
Effect of Different Extraction Methods on Major Bioactive Constituents at Different Flowering Stages of Japanese Honeysuckle (Lonicera japonica Thunb.)
*Corresponding Author(s):
Bo-Ching Chen#Department Of Natural Biotechnology, Nanhua University, Chiayi, Taiwan, Province Of China
Tel:+886 052721001,
Email:bcchen@nhu.edu.tw
Abstract
Japanese honeysuckle (Lonicera japonica Thunb.) is a traditional Chinese medicinal herb usually used to treat inflammatory disorders. The contents of two major active constituents of Japanese honeysuckle, chlorogenic acid and luteolin, vary with the plant’s development stage. This study aims to analyze the antioxidants and major active constituents of Japanese honeysuckle using different extraction methods at various flowering stages. To achieve the highest efficacy of major active constituents, methanol extraction was better than water extraction. After treatment with microwaves for 3 Min (M3), the mean contents of chlorogenic acid and luteolin obtained using methanol were 42.96 and 1.22 mg/g DW, respectively, which were highest of all treated groups. Of the different flower bud stages, total phenolic content was highest during the Silver Flower (SF) stage, with mean values of 26.91 and 25.82 mg/g DW for water and methanol extraction, respectively. The highest trolox content was also observed at this stage, with mean values of 27.02 and 28.62 mg/g DW for methanol and water extraction, respectively. The level of chlorogenic acid increased with the development of flower buds from the Juvenile Bud (JB) stage to the Gold Flower (GF) stage. The highest value obtained using methanol extraction was 24.45 mg/g DW at the SF stage and the highest values obtained using water extraction were 11.28 and 11.25 mg/g DW at the White Bud (WB) and SF stages, respectively. On the other hand, the content of luteolin obtained using methanol extraction decreased with flower bud development. The highest luteolin levels (0.60 mg/g DW) were found at the JB and Green Bud (GB) stages. For water extraction, however, luteolin content increased with flower bud development, reaching its highest level (0.142 mg/g DW) at the GF stage. The present results can be applied to determine the optimal harvest stage and postharvest treatment method for Japanese honeysuckle.
Keywords
Chlorogenic acid; Extraction method; Flower bud stages; Lonicera japonica thunb.; Luteolin
INTRODUCTION
In Asia, Chinese Traditional Medicine (CTM) has been used to treat various diseases for thousands of years. Functional phytochemicals are acquired from many natural herbal sources with potent bioactivity. Lonicera (Caprifoliaceae) is a traditional medicinal herb genus comprised of more than 150 species that is widely distributed in Eastern Asia, including China, Japan, Korea and Taiwan [1]. They are perennial climbing plants with white-to-golden flowers and a rich, sweet smell. The Japanese honeysuckle (L. japonica Thunb., also known as Jin Yin Hua in Chinese) is a main species of Lonicera widely used in CTM [2].
The dried flower buds of L. japonica Thunb. have been used not only for tea but also for treating wind-heat, fever and respiratory tract infection [3]. Furthermore, L. japonica Thunb. is often combined with other medicinal herbs to treat many diseases [4]. For example, it is often combined with Gingyo-San and used as a traditional antipyretic treatment for the common cold and influenza [5]. When combined with Shuang-Huang-Lian, it is used to treat respiratory diseases [6,7] and when combined with Jiangtang tablets, it is an anti-diabetic treatment [8]. Several pharmacological studies have demonstrated that the constituents of L. japonica Thunb. Possess much bioactivity and many functions, such as antibacterial [9], anti-inflammatory [10], antioxidant [11] antiviral [12] and hepatoprotective functions [13, 14]. Recently, due to the outbreak of COVID-19 worldwide, L. japonica Thunb. has received increasing attention because it is reported to be capable for the prevention of COVID-19.
Phytochemicals are natural chemicals originally extracted from alga, vegetables, fruits and herbs. Dietary phytochemicals may have beneficial health effects, such as protection against disease and regulatory gene expression [15,16]. Several studies have indicated that L. japonica Thunb. is rich with phytochemicals, including volatiles (linalool, epoxylinalool, geraniol, α-trepineol, etc.) [17,18], phenolic acids (chlorogenic acid, isochlorogenic acid, caffeic acid, etc.) [19] and flavonoids (luteolin, rutin, quercetin, etc.) [20]. According to the Pharmacopoeia of the People's Republic of China (2005 version), the indicator constituents of L. japonica Thunb. (i.e., chlorogenic acid and luteolin) should keep above 1.5 % and 0.05 %, respectively. Medicinally, chlorogenic acid functions primarily as an anti-tumor [21], anti-inflammatory [22] and hypoglycemic agent [23]. Luteolin has been reported to have prominent antioxidant [24], anti-inflammatory [25,26] and anti-cancer effects [27].
The quality and quantity of phytochemicals in plant tissue or organs may be affected by many factors, such as fertilization, growing season and harvest time. Figueiredo et al. indicated that several factors affect the volatile components and essential oil of plant extracts [28]. Ramakrishna and Ravishankar demonstrated that the secondary metabolites of plants are influenced by abiotic stress [29]. Jaime et al., found that temperature changes the volatile accumulation of Salvia lavandulifolia [30]. Novak et al. indicated that thymol increases with decreasing temperatures, while carvacrol increases with increasing temperatures in Origanum spp [31]. The accumulation of different compounds in Eleutherococcus senticosu is affected by light quality [32], whereas light intensity affects anthocyanin accumulation in Melastoma malabathricum [33]. In addition, the phenolic content of blueberries is influenced by maturation status; all phenolic compounds are degraded in fully ripe berries [34]. For strawberries, two active compounds comprised of anthocyanin and cinnamic acid derivatives are increased when the fruits develop from white to red [35].
Previous research has demonstrated that the content of phytochemical compounds and constituents of floral organs can be influenced by maturation status or development stage. For example, Acacia cyclops is most volatile at the yellow bud stage, which increases aliphatic compounds and irregular terpene content and decreases monoterpenoid levels [36]. After stage 3 (when the petals start to split), when Camellia sinensis has more phytochemical compounds than at other stages, the plant’s phytochemical compounds decreased until the full bloom stage [37]. The maximum contents of chlorogenic acid, cichoric acid, echinacoside and isobutylamide in Echinacea purpurea occur at stages 1, 1, 2 and 3, respectively, but total content is highest at stage 3 [38]. The maximum contents of gentiopicroside, sweroside, swertiamarin and longanic acid in Gentiana macrophylla occur at stages 2, 3, 3 and 4, respectively [39]. The most flavonols and anthocyanin in Helleborus niger are present at stages B and POF, respectively [40]. Ascorbic acid, β-Carotene, flavone and rutin in Hemerocallis fulva increase with the stage of development, whereas flavan-3-ol, quercetin and wogonin decrease with the stage of development [41,42]. The contents of anthocyanin and flavonol in Lilium increase during the late stages of flower bud development [43]. The anthocyanin content of Paeonia suffruticosa increases with development stage after the half-opening or full-opening stage [44]. Sandersonia aurantiaca has the highest flavonol content at stage 1, but the content does not have a monotonic relation with development stage [45].
This study aims to determine the antioxidant and major phytochemical compounds (chlorogenic acid and luteolin) of L. japonica Thunb. at different flowering stages. Furthermore, the effects of different extraction methods on major active constituents’ content at different flowering stages are also investigated. The results of the present study can provide strong evidence regarding the optimal harvest period and postharvest treatment strategy to enhance the value of CTM.
MATERIALS AND METHODS
Chemical compounds
Chlorogenic acid (Sigma, USA), DPPH (1, 1-diphenyl-2-picrylhydrazyl) (Sigma, USA), folin-ciocalteu reagent (Merck, Germany), gallic acid (Sigma, USA), luteolin (Sigma, USA), HPLC-grade methanol (Burdick & Jackson, USA), phosphoric acid (Merck, Germany), sodium carbonate (Merck, Germany) and trolox (6-Hydroxy-2, 5, 7, 8-tetramethylchroman-2-carbonsaure, 97 %) (Sigma, USA) were used in this study.
Plant material preparation
Plant management and harvest: Lonicera japonica Thunb. was planted organically in the screen house of the Department of Post-Modern Agriculture at MingDao University (Changhua, Taiwan). The development of flower buds from visible to wilted can be separated into six stages (Figure 1):
i. Juvenile Bud (JB): Small green buds of 0.8-1.5 cm;
ii. Green Bud (GB): Green buds of 2.1-3.0 cm;
iii. Green-White Bud (GWB): Shallow green buds of 3.1-4.0 cm;
iv. White Bud (WB): White buds getting ready to burst sized 3.8-4.9 cm;
v. Silver Flower (SF): White flower blooms of 4.4-5.2 cm; and
vi. Gold Flower (GF): Yellow petals of 4.2-5.2 cm.
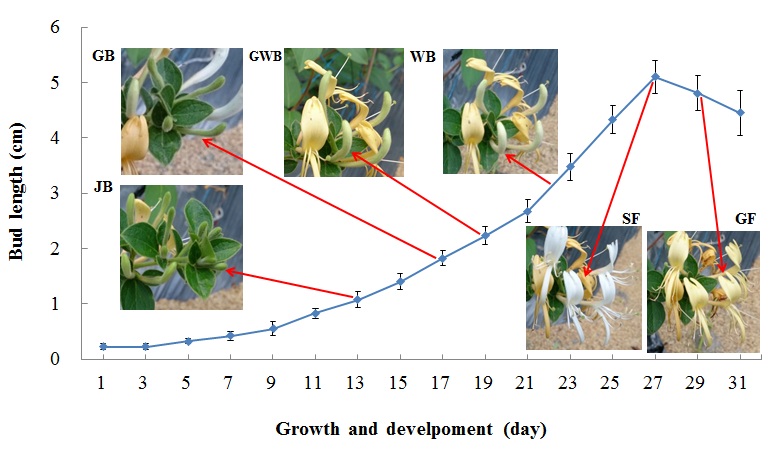 Figure 1: Different growth and developemt stages of L. japonica.
Figure 1: Different growth and developemt stages of L. japonica.
JB: Juvenile Bud, GB: Green Bud, GWB: Green-White Bud, WB: White Bud, SF: Silver Flower and GF: Gold Lower.
Flower buds at different stages were harvested in October 2014. Five hundred flowers were collected for each group. The fresh weight was recorded and the dry weight was investigated after the flowers were oven-dried at 40°C for 96 hours until fully dehydrated. The water content was calculated using the following equation:
Water content (%) = [(fresh weight-dry weight/fresh weight)]×100. (1)
The flower bud was then ground into fine powder and stored in a cabinet drier for extraction experiments.
Extraction
Different extraction methods: Water (W) or Methanol (MeOH, M) were used as solvents for the extraction of phytochemical compounds. The powder of L. japonica flowers was fully mixed with the solvent at a ratio of 1:10 (m/v). Five treatments, including water bath treatment at 55°C (H55) and 95°C (H95) for 1 hour, ultrasonic treatment (40 KHz) for 1 hour (US) and microwave treatment (500 w) for 1 Minute (M1) and 3 Minutes (M3), were performed. The treated samples were centrifuged at 6,000 rpm for 10 minutes (Hermle Z 323 K, Hermle, USA) and then filtrated (ADVANTEC No.1, TOYO) into tubes. The samples extracted using water and methanol were stored at 4 and -20°C, respectively.
Different flower bud stages: Flower buds at six development stages (i.e. JB, GB, GWB, WB, SF and GF) were treated using Water (W) or Methanol (M) as described in section 2.2.2.1.
Antioxidant activity analysis
Determination of total phenolic content: The total phenolic content of the extract was determined according to the method proposed by Sato et al. [46]. Fresh gallic acid solution (0-500 mg L-1) was prepared with 80 % methanol and 20 % sodium carbonate. Twenty-µL extracted samples of L. japonica were mixed with 1 mL Folin-Ciocalteu reagent and left to stand for 5 min. The mixture was added to 0.9 mL of 20 % sodium carbonate and left to stand for 10 min. Then, it was centrifuged at 3,000 rpm for 10 min. The absorbance was measured to be 760 nm. The total phenolic content was calculated from the calibration curve and expressed as mg of gallic acid equivalent to g in dry weight.
Determination of DPPH radical scavenging activity: Analysis of DPPH (1,1-diphenyl-2-picryl-hydrazyl) radical scavenging activity was conducted according to Shimada et al.’s method [47]. Fresh DPPH-methanol solution (26.68 µM) was prepared and stored at 4°C until use. Four mL of the solution was mixed with 20 µL of extracted solutions of L. japonica. The mixture was incubated in the dark at 4°C. Methanol solution was used as a blank. The absorbance change at 517 nm was measured 10 min later. All measurements were performed in triplicate. DPPH radical scavenging activity and 50 % scavenging concentration (EC50) were calculated using Equations (2) and (3), respectively. The relative trolox content of samples was calculated from the calibration curve and expressed as trolox equivalents.
DPPH radical scavenging effect (%) = [(A517 blank - A517 Sample/A517 blank)] ×100 (2)
EC50 (mg mL-1) = (0.05×50/DPPH radical scavenging effect (%)) ×1000 (3)
Determination of chlorogenic acid and luteolin contents
In the present study, chlorogenic acid and luteolin contents were determined by HPLC according to the method used by Lu et al. with some modification [48]. Briefly, quantification of chlorogenic acid and luteolin in the flower buds of L. japonica was performed using the Waters 1525 binary HPLC Pump (Waters Corp.) equipped with a Waters 2487 UV-Visible Detector (Waters Corp.). The standard solutions and extracts were separated on a SunFire C18 (5 µm 4.6x250 mm, Waters Corp.). The mobile phase was a methano l-0.4 % phosphoric acid solution (50:50, v/v), the flow rate was 1 mL min-1, the ultraviolet spectrum change was 350 nm and the injection volume was 10 mL. Next, 100 mM of prepared chlorogenic acid and luteolin was mixed with Milli-Q® Water (Millipore, Merck, Germany) through a syringe filter with PES of 0.22 µm (Sterlitech Corp.). Then, chlorogenic acid was diluted to 0, 0.25, 0.5, 1, 1.5 and 2 mM and luteolin was diluted to 0, 0.5, 1, 2, 5 and 7.5 mM. All samples were through syringe filters with PES of 0.22 µm until use. The chlorogenic acid and luteolin contents of samples were calculated from the calibration curve.
Statistical analysis
All experiments were conducted in triplicate. All results were expressed as means ± Standard Deviation (SD). The significance of differences was determined via LSD (Least Significant Difference) using SAS version 9.4 with a significance level of p<0.05.
RESULTS AND DISCUSSION
Flower development stages
The basic characteristics of different flower bud stages of L. japonica Thunb. found during our investigation are listed in table 1. The bud length and correspondent duration of development are as follows: JB stage (0.8-1.3 cm, 12-14 d), GB stage (2.2-2.7 cm, 17-19 d), GWB stage (3.1-3.7 cm, 21-23 d), WB stage (4.0-4.8 cm, 24-26 d), SF stage (4.5-5.3 cm, 27-28 d) and GF stage (4.2-5.1 cm, 29-30 d) (Figure 1). The bud length increased with development stage until the SF stage. This result was in line with the findings proposed by Wang et al. [18].
|
Stages |
Day of Development |
Length (cm) |
Fresh Weight (g)z |
Dry Weight (g)z |
Water Content (%) |
|
JB |
12-14 |
1.08e |
2.13e |
0.49e |
77.14d |
|
GB |
17-19 |
2.68d |
6.17d |
1.39d |
77.54d |
|
GWB |
21-23 |
3.48c |
8.01c |
1.73c |
78.46c |
|
WB |
24-26 |
4.33b |
11.65b |
2.09b |
82.10b |
|
SF |
27-28 |
4.95a |
14.00a |
2.47a |
82.39ab |
|
GF |
29-30 |
4.80a |
12.76ab |
2.20b |
82.74a |
Table 1: Basic characteristics of L. japonica Thunb. at different flower development stages.
Means in the same column followed by the same letter are not significantly different at the 5 % level according to the Least Significant Difference (LSD).
ZFive hundred flowers were collected at every stage.
The bud length of L. japonica Thunb. in this study was a little longer than in Wang et al.’s study [18]. This could be the result of different management strategies, such as irrigation and fertilizer, in different cultures or other natural factors, such as soil texture, temperature and daylight length/intensity. With each development stage, the fresh weight (2.13, 6.17, 8.01, 11.65, 14.00 and 12.76 g, respectively), dry weight (0.49, 1.39, 1.73, 2.09, 2.47 and 2.20 g, respectively) and water content (77.14, 77.54, 78.46, 82.10, 82.39 and 82.74 %, respectively) of L. japonica Thunb. buds progressively increased (Table 1).
Effects of different extraction methods
The present study showed that methanol’s extraction efficiency was superior to that of water for both antioxidants (i.e. scavenging DPPH radical activity, equivalent trolox content and total phenolic content) and major compounds (i.e. chlorogenic acid and luteolin). For US treatment, methanol (3.81 %) showed significant DPPH radical scavenging activity that was 20.05 times higher than that of water (0.19 %) (Table 2). For H55 treatment, no significant difference was observed between water and methanol extraction of equivalent trolox content (3.48 and 3.48 mg/g DW, respectively). For H95 treatment, water and methanol extraction of equivalent trolox content increased to 19.75 and 28.10 mg/g DW, respectively, indicating that heat can significantly improve extraction efficiency. For microwave treatment, DPPH radical scavenging activity and equivalent trolox content was significantly influenced by both extraction solvent and treatment time (Table 2). Compared with water extraction, methanol extraction with M1 and M3 treatment increased DPPH radical scavenging activity by 1.49 and 1.40 times, respectively. In addition, compared to water extraction, methanol extraction with M3 and M1 treatment increased the equivalent trolox content by 8.42 and 11.11 mg/g DW, respectively (Table 2). Regarding total phenolic content, H95 and M1 and M3 treatment resulted in the most efficient extraction of gallic acid content for both methanol and water (Figure 2). However, the extraction efficiency of methanol extraction with M3 was not as high. Lower extraction efficiency was also observed for H55 and US treatment. For these two treatments, methanol had better extraction efficiency than water.
|
Extraction Methods |
Scavenging Effects (%) |
EC50 (mg/mL) |
Trolox Content (mg/g DW) |
|||
|
Water |
MeOHx |
Water |
MeOH |
Water |
MeOH |
|
|
H55z |
1.54c |
1.53e |
1627.61b |
1673.92a |
3.48c |
3.48c |
|
H95 |
35.64a |
57.32a |
70.43d |
43.61d |
19.75a |
28.10a |
|
U |
0.19d |
3.81d |
13316.67a |
659.76b |
0.52c |
4.57c |
|
M1 |
19.95b |
29.73c |
125.81c |
258.88c |
12.21b |
16.10b |
|
M3 |
37.49a |
52.66b |
67.26d |
47.47d |
20.63a |
27.21a |
Table 2: Effects of different extraction methods on the DPPH radical scavenging activity of L. japonica Thunb..
Means in the same column followed by the same letter are not significantly different at the 5 % level according to the LSD.
zH: heated water bath, U: Ultrasonic, M: Microwave, 55: 55°C, 95: 95°C, 1: 1 minute and 3: 3 minutes.
x: Methanol.
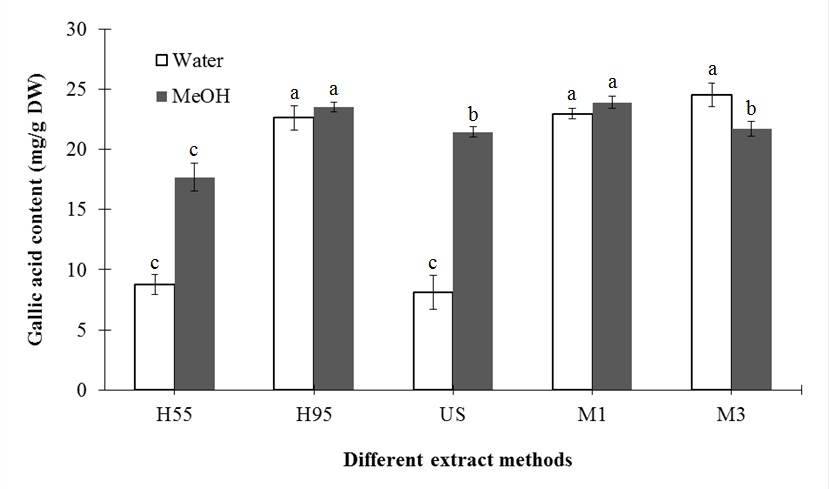 Figure 2: Effects of different extraction methods on the total phenolic content of L. japonica Thunb.
Figure 2: Effects of different extraction methods on the total phenolic content of L. japonica Thunb.
Means for the same solvent followed by the same letter are not significantly different at the 5 % level according to the LSD.
The effects of different treatment methods and extraction solvents on the major biochemical compounds, chlorogenic acid and luteolin, are displayed in figures 3 and 4, respectively. For all treatments, methanol had higher extraction efficiency than water. Similar to the results for antioxidants, the extraction efficiency of major compounds increased with temperature and time of extraction. As a result, H95 and M3 treatment with methanol extraction led to the highest content of major compounds. Compared to H55 treatment, H95 significantly increased chlorogenic acid and luteolin extraction using water (12.51 and 0.06 mg/g DW, respectively) and methanol (13.93 and 0.38 mg/g DW, respectively). In addition, compared to M1 treatment, M3 increased chlorogenic acid and luteolin extraction by 4.89 and 0.03 mg/g DW, respectively, using water and 5.58 and 0.27 mg/g DW, respectively, using methanol.
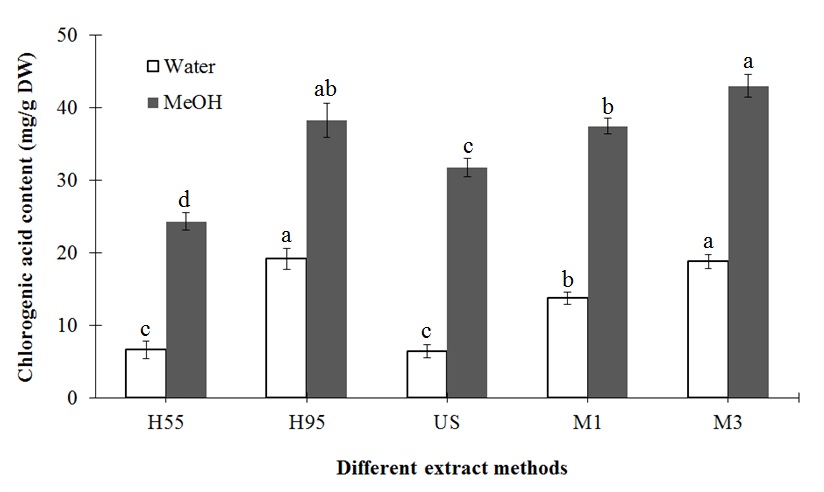 Figure 3: Effects of different extraction methods on the chlorogenic acid content of L. japonica Thunb.
Figure 3: Effects of different extraction methods on the chlorogenic acid content of L. japonica Thunb.
Means for the same solvent followed by the same letter are not significantly different at the 5 % level according to the LSD.
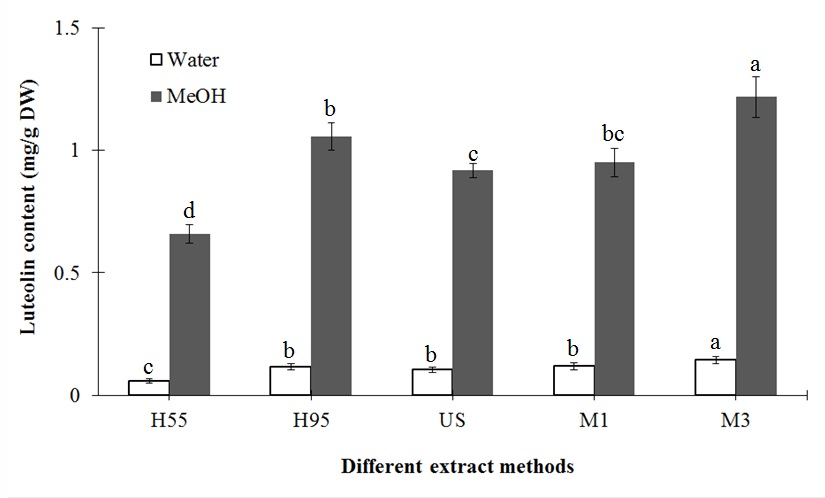
Figure 4: Effects of different extraction methods on the luteolin content of L. japonica Thunb.
Means for the same solvent followed by the same letter are not significantly different at the 5 % level according to the LSD.
As mentioned above, antioxidants and chlorogenic acid and luteolin content were substantially increased with temperature and time of extraction (Table 2; Figures 2-4). H95 and M3 treatment with methanol extraction led to the highest equivalent trolox, chlorogenic acid and luteolin contents. In comparison to heating, microwave treatment saves more time and energy. Therefore, M3 treatment was chosen for further experiments in the present study.
DPPH radical scavenging activity at different flower development stages
The results of the present study showed that DPPH scavenging radical activity and trolox content increased from the JB stage to the SF stage and then slightly decreased at the GF stage (Table 3). In other words, the highest antioxidant content was found at the SF stage (85.01 % DPPH scavenging effect and 27.02 mg/mL DW trolox content for water extraction; 90.04 % DPPH scavenging effect and 28.62 mg/mL DW trolox content for methanol extraction) and the lowest antioxidant content was observed at the JB stage (7.07 % DPPH scavenging effect and 2.29 mg/g DW trolox content for water extraction; 19.30 % DPPH scavenging effect and 6.14 mg/g DW trolox content for methanol extraction). Table 3 again shows that methanol had better overall extraction efficiency than water.
|
Stages |
Scavenging Effects (%) |
EC50 (mg/mL) |
Trolox content (mg/mL) |
|||
|
Water |
MeOH |
Water |
MeOH |
Water |
MeOH |
|
|
JB |
7.07e |
19.30e |
353.42a |
130.06a |
2.29e |
6.14e |
|
GB |
26.37d |
27.91d |
94.82b |
89.62b |
8.38d |
8.87d |
|
GWB |
53.90c |
64.28c |
46.40c |
38.89c |
17.14c |
20.43c |
|
WB |
80.70b |
89.77a |
30.99d |
27.85d |
25.65b |
28.53a |
|
SF |
85.01a |
90.04a |
29.41d |
27.76d |
27.02a |
28.62a |
|
GF |
81.47b |
87.62b |
30.69d |
28.53d |
25.90b |
27.85b |
Table 3: DPPH radical scavenging activity and trolox content of L. japonica Thunb. at different flower development stages.
Means in the same column followed by the same letter are not significantly different at the 5 % level according to the LSD.
Total phenolic content at different flower development stages
Total phenolic content at different flower development stages followed the same trend as DPPH radical scavenging activity; however, no significant difference was found between water and methanol extraction (Figure 5). Total phenolic content was highest at the SF stage (26.91 and 25.82 mg/g DW for water and methanol extraction, respectively) and then decreased dramatically at the GF stage (13.55 and 14.46 mg/g DW for water and methanol extraction, respectively).
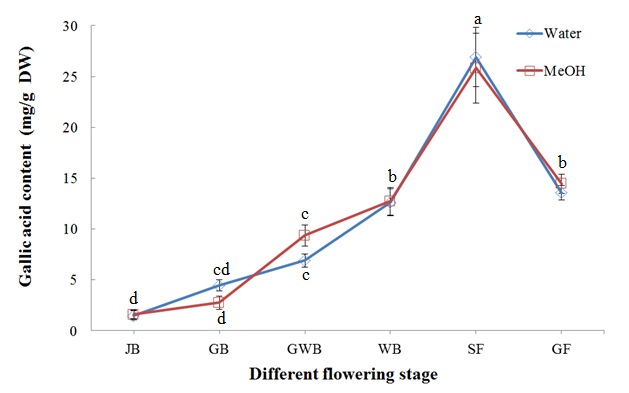 Figure 5: Total phenolic content of L. japonica Thunb. at different flower development stages Means for the same solvent followed by the same letter are not significantly different at the 5 % level according to the LSD.
Figure 5: Total phenolic content of L. japonica Thunb. at different flower development stages Means for the same solvent followed by the same letter are not significantly different at the 5 % level according to the LSD.
Chlorogenic acid content at different flower development stages
Chlorogenic acid is a major biochemical compound that accounts for approximately 1-2 % of the composition of dried L. japonica buds. In the present study, chlorogenic acid content substantially increased with flower development stage until the SF stage, similar to antioxidant content (Figure 6). The highest chlorogenic acid content obtained using methanol extraction (24.45 mg/g DW) was found at the SF stage and the highest obtained using water extraction (11.28 mg/g DW) was found at the WB stage. The maximum extraction efficiency of methanol was 2.17 times higher than that of water. At the GF stage, the chlorogenic acid content was decreased to 15.94 and 8.20 mg/g DW for methanol and water extraction, respectively.
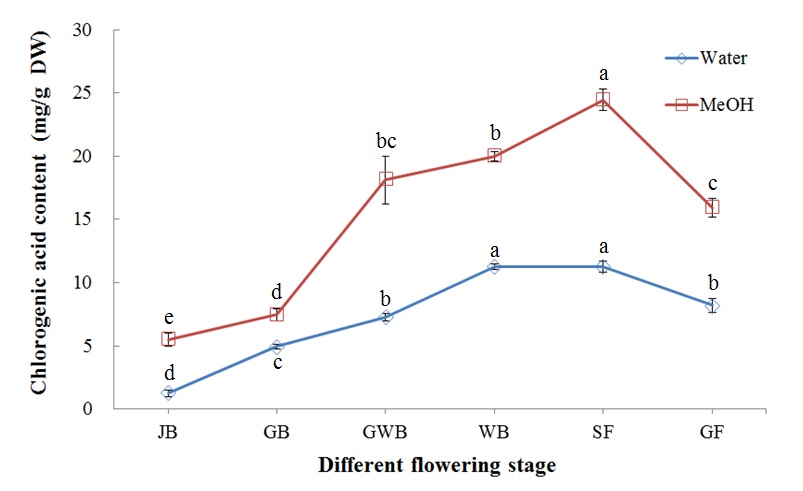 Figure 6: Chlorogenic acid content of L. japonica Thunb. at different flower development stages.
Figure 6: Chlorogenic acid content of L. japonica Thunb. at different flower development stages.
Means for the same solvent followed by the same letter are not significantly different at the 5 % level according to the LSD.
Luteolin content at different flower development stages
The efficiency of methanol extraction of luteolin was 2.08 to 4.23 times higher than that of water (Figure 7). Luteolin content slightly increased with flower development stage and reached its highest value (0.192 mg/g DW) at the GF stage after water extraction. However, it decreased from 0.60 mg/g DW at the JB stage to 0.4 mg/g DW at the GF stage.
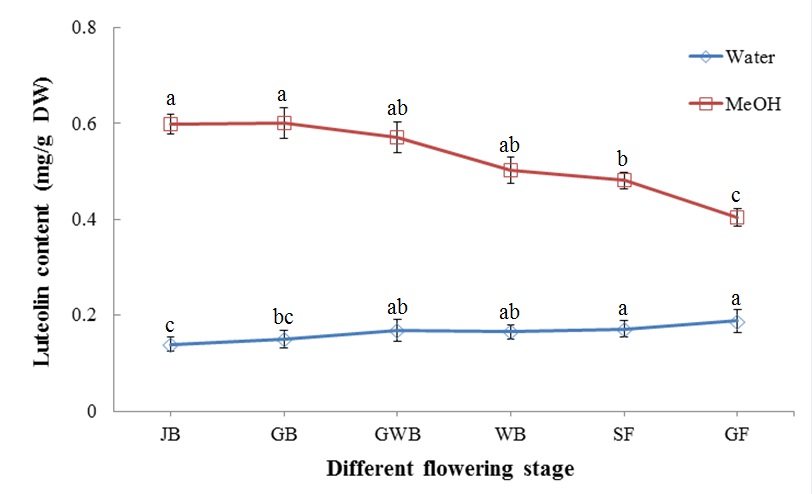 Figure 7: Luteolin content of L. japonica Thunb. at different flower development stages.
Figure 7: Luteolin content of L. japonica Thunb. at different flower development stages.
Means for the same solvent followed by the same letter are not significantly different at the 5 % level according to the LSD.
Microwave extraction is an approach that heats the solvent by microwave energy, disruption of hydrogen bonds, and enforcement of ion migration [49]. This approach is widely applied to rapidly extract biochemical compounds from many plants, such as the green tea [50], pistachio [51] and tomato plants [52]. In the present study, M3 treatment with methanol extraction led to the highest chlorogenic acid content (40 mg/g DW) in L. japonica buds (Figure 3). Zhang et al. used microwaves to extract chlorogenic acid from L. japonica and proposed that the maximum extraction content could reach 61.4 mg/g [53]. With regards to the extracting solvent, the results of the present study revealed that methanol was better than water for increasing antioxidant activity and major active constituents’ content. Similar results were also obtained for the antioxidant activity of olive leaves [54] and the total phenolic content of cactus flowers [55].
It is generally recognized that the biochemical compounds of plants change depending on the flower development stage [36,39,40]. In this study, antioxidant activity and major biochemical compounds (except luteolin) varied with the flower development stages of L. japonica and reached their highest value at the SF stage. According to Joshi et al., antioxidant activity in Camellia sinensis flowers increased with development stage and reached its maximum value when petals started to split, whereas chlorogenic acid content was highest at the stage of flower opening [37]. This result was in line with the present study. In a study investigating Echinacea purpurea, the maximum chlorogenic acid content was observed at the initial stage of flower bud development [38]. However, Zhu and Xiong found the highest chlorogenic acid content during the GWB stage of L. japonica [56]. Wang et al. also investigated the chlorogenic acid content at different development stages of L. japonica flowers and found that the highest content was present at the GB stage and decreased thereafter [18]. The present study found the highest chlorogenic acid content at the WB and SF stages. This controversy may be due to several factors, such as plant species, growing environment, growing season and extraction methods. Therefore, further studies are necessary to study the effects of these factors on the extraction of bioactive components when determining optimal harvest time for commercial demand.
CONCLUSION
Analysis of various treatment methods showed that the M3 treatment was the most efficient for extraction of antioxidant and bioactive components of Japanese honeysuckle (Lonicera japonica Thunb.). Except for luteolin content, methanol had better extraction efficiency than water. We believe that the SF stage is the optimal harvest stage because DPPH scavenging radical activity, equivalent trolox content and chlorogenic acid content were at their highest. The present results can be applied to determine the best harvest time and treatment method for enhancing the value of Chinese Traditional Medicine (CTM). However, further investigations are still needed to study the influence of environmental and biogeochemical factors on the bioactive components of L. japonica Thunb.
ACKNOWLEDGEMENT
This research was sponsored by the Ministry of Science and Technology, Taiwan under grant No. MOST 108-2313-B-343 -001 -MY3.
REFERENCES
- Schierenbeck KA (2004) Japanese honeysuckle (Lonicera japonica) as an invasive species; history, ecology and context. Crit Rev Plant Sci 23: 391-400.
- Peng LY, Mei SX, Jiang B, Zhou H, Sun HD (2000) Constituents from Lonicera japonica. Fitoterapia 71: 713-715.
- Qian ZM, Li HJ, Li P, Ren MT, Tang D (2007) Simultaneous qualitation and quantification of thirteen bioactive compounds in Flos lonicerae by high-performance liquid chromatography with diode array detector and mass spectrometry. Chem Pharm Bull 55: 1073-1076.
- Yang CC, Fang JY, Hong TL, Wang TC, Zhou YE, et al. (2013) Potential antioxidant properties and hepatoprotective effects of an aqueous extract formula derived from three Chinese medicinal herbs against CCl4-induced liver injury in rats. Int Immunopharmacol 15: 106-113.
- Kobayashi M, Davis SM, Utsunomiya T, Pollard RB, Suzuki F (1999) Antiviral effect of gingyo-san, a traditional Chinese herbal medicine, on influenza A2 virus infection in mice. Am J Chin Med 27: 53-62.
- Zhang TB, Yue RQ, Xu J, Ho HM, Ma DL, et al. (2015) Comprehensive quantitative analysis of Shuang-Huang-Lian oral liquid using UHPLC-Q-TOF-MS and HPLC-ELSD. J Pharm Biomed Anal 102: 1-8.
- Gao Y, Fang L, Cai R, Zong CJ, Chen X, et al. (2014) Shuang-Huang-Lian exerts anti-inflammatory and anti-oxidative activities in lipopolysaccharide-stimulated murine alveolar macrophages. Phytomedicine 21: 461-469.
- Chaung YX, Ge AH, Donnapee S, Li J, Bai Y, et al. (2015) The multi-targets integrated fingerprinting for screening anti-diabetic compounds from a Chinese medicine Jinqi Jiangtang Tablet. J Ethnopharmacol 164: 210-222.
- Xiong JH, Li SC, Wang WJ, Hong YP, Tang KJ, et al. (2013) Screening and identification of the antibacterial bioactive compounds from Lonicera japonica Thunb. leaves. Food Chem 138: 327-333.
- Cheng CY, Ma XQ, Kwan HY, Tse KW, Cao HH, et al. (2014) A herbal formula consisting of Rosae Multiflorae Fructus and Lonicerae Japonicae Flos inhibits inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. J Ethnopharmacol 153: 922-927.
- Seo ON, Kim GS, Park S, Lee JH, Kim YH, et al. (2012) Determination of polyphenol components of Lonicera japonica Thunb. Using liquid chromatography-tandem mass spectrometry: Contribution to the overall antioxidant activity. Food Chem 134: 572-577.
- Zhou Z, Li XH, Liu JX, Dong L, Chen Q, et al. (2015) Honeysuckle-encoded atypical microRNA2911 directly targets influenza a viruses. Cell Res 25: 39-49.
- Jeong CS, Suh IO, Hyun JE, Lee EB (2003) Screening of hepatoprotective activity of medicinal plant extracts on carbon tetrachloride-induced hepatotoxicity in rats. Nat Prod Bull Res 9: 87-90.
- Sun CH, TengY, Li GZ, Yoshioka S, Yokota J, et al. (2010) Metabonomics study of the protective effects of Lonicera japonica extract on acute liver injury in dimethylnitrosamine treated rats. J Pharm Biomed Anal 53: 98-102.
- Lin HW, Liu CW, Yang DJ, Chen CC, Chen SY, et al. (2017) Dunaliella salina alga extract inhibits the production of interleukin-6, nitric oxide and reactive oxygen species by regulating nuclear factor-κB/Janus kinase/signal transducer and activator of transcription in virus-infected. J Food Drug Anal 25: 908-918.
- Wiseman H (2013) Encyclopedia of Human Nutrition (3rd Edn). Reference Module in Biomedical Sciences. Elsevier Inc., New York, USA. Pg No: 47-51.
- Wang YP, Xue XY, Zhang FF, Xu Q, Liang XM (2008) Separation and analysis of volatile oil from Lonicera japonica Thunb. World Sci Technol 10: 45-55.
- Wang LM, Li MT, Yan YY, Ao MZ, Wu G, et al. (2009) Influence of flowering stage of Lonicera japonica Thunb. on variation in volatiles and chlorogenic acid. J Sci Food Agric 89: 953-957.
- Lee EJ, Kim JS, Kim HP, Lee JH, Kang SS (2010) Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chem 120: 134-139.
- Wang XQ, Wei FY, Wei ZF, Zhang L, Luo M, et al. (2014) Homogenate-assisted negative-pressure cavitation extraction for determination of organic acids and flavonoids in honeysuckle (Lonicera japonica Thunb.) by LC-MS/MS. Sep Purif Technol 135: 80-87.
- Yip ECH, Chan ASL, Pang H, Tam YK, Wong YH (2006) Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biol Toxicol 22: 293-302.
- Hwang SJ, Kim YW, Park Y, Lee HJ, Kim KW (2014) Anti-inflammatory effects of chlorogenic acid inlipopolysaccharide-stimulated RAW 264.7 cells. Inflamm Res 63: 81-90.
- Bassoli BK, Cassolla P, Borba-Murad GR, Constantin J, Salgueiro-Pagadigorria CL, et al. (2008) Chlorogenic acid reduces the plasma glucose peak in the oral glucose tolerance test: Effects on hepatic glucose release and glycaemia. Cell Biochem Funct 26: 320-328.
- Hu C, Kitts DD (2004) Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol Cell Biochem 265: 107-113.
- Chen CC, Chow MP, Huang WC, Lin YC, Chang YJ (2004) Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of Intercellular Adhesion Molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kappa B: structure-activity relationships. Mol Pharmacol 66: 683-693.
- Liu CW, Lin HW, Yang DJ, Chen SY, Tseng JK, et al. (2016) Luteolin inhibits viral-induced inflammatory response in RAW264.7 cells via suppression of STAT1/3 dependent NF-κB and activation of HO-1. Free Radic Biol Med 95: 180-189.
- Lee HJ, Wang CJ, Kuo HC, Chou FP, Jean LF, et al (2005) Induction apoptosis of luteolin in human hepatoma HepG2 cells involving mitochondria translocation of Bax/Bak and activation of JNK. Toxicol Appl Pharmacol 203: 124-131.
- Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJC (2008) Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Frag J 23: 213-226.
- Ramakrishna A, Ravishankar GA (2011) Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 6: 1720-1731.
- Jaime UA, Jesús PP, David HP (2014) Temperature stress causes different profiles of volatile compounds in two chemotypes of Salvia lavandulifolia Vahl. Biochem Syst Ecol 54: 166-171.
- Novak J, Lukas B, Franz C (2010) Temperature influences thymol and carvacrol differentially in Origanum spp. (Lamiaceae). J Essent Oil Res 22: 412-415.
- Shohaela AM, Alib MB, Yua KW, Hahna EJ, Islamc R, et al. (2006) Effect of light on oxidative stress, secondary metabolites and induction of antioxidant enzymes in Eleutherococcus senticosus somatic embryos in bioreactor. Process Biochem 41: 1179-1185.
- Chan LK, Koay SS, Boey PL, Bhatt A (2010) Effects of abiotic stress on biomass and anthocyanin production in cell cultures of Melastoma malabathricum. Biol Res 43: 127-135.
- Ines E, Susanne HK, Sascha R (2015) Blueberry phenolic compounds: Fruit maturation, ripening and post-harvest effects. In: Preedy VR (Ed.) Processing and Impact on Active Components in Food. Academic Press, New York, USA. Pg no: 173-180.
- Kjersti A, Remberg SF (2015) Strawberry phenolics and impact of ripening. In: Preedy VR (Ed.) Processing and Impact on Active Components in Food. Academic Press, New York, USA. Pg no: 157-164.
- Kotze MJ, Jürgens A, Johnson SD, Hoffmann JH (2010) Volatiles associated with different flower stages and leaves of Acacia cyclops and their potential role as host attractants for Dasineura dielsi (Diptera: Cecidomyiidae). S Afr J Bot 76: 701-709.
- Joshi R, Poonam, Gulati A (2011) Biochemical attributes of tea flowers (Camellia sinensis) at different developmental stages in the Kangra region of India. Sci Hortic 130: 266-274.
- Letchamo W, Livesey J, Arnason TJ, Bergeron C, Krutilina VS (1999) Cichoric acid and isobutylamide content in Echinacea purpurea as influenced by flower developmental stages. In: Janick J (Ed.) Perspectives on New Crops and New Uses. ASHS Press, Alexandria, Virginia, USA. Pg no: 494-498.
- Niu XX, Chen XW, Su H, Eneji AE, Guo YH, et al. (2014) Changes of secondary metabolites and trace elements in Gentiana macrophylla flowers: A potential medicinal plant part. Chin Herb Med 6: 145-151.
- Valentina S, Maja MP, Franci S (2013) Sepal phenolic profile during Helleborus niger flower development. J Plant Physiol 170: 1407-1415.
- Fu MR, He ZP, Zhao YY, Yang J, Mao LC (2009) Antioxidant properties and involved compounds of daylily flowers in relation to maturity. Food Chem 114: 1192-1197.
- Kao FJ, Chiang WD, Liu HM (2015) Inhibitory effect of daylily buds at various stages of maturity on nitric oxide production and the involved phenolic compounds. LWT-Food Sci Technol 61: 130-137.
- Lai YS, Shimoyamada Y, Nakayamab M, Yamagishi M (2012) Pigment accumulation and transcription of LhMYB12 and anthocyanin biosynthesis genes during flower development in the Asiatic hybrid lily (Lilium spp.). Plant Sci 193-194: 136-147.
- Zhang C, Wang WN, Wang YJ, Gao SL, Du DN, et al. (2014) Anthocyanin biosynthesis and accumulation in developing flowers of tree peony (Paeonia suffruticosa) ‘Luoyang Hong’. Postharvest Biol Technol 97: 11-22.
- Lewis DH, Bloor SJ, Schwinn KE (1998) Flavonoid and carotenoid pigments in flower tissue of Sandersonia aurantiaca Hook. Sci Hortic 72: 179-192.
- Sato M, Ramarathnam N, Suzuki Y, Ohkubo T, Takeuchi M, et al. (1996) Varietal differences in the phenolic content and superoxide radical scavenging potential of wines from different sources. J Agric Food Chem 44: 37-41.
- Shimada K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthan on the antioxidation of soybean oil incycodextrin emulsion. J Agric Food Chem 40: 945-948.
- Lu JQ, Zhan XL, Xu YT, Wan W, Cao RB, et al. (2008) Determination of chlorogenic acid and luteolin in Dendranthema indicum with HPLC. Chin J Hosp Pharm 28: 1629-1631.
- Mandal V, Mohan Y, Hemalatha S (2007) Microwave assisted extraction an innovative and promising extraction tool for medicinal plant research. Pharmacog Rev 1: 8-14.
- Pan X, Niu G, Liu H (2003) Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem Eng Prog 42: 129-133.
- Rajaei A, Barzegar M, Hamidi Z, Sahari MA (2010) Optimization of extraction conditions of phenolic compounds from pistachio (Pistachia vera) green hull through response surface method. J Agr Sci Tech 12: 605-615.
- Pinela J, Prieto MA, Carvalho AM, Barreiro MF, Oliveira MBPP, et al. (2016) Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: A nutraceutical-oriented optimization study. Sep Purif Technol 164: 114-124.
- Zhang B, Yang R, Liu CZ (2008) Microwave-assisted extraction of chlorogenic acid from flower buds of Lonicera japonica Thunb. Sep Purif Technol 62: 480-483.
- Rafiee Z, Jafari SM, Alami M, Khomeiri M (2012) Antioxidant effect of microwave-assisted extracts of olive leaves on sunflower oil. J Agr Sci Tech 14: 1497-1509.
- Ammar I, Ennouri M, Attia H (2015) Phenolic content and antioxidant activity of cactus (Opuntia ficus-indica L.) flowers are modified according to the extraction method. Ind Crops Prod 64: 97-104.
- Zhu XQ, Xiong Y (2005) Study on the best harvesting time of Lonicera japonica Thunb. Prac For Technol 5: 36-37.
Citation: Liu CW, Chen BC, Chen TM, Chang YY (2020) Effect of Different Extraction Methods on Major Bioactive Constituents at Different Flowering Stages of Japanese Honeysuckle (Lonicera japonica Thunb.). J Agron Agri Sci 3: 021.
Copyright: © 2020 Cheng-Wei Liu, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

