
Does a Perfect and Simple Peritoneal Dialysis Catheter Exist but we are Unaware of? Insights about the Current Guidelines and Literature Review
*Corresponding Author(s):
Mohamed A NasreldinKing Fahad Hospital Of The University, Department Of Nephrology, Alkhobar, Saudi Arabia
Tel:+966 563577205,
Email:nephrologistm@yahoo.com / m6042@yahoo.com.au
Abstract
Background
Peritoneal dialysis is an effective treatment for end-stage renal disease patients who require renal replacement therapy but unfortunately the use of it is still underutilized worldwide despite its several advantages over hemodialysis and cost efficiency for heath policies. Modalities include chronic ambulatory peritoneal dialysis and Automated Peritoneal Dialysis (APD) are becoming more popular nowadays and the awareness about the use of PD has attracted the attention of both nephrology physicians and patients because of the current covid-19 pandemic where frequent hospital exposures like in hemodialysis can increase the risk of being infected. The aim of this review is to look at the current literature and guidelines to find what is the best PD catheter is recommended to be used because a successful peritoneal dialysis depends greatly on a successful insertion of a well-designed and functional PD catheter. This review focuses on the innovated three cuffs Saudi PD catheter that most of the nephrology physicians worldwide are unaware of its existence.
Methods
This article will compare in general the conclusions of the current recommendations and ISPD (international society of peritoneal dialysis) guidelines of which PD catheter to choose and the results of a three year experience of using the innovated three cuffs Saudi catheter which included 153, three-cuff PD catheter insertions in 150 incident PD patients. The study was carried out in our PD center and extended from December 2012 till January 2016 with a mean follow-up period of 15 months. All patients used Automated Peritoneal Dialysis (APD). The analysis included survival rate, functionality and complications of the innovated catheter.
Results
Four patients had inguinal hernia and 1 had omental wrapping. Catheter migration, however, was 0.0% with our 3-cuff PD catheter using our new technique. A total of 25 catheters had to be removed. Indications for catheter removal were successful transplantation (n=7), hernia (n=4), omental capture (n=1), ultrafiltration failure (n=2), Psychological causes (n=4), abdominal surgery (n=1), severe tunnel infection (n=3), and unresolved peritonitis (n=3). The rate of peritonitis was as low as 0.106 per patient-year equivalent to 1 episode of peritonitis per 112 patient-months. At the end of the study, catheter survival was 91.3%.
Conclusion
When compared with the results conclusion of other studies on which depended the 2019 ISPD guidelines for creating and maintaining optimal PD access, we find that the low entry-site of the innovated PD catheter apparently prevents catheter migration and the 3-cuffs most likely help protect against peritonitis. These findings are more than enough to include this catheter in the future in the ISPD guidelines.
Keywords
Catheter survival; ISPD catheter guidelines; Migration; Peritoneal dialysis; Peritonitis; Three-cuff PD catheter
INTRODUCTION
The use of different forms of peritoneal dialysis such as continuous cyclical peritoneal dialysis and automated peritoneal dialysis has gained popularity in the recent years among nephrologists and patients in both developed and developing countries, though the use of peritoneal dialysis in general when compared to hemodialysis is still underutilized due to several factors such as health policy, lack of experience, lack of awareness of this modality among end stage renal disease patients, medical insurance preferences and etc. The only absolute contraindication to select peritoneal dialysis is lack of a functional peritoneal membrane. Therefore, almost all barriers are relative, depending upon the motivation of the patient and the clinical experience of the physician and the dialysis center. Initiating Peritoneal dialysis has several benefits over hemodialysis when selected as the first choice modality for feasible patients. These benefits include but not limited to Fewer negative side effects such as nausea, vomiting, cramping, and weight gain than with hemodialysis. Provides continuous treatment, which acts more like natural kidneys, avoid vascular access, blood borne infections, time freedom and most importantly the survival rate for patients treated with Peritoneal dialysis is now equivalent to that with in-center hemodialysis. Notably, tremendous efforts and progress has been made in developing interventions that substantially reduce the risk of PD-related complications such as peritonitis, exit site infections, peritoneal membrane failure, catheter migration and malfunction.
Unfortunately, the improvement in peritoneal dialysis programs have been unequal among individual centers, primarily because of unequal clinical application of knowledge gained from researches. Thus, there are lots of areas in need of innovation as our PD centers continue to strive to improve the health and outcomes of patients treated with PD. When a patient with end stage renal disease is evaluated and selected to initiate peritoneal dialysis, the first step will be placing an abdominal peritoneal dialysis catheter (PD catheter) which is the topic of my article.
Several kinds of PD catheters are available for chronic Peritoneal Dialysis (PD) as shown in the figure 1 below. The double-cuff straight Tenckhoff catheter, a silicone catheter with a straight intra-abdominal portion, is the most used [1]. The Missouri Swan Neck catheter and the Toronto Western catheter have also been used extensively [2]. All most all available catheters are made of silicone. Although polyurethane catheters are also available, there is limited long term experience with these catheters for PD anyway. Regardless of type, overall catheter survival is approximately 88 percent at one year, with removal rates of 15 percent per year. This information represents the current knowledge at the present time about the available and most used PD catheters.
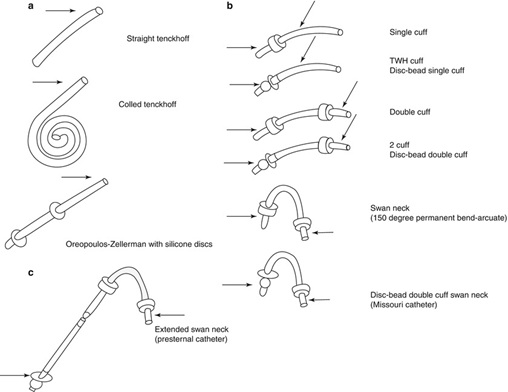 Figure 1: Several kinds of PD catheters.
Figure 1: Several kinds of PD catheters.
For simple understanding one can classify the types of PD catheters into two main types:
- Straight Tenkhoff catheter which is a simple catheter to insert by using various techniques, but it has a relative high risk of migration.
- Coiled Tenkhoff catheter which is the most popular one nowadays because it has a slightly reduced risk of migration and can also be inserted by a variety of techniques, but migration of its coiled end can cause severe abdominal pain.
How to choose a PD catheter for your patient
In today's market PD catheters vary by the design of their intra-abdominal segment (straight versus coiled), subcutaneous configuration (straight versus swan neck), number of cuffs (single versus double), tip (weighted versus not weighted), as well as the material from which they are manufactured (silicone versus polyurethane). The use of each one of these depends upon the preference and experience of the physician inserting the catheter. Most physicians prefer the double-cuff swan neck (unweighted) Tenckhoff catheter with a coiled intra-abdominal segment and of course this preference is based upon their clinical experience using this catheter. double-cuff catheters with either a straight or swan neck subcutaneous segment and a straight intra-abdominal segment may be reasonable alternatives [3].
the International Society of Peritoneal Dialysis (ISPD) [4], peritonitis recommendations stated that there is no specific catheter is definitively better than the standard silicone Tenckhoff catheter for the prevention of peritonitis [5]. In 2019 ISPD guidelines for creating and maintaining optimal PD access recommend silicone double-cuffed catheters of various types, including the standard Tenckhoff catheter [6]. To conclude this part, it is worth to mention a survey of United States and Canadian nephrologists found all most universal use of double-cuff catheters with close even distribution between preformed bend and straight-cuff segments.
Literature review of currently used PD catheters peculiarities
A 2019 Cochrane meta-analysis demonstrated that the selection between straight and coiled catheters made very little or no difference in terms of the risk of peritonitis, exit-site infection, catheter failure, or patient death [7]. The use of double-cuff catheters over single-cuff catheters. Double-cuff catheters have the fewer complications, a longer time before first peritonitis episode, and longer survival [8]. A catheter design in which the tip is weighted with 12 grams of tungsten known as self-locating catheter may reduce the incidence of catheter migration by keeping the catheter tip firmly in the pelvis [9]. This catheter is not widely used because of its complicated insertion despite the facts that observational studies revealed that self-locating catheters tend to have lower rates of migration, flow problems, shift to hemodialysis, peritonitis, exit-site infections, cuff extrusions, obstruction, and leak compared with conventional catheters [10]. A meta-analysis conducted in 2014showed no difference in catheter migration, leakage, removal, dysfunction, and one- and two-year survival rates between the two types of catheters [11]. As anyone can see up to this point of discussion there were no significant differences between the commonly used catheters in terms of major faced problems and challenges as well as there is no consensus in the current literature concerning the superiority of one particular catheter design and length over the other to prevent or reduce in particular catheter migration.
When a clinician decides to place a catheter there are several factors need attention namely the location of the exit site, size of the exit site, use of prophylactic antibiotics, implantation technique, postoperative care of the catheter, and temporal needs for dialysis. From my point of view the best clinician who can take all these aspects into consideration is a nephrologist rather than a surgeon or intervention radiologist because of their ability to manage most of the complications. The other reasons for this are beyond the scope of this article. However Published data on PD catheter placement by nephrologists does not show a higher incidence of complications than with those placed by surgeons.
To end this part of review we may say that up to this date there are no specific recommendations or consensus from the ISPD or other bodies regarding of what is the best or highly recommended catheter to use in the absence of strong evidence to favor one catheter over the other. This fact has been remaining valid for the last 20 years.
The new three cuffs forgotten PD catheter
The two cuffs Tenkhoff catheter was completely modified and innovated by my colleague professor Abdullah Alhwiesh, at the university of Dammam in Saudi Arabia in 2016. I would like to share our experience with this catheter hoping to expand its use in the future all over the world and to find its deserved place in the current guidelines and recommendation and close the gap of existing knowledge in regard to this matter.
What is the three cuffs Saudi PD catheter?
The Saudi PD catheter is silicone-rubber and about 57cm in lengths. The first cuff is located close to the coiled part as shown in the figure 2A below.
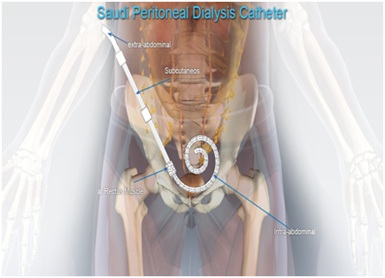 Figure 2A: The first cuff is located close to the coiled.
Figure 2A: The first cuff is located close to the coiled.
As one can see the first cuff is located near the coiled part about 2cm meaning that only the functional part of the catheter is placed in the peritoneal cavity. The second cuff is 10cm from the first cuff and third cuff is 5cm from the second cuff. Both the second and third cuff are located subcutaneously as shown in the figure 2B below.
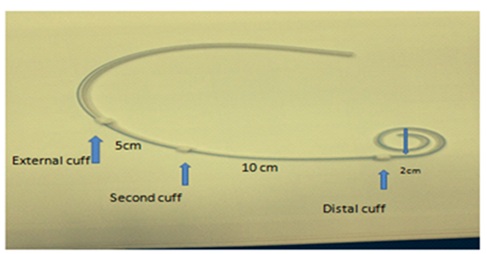 Figure 2B: The second cuff is 10cm from the first cuff and third cuff is 5cm from the second cuff.
Figure 2B: The second cuff is 10cm from the first cuff and third cuff is 5cm from the second cuff.
Method of insertion
The procedure was carried out with patient in the supine position under general anesthesia using aseptic precautions. A verses needle was used to create pneumo-peritoneum at pressure of 10-12mmHg. A 5-mm port was inserted in right hypochondrial region at midclavicular line, 2cm below the costal margin for laparoscopic camera (30degree). Diagnostic laparoscopy was then performed to rule out adhesions or herniations (Figure 3 (A,B)). The operating table was then placed in about 30degree trend elenburg position. Under direct vision, the three-cuff Saudi catheter was passed caudally through the pull-apart sheath over a 90cm stylet into the peritoneal cavity. Above the pubis the tip of the catheter was placed in the true pelvis (towards the urinary bladder), with the distal cuff in the rectus sheath before removing the stylet. The distal cuff of the catheter was secured with purse-string suture on the fascia anterior to the rectus muscle by using 2/0 absorbable vicryl stitches. The tip of catheter was in the pouch of Douglas and the rectovesical pouch in female and male respectively. The pull-apart sheath was removed, leaving the catheter in the peritoneal cavity (Figure 3 (B,C)). Following that, a subcutaneous tunnel was created for the catheter with selection of a midway point at the umbilical-crestal line to be the output of the catheter. The end of the catheter attached to the stylet was advanced into the tunnel and pulled out from the above-mentioned point; the second cuff about 10 cm from the distal one and the proximal cuff 2cm from the exit site. Figure 3 and E illustrates the final position of the 3-cuff PD catheter. The function of the catheter was checked by flushing normal saline to rule out kinking or obstruction. The skin incisions of the camera port and entrance were sutured. Xylocaine with adrenaline diluted in normal saline was injected in the incision site and in the tunnel space as demonstrated in the pictures below.
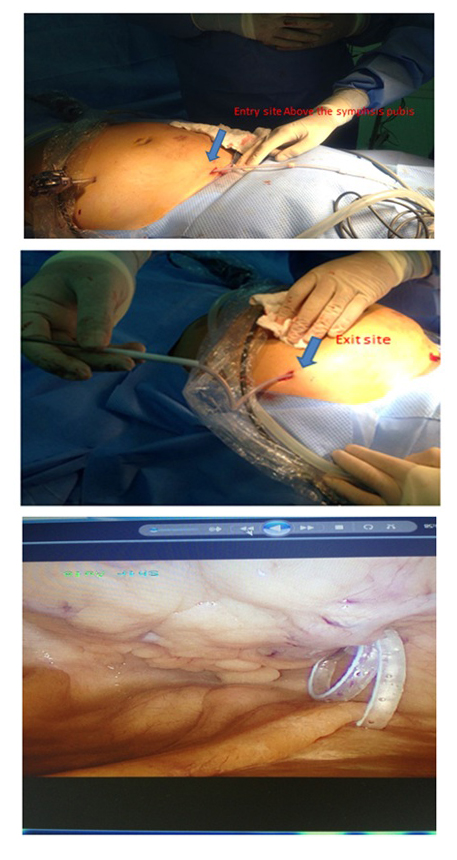 Figure 3: Diagnostic laparoscopy was then performed to rule out adhesions or herniations.
Figure 3: Diagnostic laparoscopy was then performed to rule out adhesions or herniations.
Pouch douglas
Pelvic X-ray showing the Saudi catheter in position (Figure 4).
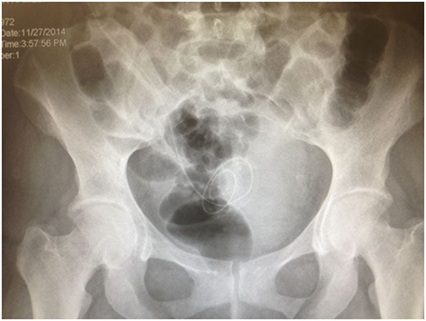 Figure 4: Pelvic X-ray showing the Saudi catheter in position.
Figure 4: Pelvic X-ray showing the Saudi catheter in position.
Let us compare between the modified three cuffs Saudi catheter and the most commonly used and popular the two cuffs classical Tenkhoff catheter in the illustrations below (Figure 5).
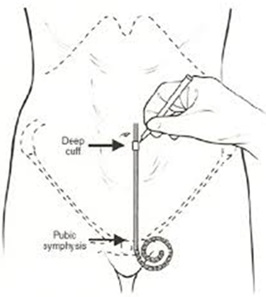 Figure 5: Classical Tenkhoff catheter.
Figure 5: Classical Tenkhoff catheter.
Notice the distance between the functional coiled part and the first cuff. Thanks to this unnecessary and non, functional part the catheter may migrate (Figure 6).
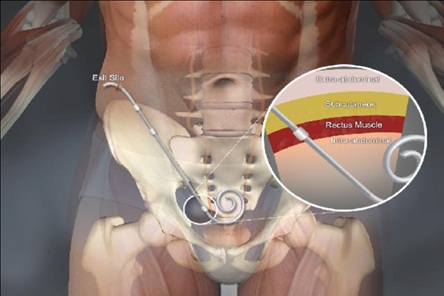 Figure 6: PD catheter function.
Figure 6: PD catheter function.
Above is the three cuffs Saudi catheter as one can notice the major two steps here. The first step was the innovation of a three cuffs catheter with the first cuff only 2cm from the coiled part as compared to the classical Tenkhoff catheter meaning that the unnecessary and nonfunctional part is significantly reduced so it is basically impossible for this catheter to migrate and the second step was the low entry technique. Both steps help maximize PD catheter function as we will see later.
Experience with the three cuffs Saudi PD catheter
A three years, experience of using this innovated catheter at our PD center was published in 2017 [12]. The aim was to assess a 3-year experience with the new, three-cuff Peritoneal Dialysis (PD) catheter with the low-entry technique and to study its effect on infectious and non-infectious complications as well as its impact on catheter survival.
METHODS
This was an observational study which had been carried out at a university hospital over 3 years. The study involved 153, three-cuff PD catheter insertions in 150 incident PD patients. The study was carried out in our PD center and extended from December, 2012 till January 2016 with a mean follow-up period of 15 months. All patients used Automated Peritoneal Dialysis (APD). Throughout the study, we analyzed survival rate, function and complications profile of our innovated catheter. The table 1, below shows the demographic characteristics of patients.
|
Age, Years (mean±SD) |
52.2±18.7 |
|
Gender (F/M), n (%) |
80(53.3)/70(46.7) |
|
DM, n (%) |
61(40.7) |
|
HNT, n (%) |
88(58.7) |
|
BMI, mean |
26.8±2.5 |
|
Total APD, mean (months) |
15.4±5.8 |
|
GFR, ml/min (mean) |
8.7±2.0 |
|
Serum BUN, mmol/L (mean) |
28.4±11.5 |
|
Serum Cr, µmol/L (mean) |
838.5±31.3 |
|
Serum albumin, g/dl (mean) |
3.6±1.4 |
|
Hgb, g/dl (mean) |
9.1±1.8 |
|
Primary renal disease, n (%) |
|
|
Diabetic nephropathy |
47 (31.3) |
|
Glomerulonephritis |
28 (18.7) |
|
Hypertensive renal disease |
24 (16.0) |
|
Interstitial nephritis |
15 (10.0) |
|
Systemic disease |
14 (9.3) |
|
Hereditary renal disease |
8 (5.3) |
|
Unknown |
14 (9.3) |
Table 1: Demographic characteristics of patients.
Note: DM: Diabetes Mellitus; HNT: Hypertension; BMI: Body Mass Index; GFR: Glomerular Filtration Rate; BUN: Blood Urea Nitrogen; Cr: Creatinine; Hgb: Hemoglobin.
RESULTS
Four patients had inguinal hernia and 1 had omental wrapping. Catheter migration, however, was 0.0% with our 3-cuff PD catheter using our new technique. A total of 25 catheters had to be removed. Indications for catheter removal were successful transplantation (n=7), hernia (n=4), omental capture (n=1), ultrafiltration failure n=2), Psychological causes (n=4), abdominal surgery (n=1), severe tunnel infection (n=3), and unresolved peritonitis (n=3). The rate of peritonitis was as low as 0.106 per patient-year equivalent to 1 episode of peritonitis per 112 patient-months. At the end of the study, catheter survival was 91.3%. The table below show the percentage of each complication. The most interesting fact in this table and as expected the rate of catheter migration was zero and the credits here go the new catheter design that helped to solve this problem completely. The results are shown in the following tables 2,3 and figure 7.
|
Complication |
N (%) |
|
Bowel perforation |
0 (0.0) |
|
Hemorrhage* |
0 (0.0) |
|
Ultrafiltration failure |
2 (1.3) |
|
Omental wrapping |
1 (0.7) |
|
Catheter migration |
0 (0) |
|
Early leakage** |
10 (6.7) |
|
Late leakage |
3 (2.0) |
|
Transient obstruction |
6 (4.0) |
|
Catheter replacement |
3 (2.0) |
Table 2: Percentage of each complication.
|
Successful kidney transplant |
7 (4.7%) |
|
Hernia |
4 (2.7%) |
|
Ultrafiltration failure |
2 (1.3%) |
|
Omental wrapping |
1 (0.7%) |
|
Psychological |
4 (2.7%) |
|
Abdominal surgery |
1 (0.7%) |
|
Severe tunnel infection |
3 (2.0%) |
|
Unresolved peritonitis |
3 (2.0%) |
|
Total number |
25 (16.8%) |
|
Technical survival* |
137 (91.3%) |
Table 3: Causes of catheter removal during the study period.
Note: *Technical survival after excluding catheter removal because of kidney transplant, abdominal surgery and psychological causes.
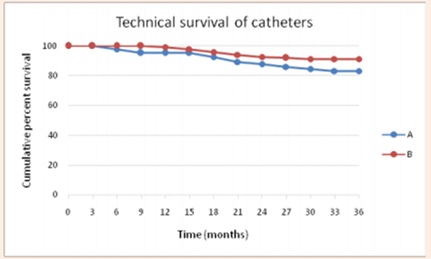 Figure 7: Technical survival of the 3-cuff PD catheter. A: Survival after exclusion of transplantation, abdominal surgery and psychological causes; B: Overall technical survival.
Figure 7: Technical survival of the 3-cuff PD catheter. A: Survival after exclusion of transplantation, abdominal surgery and psychological causes; B: Overall technical survival.
This study showed that patients who underwent the innovated catheter placement had fewer episodes of peritonitis, tunnel infection, cuff extrusion, catheter malfunction, obstruction, and leakage and without a single case of catheter migration or displacement. These factors had positive and significant impacts on catheter survival as proved by our long-term study. In our opinion, our results are logical consequence of maintaining the correct position of the catheter tip in the Douglas cavity. If the tip remains in the bottom of this cavity, catheter displacement and cuff extrusion are unlikely, tunnel infection decreases, good catheter function is conserved (omentum is absent in the Douglas cavity), and therefore episodes of peritonitis subsequently decrease. We believe as I mentioned in the beginning of this article that sharing experience between PD centers is vital for development of this underutilized service globally. A randomized controlled multi center studies in the future may be needed to see this catheter in the guidelines with a strong evidence based recommendation which personally I think it can achieve it, thanks to the reflective thinking of professor Alhwiesh [13-52].
CONCLUSION
The innovated three cuffs Saudi PD catheter is safe and easy to insert and remove. PD catheter migration is still a challenging burden associated with high rates of hospitalization, high cost and shifting to hemodialysis. PD catheter migration has been a real problem since the design of the first PD catheter and all attempts made to eliminate it either had failed or resulted in unwanted complications. Thus, the three cuffs PD catheter must find its way in the current guidelines among the other existing catheters to help more ESRD patients on PD not to have the risk of catheter migration and nephrologists to address other issues in their PD patients than PD catheter migration.
REFERENCES
- Cruz C (1992) Clinical experience with a new peritoneal dialysis access device. In: Current Concepts in Peritoneal Dialysis. Ota K, Maher J (eds.). Elsevier, Amsterdam, The Netherlands.
- Esson ML, Quinn MJ, Hudson EL, Teitelbaum I (2000) Subcutaneously tunnel led peritoneal dialysis catheters with delayed externalization: Long-term follow-up. Adv Perit Dial 16: 123-128.
- Eklund BH (1995) Surgical implantation of CAPD catheters: Presentation of midline incision-lateral placement method and a review of 110 procedures. Nephrol Dial Transplant 10: 386-390.
- Li PKT, Szeto CC, Piraino B, de Arteaga J, Fan S, et al. (2016) ISPD peritonitis recommendations: 2016 update on prevention and treatment. International Society for Peritoneal Dialysis 38: 481-508.
- Bay WH, Cerilli GJ, Perrine V, Powell S, Erlich L (1983) Analysis of a new technique to stabilize the chronicperitoneal dialysis catheter. Am J Kidney Dis 3: 133-135.
- Singh N, Davidson I, Minhajuddin A, Gieser S, Nurenberg M, et al. (2010) Risk factors associated with peritoneal dialysiscatheter survival: A 9-year single-center study in 315 patients. J Vasc Access 11: 316-322.
- Handt AE, Ash SR (1984) Longevity of Tenchoff catheters placed by the VITEC peritoneoscopic technique. Perspect Perit Dial 2: 30-33.
- Flanigan M, Gokal R (2005) Peritoneal catheters and exit-sitepractices toward optimum peritoneal access: A review of current developments. Perit Dial Int 25: 132-139.
- Cavagna R, Tessarin C, Tarroni G, Casol D, De SilvestroL, et al. (1999) The self-locating catheter: Clinical evaluation and comparison with the Tenckhoff catheter. Perit Dial Int 19: 540-543.
- Rodriguez-Carmona A, Garcia-Falcon T, Perez-Fontan M, Bouza P, Adeva M, et al. (1996) Survival on chronic peritoneal dialysis: Have results improved in the 1990s? Perit Dial Int 1: 410-413.
- Oreopoulos DG, Izatt S, Zellerman G, Karanicolas S, Mathews RE (1976) A prospective study of the effectiveness of three permanent peritoneal catheters. Proc Clin Dial Transplant Forum 6: 96-100.
- Ishizaki M, Suzuki K, Kurosawa K, Shishido Y, Takahashi H (1988) Swan neck Sendai catheter: A modification of the swan neck Tenckhoff catheter. Perit Dial Int 8: 221-225.
- Twardowski ZJ, Prowant BF, Nichols WK, Nolph KD, Khanna R (1992) Six years' experience with swan neck catheter. Perit Dial Int 12: 384-389.
- Sieniawska M, Roszkowaska-Blaim M, Warchol S (1993) Preliminary results with the swan neck presternal catheter for CAPD in children. Adv Perit Dial 9: 321-324.
- Valli A, Crescimannu U, Midri R, Khaled Arw, Peter Riegler, et al. (1983) 18 Months’ experience with a new (Valli) catheter for peritoneal dialysis. Perit Dial Bull 3: 22-24.
- Ash SR, Sutton JM, Mankus RA, Rossmann J, Ridder V, et al. (2002) Clinical trials of the T-fluted (Ash Advantage) peritoneal dialysis catheter. Adv Ren Replace Ther 9: 133-143.
- De Paolo N, Petrini G, Garosi G, Buoncristiani U, Brardi S, et al. (1996) A new self-locating peritoneal catheter. Perit Dial Int 16: 623-627.
- Russo R, Gente M, Corciulo R (2011) Difficulties in removing the self-locating peritoneal catheter. Perit Dial Int 31: 500-502.
- Bergamin B, Senn O, Corsenca A, Dutkowski P, Weber M, et al. (2010) Finding the right position: A three-year, single-center experience with the “self-locating” catheter. Perit Dial Int 30: 519-523.
- Amici G, Bernacconi T, Bonforte G (2013) The peritoneal dialysis catheter. J Nephrol 26: 4-75.
- Alhwiesh AK (2016) Modified peritoneal dialysis catheter with a new technique: Farewell to catheter migration Saudi. J Kidney Dis Transpl 27: 281-289.
- Schleifer CR, Ziemek H, Teehan BP, LB Robert, HS Miles, et al. (1987) Migration of peritoneal catheters: personal experience and a survey of 72 other units. Perit Dial Bull 7: 189-193.
- Nicholson ML, Donnelly PK, Burton PR, Veitch PS, Walls J (1990) Factors influencing peritoneal catheter survival in CAPD. Ann R Coll Surg Engl 72: 366-372.
- Joffe P, Christensen AL, Jensen C (1991) Peritoneal cathetertip location during non-complicated continuous ambulatory peritoneal dialysis. Perit Dial Int 11: 261-264.
- Biermann MH, Kasperbauer J, Kusek A, Michael DH, Robert JF, et al. (2016) Peritoneal catheter survival and complications in end-stage renal disease.
- Vogt K, Binswanger U, Buchman P, Baumgartner D, Keusch G, et al. (1987) Catheter related complications during CAPD: A retrospective study on sixty-two double-cuff Tenckhoff catheters. Am J Kidney Dis 10: 47-51.
- Nielsen PK, Hemmingsen C, Lagefoged J (1994) A consequetive study of 646 peritoneal dialysis catheters. Perit Dial Int 14: 170-172.
- Moon JY, Song S, Jung KH, Park M, Lee SH, et al. (2008) Fluoroscopically guided peritoneal dialysis catheter placement: Long-term results from a single center. Perit Dial Int 28: 163-169.
- Scott PD, Bakran A, Pearson R, Raid H, Parrot N, et al. (1994) Peritoneal dialysis access. Prospective randomized trial of 3 different peritoneal catheters-preliminary report. Perit Dial Int 14: 289-290.
- Bay WH, Cerilli GJ, Perrine V, Powell S, Erlich L (1983) Analysis of a new technique to stabilize the chronic peritoneal dialysis catheter. Am J Kidney Dis 3: 133-135.
- Tsimoyiannis EC, Siakas P, Glantzounis G, Toli C, Sferopoulos G, et al. (2000) Laparoscopic placement of Tenckhoff catheter for peritoneal dialysis. Surg Laparosc Endosc Percutan Tech 10: 218-221.
- Davis R, Toung J, Diamond D (1982) Management of peritoneal dialysis catheter dysfunction. Am J Nephrol 2: 285-290.
- Lye WC, Kour NW, van der Straaten JC, Leong SO, Lee EJ, et al. (1996) A prospective randomized comparison of the swan-neck coiled and straight Tenckhoff catheters in patients on CAPD. Perit Dial Int 16: 333-335.
- Eklund BH, Honkanen EO, Kala AR, Kyllönen LE (1994) Catheter configuration and outcome in patients on continuous ambulatory peritoneal dialysis: A prospective comparison of two catheters. Perit Dial Int 14: 70-74.
- Nielsen PK, Hemmingsen C, Lagefoged J (1994) A consequetive study of 646 peritoneal dialysis catheters. Perit Dial Int 14: 170-172.
- Di Paolo N, Petrini G, Garosi G, Buoncristiani U, Brardi S, et al. (1996) A new self-locating peritoneal catheter. Perit Dial Int 16: 623-627.
- Bergamin B, Senn O, Corsenca A, Dutkowski P, Weber M, et al. (2010) Finding the right position: A three-year, single-center experience with the “self-locating” catheter. Perit Dial Int 30: 519-523.
- Lu CT, Watson DI, Elias TJ, Faull RJ, Clarkson AR, et al. (2003) Laparoscopic placement of peritoneal dialysis catheters: 7 years' experience. ANZ J Surg 73: 109-111.
- Moncrief JW, Popovich RP, Broadrick LJ, He ZZ, Simmons EE, et al. (1993) The Moncrief-Popovich catheter. A new peritoneal access technique for patients on peritoneal dialysis. ASAIO J 39: 62-65.
- de Alvaro F, Selgas R, Bajo MA, Serrano P, Fernandez-Reyes MJ, et al. (1994) Moncrief’s technique for peritoneal catheter placement: experience of a CAPD unit. Adv Perit Dial 10: 199-202.
- Park MS, Yim AS, Chung SH, Lee EY, Cha MK, et al. (1998) Effect of prolonged subcutaneous implantation of peritoneal catheter on peritonitis rate during CAPD: A prospective randomized study. Blood Purif 16: 171-178.
- Danielsson A, Blohme L, Tranaeus A, Hylander B (2002) A prospective randomized study of the effect of a subcutaneously “buried” peritoneal dialysis catheter technique versus standard technique on the incidence of peritonitis and exit-site infection. Perit Dial Int 22: 211-219.
- Prischl FC, Wallner M, Kalchmair H, Povacz F, Kramar R (1997) Initial subcutaneous embedding of the peritoneal dialysiscatheter-a critical appraisal of this new implantation technique. Nephrol Dial Transplant 12: 1661-1667.
- Nielsen PK, Hemmingsen C, Friis SU, Ladefoged J, Olgaard K (1995) Comparison of straight and curled Tenckhoff peritoneal dialysis catheters implanted by percutaneous technique: A prospective randomized study. Perit Dial Int 15: 18-21.
- Crabtree JH, Fishman A (1996) Laparoscopic epiplopexy of the greater omentum and epiploic appendices in the salvaging of dysfunctional peritoneal dialysis catheters. Surg Laparosc Endosc 6: 176-180.
- Gadallah MF, Pervez A, el-Shahawy MA, Sorrells D, Zibari G, et al. (1999) Peritoneoscopic versus surgical placement of peritoneal dialysis catheters: A prospective randomized study on outcome. Am J Kidney Dis 33: 118-122.
- Canada-USA (CANUSA) peritoneal dialysis group (1996) Adequacy of dialysis and nutrition in continuous peritoneal dialysis: associated with clinical outcome. J Am Soc Nephrol 7: 198-207.
- Ghali JR, Bannister KM, Brown FG, Rosman JB, Wiggins KJ, et al. (2011) Microbiology and outcomes of peritonitis in Australian peritoneal dialysis patients. Perit Dial Int 31: 651-662.
- Kavanagh D, Prescott GJ, Mactier RA (2004) Peritoneal dialysis-associated peritonitis in Scotland (1999-2002). Nephrol Dial Transplant 19: 2584-2591.
- Fang W, Ni Z, Qian J (2014) Key factors for a high-quality peritoneal dialysis program-the role of the PD team and continuous quality improvement. Perit Dial Int 34: 35-42.
- Nishina M, Yanagi H, Kakuta T, Endoh M, Fukagawa M, et al. (2014) A 10-year retrospective cohort study on the risk factors for peritoneal dialysisrelated peritonitis: A single-center study at Tokai University Hospital. Clin Exp Nephrol 18: 649-654.
- USRDS (1992) US Renal Data System Annual Data Report IV. Catheter-related factors and peritonitis risk in CAPD patients. Am J Kidney Dis 20: 48-54.
Citation: Nasreldin MA, Alhwiesh A, Saeed I, Alotaibi K, Ahmed M, et al. (2020) Does a Perfect and Simple Peritoneal Dialysis Catheter Exist but we are Unaware of? Insights about the Current Guidelines and Literature Review. J Nephrol Renal Ther 6: 040.
Copyright: © 2020 Mohamed A Nasreldin, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

