
Ecophysiology of Leaf Senescence
*Corresponding Author(s):
Fábio Santos MatosPlant Productivity Postgraduate Program, State University Of Goiás, Ipameri, Goiás, Brazil
Tel:+55 64984554834,
Email:fabio.agronomia@hotmail.com
Abstract
In addition to reducing leaf number, leaf senescence has an important role in nutrient remobilization. This phenomenon does not necessarily signal the final stage of the plant life cycle. This is because arboreal and cultivated plants employ various physiological mechanisms in response to senescence. This review addresses several abiotic factors, including photoperiod, temperature, nitrogen and water deficit, all of which alter plant metabolism and trigger leaf senescence. In addition to associating these events with physiological responses in perennial and deciduous plants, we focus particular attention on Jatropha curcas L, a species that is economically important to the latex and biodiesel industries. This species is an important line of research for our group.
Keywords
Deciduous; Jatropha curcas; Nutrients; Perennial plants; Photoperiod; Plant physiology; Temperature; Water deficit
INTRODUCTION
Senescence in higher plants is the final stage of development that leads to the death of a cell, tissue, organ or organism [1]. Nevertheless, senescence should not be seen as a process of deterioration, but rather as an integral part of a development program, as it is the end of life of an organ (leaves, sepals, petals and fruits) with controlled dismantling for remobilization of nutrients and reserves [2].
Under optimal developmental conditions, leaf longevity is age-dependent. Nevertheless, the process may be induced by environmental stimuli with subsequent endogenous changes and consequent resource cycling to newly developed organs [3,4]. During senescence, carbon assimilation is metabolically replaced by catabolism of chlorophyll, proteins, membrane lipids and RNA [5]. Ultra structural studies show that chloroplasts are the first organelles to be dismantled, while mitochondria and the nucleus remain intact until the final stages of leaf senescence [6].
Increased catabolic activity is responsible for converting cellular material accumulated during leaf growth into exportable nutrients that are supplied to seed development or other growing organs [7]. The concomitant degeneration and remobilization processes are therefore extremely organized and highly coordinated [2].
Reductions in photosynthetic rate, chlorophyll content and quantum efficiency of FSII are physiological symptoms of leaf senescence [8]. These changes are orchestrated with metabolic changes, as stromal enzymes are degraded in the early stages of senescence with a decrease in carbon assimilation capacity. The enzymes involved in nitrogen and carbon assimilation are lost and the amino acids derived from their catabolism are possibly exported via phloem with or without transformation [9].
Short-cycle species (maize, rice, lettuce, soybean, tobacco) bear fruit only once (monocarpic plants) and die even if environmental conditions remain favorable for development [10]. In this group, there are not only herbaceous plants, but also many species of agaves and clumping bamboo that can take dozens of years to flourish and fruit; however, when they do, they die afterwards [11]. For trees and other perennials present in the Brazilian Cerrado, leaf senescence can be followed by the splendid autumn scenery of plant color changes through the flowering of Ipês (Handroanthus spp.).
In annual plants, the nutrients resulting from catabolism in the senescence process are provided for seed development. In perennials, nutrients are reallocated to stems or roots that are used as storage resources for initiation of new leaves or flowers in the next season [7]. Therefore, despite the fact that leaf senescence is a deleterious mechanism for the leaf, it can be seen as an altruistic process, as it contributes to plant adaptation to prevailing environmental conditions, ensuring optimal offspring production and better plant survival in its temporal regions and space niches [11].
In Jatropha curcas L., the leaf senescence process is closely interconnected with larger phenological events at the whole plant level; it is not merelya leaf degenerative process. In the Brazilian Cerrado, the cold period (April to August) is characterized by temperature reduction, low precipitation and relative humidity. During this period, J. curcas plants undergo leaf senescence, allowing nutrient remobilization and reserve allocation to the stem. This allows for higher production of secondary compounds.
During the cold period, the leafless plant and in the absence of fruit directs its reserves to latex production. The presence of nitrogen and amino acids in J. curcas latex supports this statement; because the initial target of senescence is chloroplast and in this organelle is about 75 % of the nitrogen present in mesophyll cells. According to Almeida et al. [12] and Matos et al. [13], the highest latex production in J. curcas plants occurs in the months following the onset of leaf senescence, between May and October, as shown in figure 1. Thus, physiologically, latex production and leaf senescence are closely interconnected.
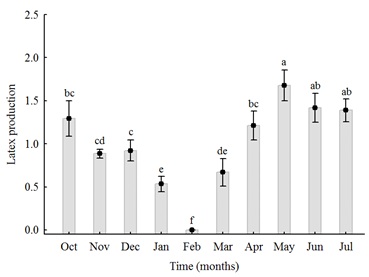 Figure 1: Latex production by Jatropha curcas L. over 10 months. Bars represents means ± SE (n = 6). Means followed by the same letter do not differ by Kruskal-Wallis test (H(9,N – 180) = 88.06; p < 0.01) [13].
Figure 1: Latex production by Jatropha curcas L. over 10 months. Bars represents means ± SE (n = 6). Means followed by the same letter do not differ by Kruskal-Wallis test (H(9,N – 180) = 88.06; p < 0.01) [13].
Leaf senescence is under hormonal control and may be triggered as a result of the end of the organ’s phenological cycle or as a result of deviations from normal development under stress, including air temperature variations, changes in water and nutrient availability (especially nitrogen), and variations in the photoperiod. In addition, infections can also result in leaf senescence [14].
PHOTOPERIOD
Solar radiation is a necessary energy source for photosynthesis in plant species. From the thermodynamic point of view, photosynthesis is endothermic reactions that is not spontaneous, and therefore requires energy input to initiate the process of assimilate production. Agroclimatic zoning provides information on areas suitable for growing plant species based on the requirement of abiotic conditions for the growth and development of these plants. However, plant cultivation in unfit areas, such as those with inadequate light conditions, can result in stress and can trigger leaf senescence. The deterioration process can occur prematurely in plant leaves with above-optimal leaf area indexes that have a microclimate inside the canopy with radiation availability below the compensation point for low leaves [15].
Leaf senescence can be induced by the interaction of photoperiod and temperature or by isolated factors. This is because, in many cases, changes in photoperiod do not affect the induction of resulting leaf senescence from variations in air temperature [16,17]. It is also possible that the abiotic factors of senescence activation differ among deciduous species, because some plants depend on the interaction between environmental factors for leaf senescence. Lang et al. [18], evaluating 27 woody and herbaceous plant species between 1981 and 2012, observed that photoperiod reduction increased leaf senescence of plant species by 61.2 %, while low temperatures increased this process by approximately 39 %.
Leaf longevity is closely related to crop growth and yield and the use of models to identify the timing of onset of temperature and photoperiod senescence has been an important tool for studying leaf longevity and plant phenology [19]. Photoperiodic changes throughout the seasons have been identified as determinants for the control of leaf senescence. In species such as Fagus sylvatica, the effects of temperature are secondary to the importance of photoperiod in senescence, considering plant phenology [20]. Photoperiod reduction triggers leaf senescence in several woody species, such that the deterioration process is dependent on a minimum photoperiod below which the mechanism is activated [18].
The main activating hormone of leaf senescence and abscission is ethylene. However, under photoperiod variation, it is possible that Abscisic Acid (ABA) plays a secondary role in leaf senescence. In Spondias tuberosa, endemic to the Brazilian Caatinga biome, leaf senescence occurs in the period of low precipitation, temperature and photoperiod. However, in these plants, even before significant temperature variations, senescence is activated and before the onset of summer rains, the leaves are initiated. Thus, the photoperiod may be the abiotic factor of senescence activation, with water and temperature as secondary factors that accelerate the deterioration process. Under these circumstances, ABA acts as a chemical messenger of stress, restricting growth by maintaining bud dormancy and probably reducing sensitivity to other growth stimulating hormones such as gibberellins. Abscisic acid plays a pleiotropic role in plants by controlling various stress-related metabolic processes including leaf abscission and senescence, as well as plant growth [21].
TEMPERATURE
Temperature and water determine the distribution of plant species on the earth’s surface. Long-term elevated temperatures contribute to reduced water uptake because these conditions promote increased evapotranspiration and reduce soil water availability. Elevated temperatures also cause short-term cellular damage, with effects on membrane stability. Nevertheless, it is low temperatures that are responsible for activating leaf senescence in most deciduous species sensitive to thermal variations.
Cold is an important factor inducing leaf senescence. Low temperature-sensitive deciduous plants respond to these conditions by increasing electrolyte leakage, chlorophyll degradation and reactive oxygen species formation, as well as reducing enzyme activities of antioxidant metabolism [22]. Leaf longevity is closely related to the productivity of perennial plant species. The concern for green leaf maintenance and senescence retardation has increased the development of research aiming to elucidate senescence control mechanisms. According to Sun et al. [22], chlorophyll and protein content progressively decrease with cold days; however, polyamine application minimizes lipid peroxidation, membrane damage, chlorophyll and protein degradation and maximizes antioxidant enzyme activity.
J. curcas is a rustic multi-purpose plant with great importance in terms of production of oil that is converted to biodiesel, as well as for the production of latex with pharmacological value. This species has higher latex production in the period coinciding with leaf senescence and higher fruit yield, when plants have greater leaf abundance [13]. J. curcas leaf senescence is activated by low air temperature; exposure to temperatures below 10°C for one week is sufficient to activate the deterioration process and cause leaf abscission [23]. On the other hand, this species is sensitive to frost and does not grow in places with constant cold temperatures. Low temperature sensitivity was observed in such biomes as the Atlantic forest and Cerrado [12,23].
In the Brazilian Cerrado, characterized by dry autumns and winter, it is common for plants species to undergo leaf senescence during this period. Confusion as to the abiotic factor that triggers leaf deterioration and leaf abscission processes is normal, because many studies point to water deficit as the cause. However, the Cerrado tree species have deep roots allowing efficient water accumulation that permits the initiation and development of flowers even in the dry season. This suggests that water deficit may exert a secondary effect of accelerated leaf senescence, while low air temperature exerts the primary activation effect. The frequent record of minimum temperatures below 10° at latitudes between 13° 02” and 17° 34” in the Cerrado corroborates this observation.
Climate change may, over time, promote changes in the sensitivity of species to low temperatures. According to Chen et al. [24], the sensitivity of leaf senescence to temperature has been reduced over time (1980-2013), i.e. leaves of tree species that exhibit cold-activated leaf senescence are becoming less sensitive with time. It follows that climate change recorded and projected by the intergovernmental panel on climate change causes changes in temperature sensitivity of species and promotes changes in growth and productivity of commercial crops.
Leaf senescence triggered directly by high temperature is less common than senescence caused by cold. However, under extreme temperature conditions, the increase in water vapor pressure deficit contributes to the reduction of stomatal conductance and photosynthesis. The reduction in the carboxylation rate implies excess undisclosed photochemical energy which, combined with increased respiration, may result in the formation of reactive oxygen species and the sum of all the consequences mentioned may trigger leaf senescence.
NITROGEN
Nitrogen is an essential nutrient for plant metabolism. Its deficiency can trigger severe stress on plants and accelerate leaf senescence. High nitrogen levels may delay leaf senescence in non-perennial species, in addition to maintaining high photosynthetic rates during grain filling, thereby increasing yield [25,26]. Nitrogen recycling from old to new leaves is important to maintain plant growth [27]. Therefore, in non-perennial plants such as soybean, wheat, sorghum and corn, leaf senescence should not be understood as a degenerative event representing the end of the life cycle, but rather as part of a metabolic process of degradation, remobilization, transport and reserves for grains that may be accelerated or delayed by abiotic factors.
Free amino acids and small peptides are the main compounds of nitrogen and carbohydrate metabolism during leaf senescence. Protein and amino acid degradation is especially high, contributing to the allocation of nutrients to developing seeds. The genes involved in the processes and transport of biosynthetic aromatic amino acids are regulated in early stages of leaf senescence. By contrast, elevations in levels of sugars (glucose, fructose, galactose and mannose) and high rates of photosynthesis, respiration and photorespiration coincide with late senescence in wheat plants [25].
Despite the fact that fertilizers increase plant nitrogen availability and crop productivity, nitrates that leach groundwater cause pollution. Therefore, it is crucial to develop low-input use strategies such as improved plant materials for high yield under low nitrogen availability. Nevertheless, it is imperative to understand and control the nitrogen-affected leaf senescence process that, when deficient, acts as a trigger for leaf deterioration. According to Zhang et al. [28], wheat plants with higher leaf nitrogen content have delayed senescence, higher photochemical efficiency and high biomass accumulation. The ability of maize genotypes to maintain high levels of photosynthesis under nitrogen deficiency and to delay leaf senescence is an important parameter for selecting high efficiency nitrogen cultivars [26].
Nitrogen, amino acids and sugars remobilized during leaf senescence of short-cycle species such as soybean and wheat may represent important sources for grain filling; therefore, yield is directly related to the leaf senescence process. In perennial plants, remobilization may represent reserves that are stored in the stem and/or roots for later use in leaf or flower initiation. A third, however not least important pathway, is the case of multi-purpose species such as J. curcas that direct remobilized compounds and nutrients for latex production during leaf senescence, as reported by Almeida et al. [12] and Matos et al. [13].
WATER DEFICIT
The most common visual symptom of water deficit is leaf senescence, because under prolonged water limitation in the soil and atmosphere, most plants reduce leaves (the transpiration organ) to preserve water status and to prevent or delay dehydration. Throughout evolution from aquatic to terrestrial environments, where droughts are frequent and varying in intensity, plants have developed efficient survival mechanisms such as deciduous leaves. Leaf senescence is therefore an evolutionarily selected and genetically controlled developmental process that constitutes an important phase in the plant life cycle [7].
Water deficit, by accelerating leaf senescence, limits crop yields because it impairs the growth and filling of soybeans, wheat, sorghum, corn, vegetables and other species [10]. Under water restriction, plants retard dehydration by closing stomata in response to the production of the chemical messenger Abscisic Acid (ABA). Increased ABA levels also increase ethylene production and as a consequence, cause the synthesis of the enzymes that act on the cell wall and middle lamella. In this manner, ABA intensely accelerates leaf senescence, indirectly inducing abscission [29]. Leaf senescence and the fall leaves phase is characterized by ethylene’s induction of genes that encode specific hydrolytic enzymes of polysaccharides and cell wall proteins in the abscission zone [29].
Cytokines have been known for many decades as senescence-retarding hormones because the level of endogenous cytokines falls during leaf senescence and, on the other hand, exogenous application delays senescence [30]. Ethylene is the main hormone involved in leaf senescence [31]. Studies with mutant plants for ethylene-insensitive receptor proteins have been show delayed senescence [32].
Immature leaves are little sensitive to ethylene; they present high concentrations of cytokines and auxins that delay leaf senescence and abscission, respectively. The pattern of deterioration and fall of leaves under water deficit is rising, starting from mature leaves that have greater sensitivity to ethylene. Hormonal pathways play key roles in the progression of senescence; because some genes are apparently deactivated or have low activity during plant development. Under certain stimuli, increased hormone production induces the activation of hydrolytic enzyme synthesis genes and triggers leaf senescence [33].
Uneven distribution of rainfall over the crop cycle may restrict growth and/or yield of economic interest species to varying magnitudes. Rain seasonality highlights water deficit as the main abiotic factor activating leaf senescence of native and cultivated plants. In biomes such as Cerrado and Caatinga, characterized by long dry periods during the year, it is common to find leafless species in soils with low water potentials.
CONCLUSION
Leaf senescence is a genetically controlled process closely related to leaf longevity; however, can be accelerated or retarded by abiotic stimuli (water, light, nutrients, photoperiod).
The senescence process can be interpreted as a result of a deviation from the normal condition of plant development (stress) and reduction in the number of leaves negatively interfering in plant growth and yield. It can also be understood as a phenological step that marks the end leaf longevity with remobilization and orderly transport of reserves to growing organs. In this case, it is necessary to develop quantitative studies to understand the correlation between remobilized reserves and development of plant products with economic interest.
A third line of interpretation, specific to J. curcas plants, but occurring in a number of native species, states that, during predominant green leaf growth, there is higher fruit production; during the senescent leaf period, the reserves are partitioned for latex production and other secondary metabolites.
CONFLICT OF INTEREST
All authors declare no conflict of interests.
REFERENCES
- Gan S (2018) Concepts and types of senescence in plants. Methods Mol Biol 1744: 3-8.
- Kalra GB (2018) Senescence and programmed cell death. Plant Physiology, Development and Metabolism, Springer, Singapore. Pg no: 978-966.
- Daneva A, Gao Z, van Durme M, Nowack MK (2016) Functions and regulation of programmed cell death in plant development. Annu Rev Cell Dev Biol 32: 441-468.
- Kim J, Woo HR, Nam HG (2016) Toward systems understanding of leaf senescence: An integrated multi-omics perspective on leaf senescence research. Mol Plant 9: 813-825.
- Schippers JH, Schmidt R, Wagstaff C, Jing HC (2015) Living to die and dying to live: The survival strategy behind leaf senescence. Plant Physiol 169: 914-930.
- Tamary E, Nevo R, Naveh L, Levin-Zaidman S, Kiss V, et al. (2019) Chlorophyll catabolism precedes changes in chloroplast structure and proteome during leaf senescence. Plant Direct 3: 00127.
- Woo HR, Kim HJ, Lim PO, Nam HG (2019) Leaf senescence: Systems and dynamics aspects. Annu Rev Plant Biol 70: 347-376.
- Chen D, Wang S, Xiong B, Cao B, Deng X (2015) Carbon/nitrogen imbalance associated with drought-induced leaf senescence in Sorghum bicolor. PloS One 10: 0137026.
- Hörtensteiner S, Feller U (2002) Nitrogen metabolism and remobilization during senescence. J Exp Bot 53: 927-937.
- Yadava PSA, Kumar K, Singh I (2019) Senescence signalling and control in plants. Press A Ed. Pg no: 283-302.
- Kerbauy GB (2004) Fisiologia vegetal. Guanabara Koogan Rio de Janeiro. Pg no: 7.
- Almeida LM, Matos FS, Bailão EFLC, Gonçalves PJ (2019) Jatropha curcas L. Latex Production, Characterization, and Biotechnological Applications. Jatropha, Challenges for a New Energy Crop. Pg no: 437-459.
- Matos FS, Ciappina AL, Rocha EC, Almeida LM (2018) Factors that influence in Jatropha curcas L. latex production. Bragantia 77: 74-82.
- Wingler A, Roitsch T (2008) Metabolic regulation of leaf senescence: interactions of sugar signalling with biotic and abiotic stress responses. Plant Biol 10: 50-62.
- Sade N, del Mar Rubio-Wilhelmi M, Umnajkitikorn K, Blumwald E (2017) Stress-induced senescence and plant tolerance to abiotic stress. J Exp Bot 69: 845-853.
- Tao Z, Wang H, Dai J, Alatalo J, Ge Q (2018) Modeling spatiotemporal variations in leaf coloring date of three tree species across China. Agr Forest Meteorol 249: 310-318.
- Vitasse Y, François C, Delpierre N, Dufrêne E, Kremer, A, et al. (2011) Assessing the effects of climate change on the phenology of European temperate trees. Agr Forest Meteorol 151: 969-980.
- Lang W, Chen X, Qian S, Liu G, Piao S (2019) A new process-based model for predicting autumn phenology: How is leaf senescence controlled by photoperiod and temperature coupling? Agr Forest Meteorol 268: 124-135.
- Peaucelle M. Ciais P, Maignan F, Nicolas M, Cecchini S, et al. (2019) Representing explicit budburst and senescence processes for evergreen conifers in global models. Agr Forest Meteorol 266: 97-108.
- Vitasse Y, Hoch G, Randin CF, Lenz A, Kollas C, et al. (2013) Elevational adaptation and plasticity in seedling phenology of temperate deciduous tree species. Oecologia 171: 663-678.
- Killiny N, Nehela Y (2019) Abscisic acid deficiency caused by phytoene desaturase silencing is associated with dwarfing syndrome in citrus. Plant Cell Rep 38: 965-980.
- Sun X, Xie L, Han L (2019) Effects of exogenous spermidine and spermine on antioxidant metabolism associated with cold-induced leaf senescence in Zoysiagrass (Zoysia japonica). Hortic Environ Biote 60: 295-302.
- Matos FS, de Oliveria LR, de Freitas RG, Evaristo AB, Missio RF, et al. (2012) Physiological characterization of leaf senescence of Jatropha curcas L. Biomass Bioenerg 45: 57-64.
- Chen L, Huang JG, Ma Q, Hänninen H, Tremblay F, et al. (2019) Long-term changes in the impacts of global warming on leaf phenology of four temperate tree species. Global Change Biol 25: 997-1004.
- Heyneke E, Watanabe M, Erban A, Duan G, Buchner P, et al. (2019) Effect of senescence phenotypes and nitrate availability on wheat leaf metabolome during grain filling. Agronomy 9: 305.
- Schulte auf’m Erley G, Begum N, Worku M, Bänziger M, Horst WJ (170) Leaf senescence induced by nitrogen deficiency as indicator of genotypic differences in nitrogen efficiency in tropical maize. J Plant Nutr Soil Sc 170: 106-114.
- Han YL, Liao JY, Yu Y, Song HX, Rong N, et al. (2017) Exogenous abscisic acid promotes the nitrogen use efficiency of Brassica napus by increasing nitrogen remobilization in the leaves. JPlant Nutr 40: 2540-2549.
- Zhang N, Yan J, Zhang S (2019) Rht13 dwarfing gene delays foliar senescence in wheat induced by nitrogen deficiency. Pak J Bot 51: 143-147.
- Taiz L, Zeiger E, Møller IM, Murphy A (2017) Fisiologia e desenvolvimento vegetal. Artmed Editora.
- Janecková H, Husicková A, Lazár D, Ferretti U, Pospíšil P, et al. (2019) Exogenous application of cytokinin during dark senescence eliminates the acceleration of photosystem II impairment caused by chlorophyll b deficiency in barley. Plant Physiol Biochem 136: 43-51.
- Reid MS, Wu MJ (2018) Ethylene in flower development and senescence. The plant hormone ethylene, Ed. Pg no: 215-234.
- Graham LE, Schippers JH, Dijkwel PP, Wagstaff C (2012) Ethylene and Senescence Processes. Annual Plant Reviews 44.
- Velasco-Arroyo B, Diaz-Mendoza M, Santamaria ME, Gonzalez-Melendi P, Gomez-Sanchez A, et al. (2017) Senescence-associated genes in response to abiotic/biotic stresses. Progress in Botany 79: 89-109.
Citation: Matos FB, Borges Lp, Müller C (2020) Ecophysiology of Leaf Senescence. J Agron Agri Sci 3: 019.
Copyright: © 2020 Fábio Santos Matos, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

