 |
1 (11,1) |
8 (88,9) |
< 0,001 |
0 |
9 (100,0) |
0,003* |
||||||||||||||
|
Death |
No |
17 (73,9) |
6 (26,1) |
|
14 (60,9) |
9 (39,1) |
|
10 (43,5) |
13 (56,5) |
|
||||||||||||||
|
|
Yes |
1 (33,3) |
2 (66,7) |
0,215 |
1 (33,3) |
2 (66,7) |
0,556 |
1 (33,3) |
2 (66,7) |
1,000 |
||||||||||||||
|
|
|
n (%)/Mean ± SD |
p |
n (%)/Mean ± SD |
p |
n (%)/Mean ± SD |
p |
|
||||||||||||||||
|
|
|
Hematotoxicity |
|
Othertoxicity |
|
Toxicity grade 3-4 |
|
|
||||||||||||||||
|
|
|
No |
Yes |
|
No |
Yes |
|
No |
Yes |
|
|
|||||||||||||
|
CGA |
|
|||||||||||||||||||||||
|
Presence of social support |
No |
0 |
1 (100,0) |
|
0 |
1 (100,0) |
|
0 |
1 (100,0) |
|
|
|||||||||||||
|
|
Yes |
18 (72,0) |
7 (28,0) |
0,308 |
15 (60,0) |
10 (40,0) |
0,423 |
11 (44,0) |
14 (56,0) |
1,000 |
|
|||||||||||||
|
Abnormal ADL |
No |
16 (69,6) |
7 (30,4) |
|
14 (60,9) |
9 (39,1) |
|
11 (47,8) |
12 (52,2) |
|
|
|||||||||||||
|
|
Yes |
2 (66,7) |
1 (33,3) |
1,000 |
1 (33,3) |
2 (66,7) |
0,556 |
0 |
3 (100,0) |
0,239 |
|
|||||||||||||
|
Abnormal IADL |
No |
6 (60,0) |
4 (40,0) |
|
5 (50,0) |
5 (50,0) |
|
4 (40,0) |
6 (60,0) |
|
|
|||||||||||||
|
|
Yes |
12 (75,0) |
4 (25,0) |
0,665 |
10 (62,5) |
6 (37,5) |
0,689 |
7 (43,8) |
9 (56,3) |
1,000 |
|
|||||||||||||
|
Depression |
No |
12 (66,7) |
6 (33,3) |
|
12 (66,7) |
6 (33,3) |
|
9 (50,0) |
9 (50,0) |
|
|
|||||||||||||
|
|
Yes |
6 (75,0) |
2 (25,0) |
1,000 |
3 (37,5) |
5 (62,5) |
0,218 |
2 (25,0) |
6 (75,0) |
0,395 |
|
|||||||||||||
|
Cognitive impairment |
No |
13 (68,4) |
6 (31,6) |
|
11 (57,9) |
8 (42,1) |
|
9 (47,4) |
10 (52,6) |
|
|
|||||||||||||
|
|
Yes |
4 (66,7) |
2 (33,3) |
1,000 |
3 (50,0) |
3 (50,0) |
1,000 |
1 (16,7) |
5 (83,3) |
0,345 |
|
|||||||||||||
|
Visual deficit |
No |
17 (70,8) |
7 (29,2) |
|
14 (58,3) |
10 (41,7) |
|
10 (41,7) |
14 (58,3) |
|
|
|||||||||||||
|
|
Yes |
1 (50,0) |
1 (50,0) |
0,529 |
1 (50,0) |
1 (50,0) |
1,000 |
1 (50,0) |
1 (50,0) |
1,000 |
|
|||||||||||||
|
Hearing deficit |
No |
10 (62,5) |
6 (37,5) |
|
12 (75,0) |
4 (25,0) |
|
8 (50,0) |
8 (50,0) |
|
|
|||||||||||||
|
|
Yes |
8 (80,0) |
2 (20,0) |
0,420 |
3 (30,0) |
7 (70,0) |
0,043 |
3 (30,0) |
7 (70,0) |
0,428 |
|
|||||||||||||
|
Falls |
No |
12 (60,0) |
8 (40,0) |
|
12 (60,0) |
8 (40,0) |
|
8 (40,0) |
12 (60,0) |
|
|
|||||||||||||
|
|
Yes |
3 (100,0) |
0 |
0,526 |
1 (33,3) |
2 (66,7) |
0,560 |
1 (33,3) |
2 (66,7) |
0,825 |
|
|||||||||||||
|
BMI (kg/m2) |
|
26,8 ± 5,0 |
25,3 ± 1,7 |
0,462 |
27,0 ± 4,6 |
25,3 ± 3,8 |
0,488 |
27,8 ± 5,1 |
25,2 ± 3,4 |
0,239 |
|
|||||||||||||
|
Comorbidities |
|
3,35 ± 2,21 |
3,29 ± 1,38 |
0,941 |
3,20 ± 2,14 |
3,56 ± 1,74 |
0,678 |
2,91 ± 2,34 |
3,69 ±1,60 |
0,343 |
|
|||||||||||||
|
Hypertension |
No |
10 (76,9) |
3 (23,1) |
|
6 (46,2) |
7 (53,8) |
|
5 (38,5) |
8 (61,5) |
|
|
|||||||||||||
|
|
Yes |
7 (58,3) |
5 (41,7) |
0,411 |
9 (75,0) |
3 (25,0) |
0,226 |
6 (50,0) |
6 (50,0) |
0,695 |
|
|||||||||||||
|
Cardiovascular |
No |
11 (78,6) |
3 (21,4) |
|
9 (64,3) |
5 (35,7) |
|
8 (57,1) |
6 (42,9) |
|
|
|||||||||||||
|
|
Yes |
5 (50,0) |
5 (50,0) |
0,204 |
6 (60,0) |
4 (40,0) |
1,000 |
3 (30,0) |
7 (70,0) |
0,240 |
|
|||||||||||||
|
Osteoarticular |
No |
10 (71,4) |
4 (28,6) |
|
9 (64,3) |
5 (35,7) |
|
8 (57,1) |
6 (42,9) |
|
|
|||||||||||||
|
|
Yes |
7 (63,6) |
4 (36,4) |
1,000 |
6 (54,5) |
5 (45,5) |
0,697 |
3 (27,3) |
8 (72,7) |
0,227 |
|
|||||||||||||
Table 3: Toxicitypredictive data.
Abbreviations: kg: Kilograms; m: meters; LV5FU: 5-Fluorouracil + Calcium folinate; 5FU: 5-Fluorouracil; CGA: Comprehensive Geriatric Assesment; ADL: Activities of daily Living; IADL: Instrumental Activities of daily Living; BMI: Body Mass Index.
*: statistically significant
DISCUSSION
To our knowledge, this is the first study to investigate the association between evaluation parameters and the reduction of chemotherapy doses, the development of early toxicity of anticancer drugs and the analysis of compliance with treatment recommendations in older patients with CRC who have had a geriatric oncology evaluation. Very few studies have investigated the parameters associated with dose reduction of cancer therapy. In the Aparicio study [19], no geriatric parameters were associated with dose reduction. Only the presence of high alkaline phosphatases was associated with a reduction of at least 33% in intensity dose. Similarly, we did not find in the present study any significant association between geriatric markers or the number of frailty markers and dose reduction despite a high prevalence of these markers in our population (46% had at least 3 abnormal CGA parameters and 74% of patients had at least 1 frailty marker). However, in the study by Farcetet al., [11], the more frailty markers increased, the more treatment recommendations were directed towards modified treatment. However, in the aforementioned study, follow-up on the recommendations was not available. In the study by Kenis et al., [41], where 1967 older patients with cancer had CGA, oncologists were aware of the results of the geriatric assessment at the time of the treatment decision for only two-thirds of patients whereas the treatment decision was only influenced by 25%.Similarly to Kenis ‘study, we founded that treatment decision was poorly influenced despite the results of the assessment were available in the medical file of the patient. As oncologic supportive care already include some of the most frequently recommended geriatric interventions (such as dietician, psychologist or physiotherapists), their implementations are mainly follow and suitable. However, for oncologic decisions, we could assume that there are oncologist’s reluctances to follow a non-cancer specialist suggestion. Moreover, studies that demonstrate the usefulness of the geriatric team recommendations in reducing chemotoxicity are still lacking. Based on the result of our study, we organized a presentation at the weekly oncologist team meeting. Decisions to improve the 'influence’ of the geriatric team evaluation were to systematically make a phone call to each oncologist with an SMS summarizing treatment’s decisions (in addition to the all report integrated in the dpi) and to systematically informed the oncologic support team nurse that an oncologic treatment recommendations was made (in addition to the recommended geriatric interventions). In the present study we did not find any significant association between the appearance of toxicity and frailty markers or geriatric parameters. In the literature, several studies have demonstrated the predictive value of frailty markers in the development of chemotoxicity. Only one study found that grip strength predicted early chemotoxicity [26], the MOST study also confirmed the interest of this marker for predicting one-year chemotoxicity [25]. The presence of frailty markers such as undernutrition or mobility would appear to predict treatment toxicity and risk of death at one year [27,28]. Studies have also analyzed the relationships between CGA components and chemotoxicity. In several studies [19,30,36,42,43], disabilities, reduced mobility, cognitive dysfunction, social difficulties, comorbidities and polypharmacy were also significantly associated with chemotoxicity and comorbidities were associated with unscheduled hospitalizations [44-46]. Although in our population, some parameters were common, such as abnormal IADL score (56.7% of the population), co-morbidities (53.3%) or undernutrition (60%), none were significantly associated with dose reduction and early toxicity. In contrast, the patients of the present study were not very dependent according to the ADL score (10% of patients), and had few cognitive problems (present in only 24.7% of patients) and were therefore not predictive of a dose reduction and toxicity at 3 months. We rather may think that a combination of predictors, including markers of frailty, some domains of CGAs and cancer characteristics, would be more clinically informative to understand the complexity of older patients with cancer, as suggested by several studies [42,43,47]. In the MOST study, 2 simple scores combining patient characteristics with tumor and biological indices were shown to be powerful in predicting severe chemotoxicity and death in older patients with CRC [25].
The indication for adjuvant chemotherapy should be balanced against the life expectancy of the patient whose survival is expected to improve at 5 years. In stage III CRC, adjuvant chemotherapy is recommended. Post-operative chemotherapy with FOLFOX4 or XELOX is the standard treatment. In case of contraindications to oxaliplatin, chemotherapy with LV5FU or capecitabin is available [48]. In cases of metastatic CRC, for older patients in general good condition, the protocols combining 5-FU and oxaliplatin or irinotecan are the same as for young subjects (FOLFOX/FOLFIRI), in combination with targeted therapies such as anti-EGFR (Epidermal Growth Factor Receptor) (depending on KRas status) or anti-VEGF antibodies. For frail patients, capecitabin oral chemotherapy or LV5FU2 chemotherapy is available [49]. In some studies, it has been reported that older patients with cancer tend to receive less aggressive treatment [50,51]. Although the number of patients receiving adjuvant chemotherapy decreases with age, several studies show an interest in adjuvant chemotherapy in the older [52]. Under treatment strongly reduces survival of patients with certain cancers [53,54]. We found that dose reductions were applied for 44.4% of patients for whom standard treatment had been recommended. We also observed that a dose reduction had been applied for only 50% of treatments with a recommendation to adapt chemotherapy. However, in general, it is reported that more than 50% of older patients with advanced cancer have severe toxicity during the first three months of chemotherapy [55]. In our present work, we note the systematic reduction of certain molecules: irinotecan was systematically reduced in chemotherapy protocols, on average by 30%. In the multivariate analysis of Aparicio [19], significant predictive factors for grade 3-4 toxicity and dose reduction of more than 33% were found in the Irinotecanarm compared to the 5FU arm. In two other studies, the treatment regimen of irinotecan monotherapy and the combination of FOLFIRI were considered to be active regimens with increased but manageable first-line toxicity in patients over 70 years of age [56,57].
Our study has several limitations. First, it is a monocentric study, but it is long enough to have a broader view of the practices of different oncologists over the years. However, the geriatric oncology evaluation was performed by the same team of practitioners, so it would be interesting to carry out this work within another team of practitioners. Second, our study had a small number of staff: this is due to the selection on the basis of having been referred for a CGA and not on the overall population. Indeed, the final population of 30 patients is the result of a first step, the referral of patients to a CGA or not during the 1stmultidisciplinary team meeting MDT which decides on the need according to age and health problems present in some patients. However, the high prevalence of frailty markers and geriatric parameters shows that oncologists probably refer patients they consider most vulnerable to chemotherapy. This may explain why oncologists immediately prescribe a dose reduction without taking into account geriatric advice.
The important strengths of this study are the use of validated self-assessment and performance tests, the use of a truly geriatric population (mean age: 79.9 years) with a single targeted cancer type allowing in-depth analysis of chemotherapy protocols.
CONCLUSION
Our study shows that the dose changes of chemotherapy prescribed for CRC treatment are not related to the parameters and conclusions of the geriatric oncology evaluation. Some parameters such as postponement of treatment and the presence of unscheduled hospitalization are associated with early toxicity of treatment. Geriatric oncology should be considered as a sharing of knowledge between specialties, not as a new independent specialty. However, our study clearly shows that this geriatric oncology evaluation, although requested by oncologists, does not explain the decisions made for the prescription of chemotherapy. No decision tree defining the modalities of chemotherapy administration for CRC in older patients in terms of dose/intensity modification is currently available.
ACKNOWLEDGEMENT
The authors thank the oncologist’s and pharmacy’s team from their help in collecting data.
AUTHORS’ DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
All authors report no conflicts of interest.
FUNDING
This work was supported by grant from ASRO (Association Sud Est de Recherche en Oncogeriatrie).
AUTHORS CONTRIBUTION
Study Concepts: M Chermette, L Diaz, J Gigout, F Retornaz,
Study Design: M Chermette, L Diaz, F Retornaz,
Data acquisition :A Farcet, Y Rinaldi, J Gigout, N Barriere, C Julien, P Dominici, F Retornaz
Quality control of data and algorithms: M Chermette, M Grino, F Retornaz,
Data analysis and interpretation: M Chermette, M Grino, F Retornaz,
Statistical analysis: M Chermette, M Grino, F Retornaz,
Manuscript preparation: M Chermette, M Grino, F Retornaz,
Manuscript editing: M Chermette, M Grino, F Retornaz,
Manuscript review: M Chermette, L Diaz, M Grino, F Retornaz
REFERENCES
- Institut National du Cancer, I. N. du C. (2018) Epidemiologie des cancers chez les patients de 65 ans et plus.
- Netgen (2009) Cancer colorectal et sujets ages: Y a-t-il une specificite de prise en charge ? Revue Medicale Suisse.
- Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, et al. (2001) A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 345: 1091-1097.
- Moth EB, Vardy J, Blinman P (2016) Decision-making in geriatric oncology: systemic treatment considerations for older adults with colon cancer. Expert review of gastroenterology & hepatology 10: 1321-1340.
- Khan S, Alibay TA, Merad M, DiPalma M, Raynard B, et al. (2016) Détection et évaluation de la dénutrition en oncologie: quels sont les outils, pour quel type de cancer et dans quels buts? Bulletin du Cancer 103: 776-785.
- Lacau St Guily J, Bouvard É, Raynard B, Goldwasser F, Maget B, et al. (2018). NutriCancer: A French observational multicentre cross-sectional study of malnutrition in elderly patients with cancer. J Geriatr Oncol 9: 74-80.
- Mislang AR, Di Donato S, Hubbard J, Krishna L, Mottino G, et al. (2018) Nutritional management of older adults with gastrointestinal cancers: An International Society of Geriatric Oncology (SIOG) review paper. J Geriatr Oncol 9: 382-392.
- Park SE, Hwang IG, Choi CH, Kang H, Kim BG, et al. (2018) Sarcopenia is poor prognostic factor in older patients with locally advanced rectal cancer who received preoperative or postoperative chemoradiotherapy. Medicine 97: 13363.
- Barret M, Berthaud C, Taieb J (2014) La sarcopénie: un concept d’importance croissante dans la prise en charge du cancer colorectal. La Presse Medicale 43: 628-632.
- Handforth C, Clegg A, Young C, Simpkins S, Seymour MT, et al. (2015) The prevalence and outcomes of frailty in older cancer patients: A systematic review. Ann Oncol 26: 1091-1101.
- Farcet A, de Decker L, Pauly V, Rousseau F, Bergman H, et al. (2016) Frailty Markers and Treatment Decisions in Patients Seen in Oncogeriatric Clinics: Results from the ASRO Pilot Study. PLoS One 11: 0149732.
- Extermann M, Aapro M, Bernabei R, Cohen HJ, Droz JP, et al. (2005) Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 55: 241-252.
- Hassett MJ, Rao SR, Brozovic S, Stahl JE, Schwartz JH, et al. (2011) Chemotherapy-related hospitalization among community cancer center patients. Oncologist 16: 378-387.
- Zauderer M, Patil S, Hurria A (2009) Feasibility and toxicity of dose-dense adjuvant chemotherapy in older women with breast cancer. Breast Cancer Res Treat 117.
- Grønberg BH, Sundstrøm S, Kaasa S, Bremnes RM, Fløtten O, et al. (2010) Influence of comorbidity on survival, toxicity and health-related quality of life in patients with advanced non-small-cell lung cancer receiving platinum-doublet chemotherapy. Eur J Cancer 46: 2225-2234.
- Neugut AI, Matasar M, Wang X, McBride R, Jacobson JS, et al. (2006) Duration of Adjuvant Chemotherapy for Colon Cancer and Survival Among the Elderly. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, 24: 2368-2375.
- Puts MT, Santos B, Hardt J, Monette J, Girre V, et al. (2014) An update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann Oncol 25: 307-315.
- Hamaker ME, Vos AG, Smorenburg CH, De Rooij SE, van Munster BC (2012) The Value of Geriatric Assessments in Predicting Treatment Tolerance and All-Cause Mortality in Older Patients With Cancer. Oncologist 17: 1439-1449.
- Aparicio T, Jouve JL, Teillet L, Gargot D, Subtil F, et al. (2013) Geriatric factors predict chemotherapy feasibility: ancillary results of FFCD 2001-02 phase III study in first-line chemotherapy for metastatic colorectal cancer in elderly patients. J Clin Oncol 31:1464-1470.
- Kalsi T, Babic-Illman G, Ross PJ, Maisey NR, Hughes S, et al. (2015) The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br J Cancer 112: 1435-1444.
- Chaïbi P, Magné N, Breton S, Chebib A, Watson S, et al. (2011) Influence of geriatric consultation with comprehensive geriatric assessment on final therapeutic decision in elderly cancer patients. Crit Rev Oncol Hematol 79: 302-307.
- Girre V, Falcou MC, Gisselbrecht M, Gridel G, Mosseri V, et al. (2008) Does a geriatric oncology consultation modify the cancer treatment plan for elderly patients? J Gerontol A Biol Sci Med Sci 63: 724-730.
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, et al. (2001) Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56: 146-156.
- Retornaz F, Monette J, Batist G, Monette M, Sourial N, et al. (2008) Usefulness of Frailty Markers in the Assessment of the Health and Functional Status of Older Cancer Patients Referred for Chemotherapy: A Pilot Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 63: 518-522.
- Puts MT, Monette J, Girre V, Pepe C, Monette M, et al. (2011) Are frailty markers useful for predicting treatment toxicity and mortality in older newly diagnosed cancer patients? Results from a prospective pilot study. Crit Rev Oncol Hematol 78: 138-149.
- Retornaz F, Guillem O, Rousseau F, Morvan F, Rinaldi Y, et al. (2018) Predicting Chemotherapy Toxicity and Death in Older Adults with Colon Cancer: Results of MOST Study. Oncologist 25: 85-93.
- Soubeyran P, Fonck M, Blanc-Bisson C, Blanc JF, Ceccaldi J, et al. (2012) Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol 30: 1829-1834.
- Boulahssass R, Gonfrier S, Ferrero JM, Sanchez M, Mari V, et al. (2018) Predicting early death in older adults with cancer. Eur J Cancer 100: 65-74.
- Katz S (1983) Assessing self-maintenance: Activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc 31: 721-727.
- Fillenbaum GG, Smyer MA (1981) The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol 36: 428-434.
- Yesavage JA, Sheikh JI (1986) 9/Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontologist: The Journal of Aging and Mental Health 5: 165-173.
- Borson S, Scanlan JM, Watanabe J, Tu S-P, Lessig M (2005) Simplifying Detection of Cognitive Impairment: Comparison of the Mini-Cog and Mini-Mental State Examination in a Multiethnic Sample. Journal of the American Geriatrics Society 53: 871- 874.
- Cahn DA, Salmon DP, Monsch AU, Butters N, Wiederholt WC, et al. (1996) Screening for dementia of the alzheimer type in the community: The utility of the Clock Drawing Test. Arch Clin Neuropsychol 11: 529-539.
- Cook Z, Kirk S, Lawrenson S, Sandford S (2005) Use of BMI in the assessment of undernutrition in older subjects: Reflecting on practice. Proc Nutr Soc 64: 313-317.
- Retornaz F, Seux V, Sourial N, Braud AC, Monette J, et al. (2007) Comparison of the health and functional status between older inpatients with and without cancer admitted to a geriatric/internal medicine unit. J Gerontol A Biol Sci Med Sci 62: 917-922.
- Falandry C, Weber B, Savoye AM, Tinquaut F, Tredan O, et al. (2013) Development of a geriatric vulnerability score in elderly patients with advanced ovarian cancer treated with first-line carboplatin: A GINECO prospective trial. Ann Oncol 24: 2808-2813.
- Davis HS, MacPherson K, Merry HR, Wentzel C, Rockwood K (2001) Reliability and Validity of Questions About Exercise in the Canadian Study of Health and Aging. International Psychogeriatrics 13: 177-182.
- Podsiadlo D, Richardson S (1991) The timed "Up & Go": A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39: 142-148.
- Vellas BJ, Wayne SJ, Romero L, Baumgartner RN, Rubenstein LZ, et al. (1997) One-Leg Balance Is an Important Predictor of Injurious Falls in Older Persons. Journal of the American Geriatrics Society 45: 735-738.
- Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, et al. (2006) Phenotype of frailty: Characterization in the women's health and aging studies. The J Gerontol A Biol Sci Med Sci 61: 262-266.
- Kenis C, Bron D, Libert Y, Decoster L, Van Puyvelde K, et al. (2013) Relevance of a systematic geriatric screening and assessment in older patients with cancer: Results of a prospective multicentric study. Ann Oncol 24: 1306-1312.
- Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, et al. (2011) Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol 29: 3457-3465.
- Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, et al. (2012) Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 118: 3377-3386.
- Terret C (2008) How and why to perform a geriatric assessment in clinical practice. Annals of oncology 19: 300-303.
- Townsley C, Pond GR, Peloza B, Kok J, Naidoo K, et al. (2005) Analysis of treatment practices for elderly cancer patients in Ontario, Canada. J Clin Oncol 23: 3802-3810.
- Fung C, Dale W, Mohile SG (2014) Prostate cancer in the elderly patient. J Clin Oncol 32: 2523-2530.
- Sourial N, Bergman H, Karunananthan S, Wolfson C, Payette H, et al. (2013) Implementing frailty into clinical practice: A cautionary tale. J Gerontol A Biol Sci Med Sci 68: 1505-1511.
- Phelip JM, Benhaim L, Bouche O, Christou N, Desolneux G, et al. (2018) Cancer colorectal metastatique. Thesaurus National de Cancerologie Digestive.
- Francois E, Berdah J-F, Chamorey E, Lesbats G, Teissier E, et al. (2008) Use of the Folinic acid/5-Fluorouracil/Irinotecan (FOLFIRI 1) regimen in elderly patients as a first-line treatment for metastatic colorectal cancer: A Phase II study. Cancer Chemotherapy and Pharmacology 62: 931- 936.
- Giordano SH, Hortobagyi GN, Kau SW, Theriault RL, Bondy ML, et al. (2005) Breast cancer treatment guidelines in older women. J Clin Oncol 23: 783-791.
- Hébert-Croteau N, Brisson J, Latreille J, Rivard M, Abdelaziz N, et al. (2004) Compliance with consensus recommendations for systemic therapy is associated with improved survival of women with node-negative breast cancer. J Clin Oncol 22: 3685-3693.
- Iwashyna TJ, Lamont EB (2002) Effectiveness of adjuvant fluorouracil in clinical practice: a population-based cohort study of elderly patients with stage III colon cancer. J Clin Oncol 20: 3992-3998.
- Bouchardy C, Rapiti E, Fioretta G, Laissue P, Neyroud-Caspar I, et al. (2003) Undertreatment Strongly Decreases Prognosis of Breast Cancer in Elderly Women. Journal of Clinical Oncology 21: 3580-3587.
- Goodwin JS, Samet JM, Hunt WC (1996) Determinants of Survival in Older Cancer Patients. Journal of the National Cancer Institute 88: 1031-1038.
- Hurria A, Mohile S, Gajra A, Klepin H, Muss H, et al. (2016) Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol 34: 2366-2371.
- Chau I, Norman AR, Cunningham D, Waters JS, Topham C, et al. (2004) Elderly patients with fluoropyrimidine and thymidylate synthase inhibitor-resistant advanced colorectal cancer derive similar benefit without excessive toxicity when treated with irinotecan monotherapy. Br J Cancer 91: 1453-1458.
- Souglakos J, Pallis A, Kakolyri S, Mavroudis D, Androulakis N, et al. (2005) Combination of Irinotecan (CPT-11) plus 5-Fluorouracil and Leucovorin (FOLFIRI Regimen) as First Line Treatment for Elderly Patients with Metastatic Colorectal Cancer: A Phase II Oncology 69: 384-390.
Citation: Chermette M, Diaz L, Farcet A, Rinaldi Y, Gigout J, et al. (2020) Do the Oncologists Systematically follow Treatment Recommendations and Protocol Adaptations Suggested by prior Comprehensive Geriatric Assessment in Older Patients with Colorectal Cancer? J Gerontol Geriatr Med 6: 078.
Copyright: © 2020 Chermette M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

