Background
Tubercular involvement of female genital tract is probably becoming more and more of latent form now a days. This involvement is difficult to diagnose by standard test like culture, animal inoculation and histopathology. The diagnostic dilemma is due to paucity of bacillary number in genital tract. Polymerase Chain Reaction (PCR) is fast emerging as the preferred diagnostic tool for identification of tubercular assault in female genital tract. The ovarian affection of tubercular bacilli has been investigated by some investigators but the hormonal affection requires extensive investigation. Evaluation of Anti-Mullerian Hormone (AMH) as a marker of ovarian function in those cases might have some roles.
Methods
700 patients that presented with symptoms of clinically confirmed infertility at reproductive medicine unit of Calcutta fertility mission during the months of December 2015 to December 2017 were recruited for in vitro fertilization. 414 of these patients fulfilled the required inclusion criteria were advised for Polymerase Chain Reaction (PCR) along with routine ovarian function check-up for estimating the hormone assay. A group of 108 fertile subjects were included as control group.
Results
Of the 414 subjects, 230 (55.55%) were PCR positive and 184 (44.44%) subjects were PCR negative. It has been observed that AMH concentration is much lower in PCR positive group than PCR negative and fertile group with statistical significance.
Conclusion
We concluded that tubercular involvement of genital tract may lower the AMH secretion as well as ovarian reserve causing infertility.
KEY MESSAGE
Poor ovulatory response to stimulation is observed in women undergoing IVF programme who has tubercular infestation in genital tract. This is probably due to diminished ovarian function as indicated by lowering of AMH level
GTBI: Genital Tubercular Infestation
PCR: Polymerase Chain Reaction
AMH: Anti Mullerian Hormone
ATD: Anti Tubercular Drug
IVF: In Vitro Fertilization
ORT: Ovarian Reserve Test
The estimated prevalence of Female Genital Tract Tuberculosis (FGTB) is < 1% in developed countries like USA in contrast to 18% in India as per available records [1]. Initial exposure of tuberculosis bacillus is through the lungs and based on immune status in some affected persons it remains silent without any clinical symptom. There is lack of reliable confirmatory test for this silent variety as of now [2]. This is called Latent Genital Tuberculosis (LGTB) or otherwise Genital Tubercular Infestation (GTBI). This can cause infertility due to tubal obstruction, impaired implantation due to endometrial involvement and ovulatory disorder due to ovarian involvement. The ovarian reserve determining the physiologic age of the ovary is a major predictor of a woman's reproductive capacity regardless of her chronologic age. Every woman is born with a fixed number of primordial ovarian follicles and this number declines with each passing year until menopause, when only a few follicles remain. This normal decrease in the number of primordial follicles eventually results in a low ovarian reserve, which has a direct bearing on the quality of oocytes [3]. Although the processes that control ovarian aging remain unclear, it is known that women do not respond well to ovarian stimulation when they have low ovarian reserve. Hormonal markers are used to predict ovarian response, however, if an infertile woman has high levels of Follicle Stimulating Hormone (FSH) and low levels anti-Müllerian hormone on day 2 or 3 of a natural cycle, her ovarian reserve is considered to be low. Ovarian volume, ovarian blood flow and low Antral Follicle Count (AFC) in assessed on ultrasound are also considered predictive of reproductive potential. Thus AMH acts as a useful predictor of female reproductive potential and ovarian response [4-6]. In human, AMH secretion peaks a few years after puberty and diminishes subsequently with age, becoming almost undetectable at menopause and likely reflecting the age-related decline in the ovarian reserve. During ovarian reserve assessment particularly before IVF, low AMH level is observed in LGTB or GTBI patients. The tubal and endometrial involvement by tubercle bacilli is sometimes apparent at laparoscopy and hysteroscopy, but little is known about ovarian involvement. Mycobacterium tuberculosis may have toxic effects on ovarian reserve, which would explain the poor results observed during the intrauterine insemination or IVF cycle, particularly in response to ovulation induction [7]. For diagnostic confirmation of tuberculosis, tissue based culture, histopathological feature of Epiteelioid granuloma, or detection of Acid Fast Bacilli (AFB) from the smear of tissue fluid are required but it is not possible to confirm absolute diagnosis of LGTB from the characteristic features of Hystero-Salpingogram (HSG) or laparoscopy [8]. Sometimes due to the lower bacterial load, tissue based culture or histopathology also not sensitive enough [9]. There are evidences in literature that diagnosis of LGTB may be obtained by using PCR technology [2]. The efficiency of such test is also of optimal value [10]. Even without a direct involvement of ovaries, the ovarian function, especially the endrocrinological reserve is affected in latent TB cases [11].
A prospective observational study was carried out between December 2015 to December 2017, 700 infertile patients attending reproductive medicine unit of Calcutta fertility mission during this period, were recruited for IVF were screened. 414 patients fulfilled the inclusion criteria. The selected patients had PCR test of their menstrual blood as well as from endometrial aspirate in two consecutive cycles. Those with positive PCR and negative PCR in all the samples which are included in this study. Those with positive PCR and negative PCR in all the samples were included in the study. 108 fertile subjects satisfying the inclusion criteria, during the same follow-up period, were also as fertile control group. PCR test was not performed on these patients because they were conceived within six months from the counseling. The AMH levels were compared between TB-PCR positive and negative cases and also to fertile control group, to evaluate the ovarian function.
Study design
Routine check of ovarian function by estimating Serum Follicular Stimulating Hormone (FSH), AMH and antral follicular count in early follicular phase is performed in all patients are going IVF along with other tests. A routine check of presence of tubercular bacilli in Genital Tract (GTBI or LGTB) is performed by Polymerase Chain Reaction (PCR) for tuberculosis from menstrual blood collected on d2/d3 of menstrual cycle and endometrial aspirate obtained in mid-luteal phase (d21-d23), before proceeding to IVF in our centre. The day of good flow is accepted as onset of menstrual cycle. Premenstrual spotting and any such sort of things have been excluded. The dates have been categorically asked from the patient and confirmed. The latter procedure is performed under strict asepsis in two consecutive cycles to avoid a false positive result. On the other hand check of hormone of all subjects with infertility was determined at the time of recruitment. Based on hormone status of individual subject, treatment was started by counseling procedure followed by ovulation induction. From the starting of treatment within six months, 108 subjects were conceived by following discussed method and they were treated as normal control group.
Selection criteria
The cases subjected to study were selected with the following inclusion criteria - age between 25-40 years with regular menstrual periods, having no evidence of hyper-prolactinemia, hypo-thyroidismabd, hyper-androgenism and FSH value less than 12 mIU/ml and Leutinizin Hormone (LH) value less than 10 mIU/ml in early follicular phase. These patients did not have any pelvic pathology in clinical examination or Transvaginal Ultrasound (TVS). The patients with polycystic ovarian syndrome, any history of present or past intake of Anti Tubercular Drug (ATD) were excluded from the study.
Aim
Great interest has been created to find out whether LGTB or GTBI affects ovarian function. The study is aimed at estimating AMH level as indicator of ovarian reserve in LGTB affected women. Diminished ovarian reserve is a common presentation in advanced age where AMH level is low. In many situations low AMH is detected even in younger patients affected by LGTB. Whether LGTB reduces ovarian reserve or ovarian AMH secretion is the point of interest. To obtain this information this study was undertaken in our institute.
Consent
Written informed consent from study subject was also obtained prior to sample collection at the Calcutta Fertility Mission.
Collection of sample
PCR (Polymerase Chain Reaction) study
After one year only infertile subjects were advised to attend to the clinic on the 2nd day and in between 21st to 23rd day of natural cycle. Samples were collected by using IUI cannula (intrauterine insemination) via cervical os without anesthesia. After collecting menstrual blood or endometrial tissue, the contents were transferred to the lysis buffer for Deoxyribonucleic Acid (DNA) extraction followed by multiplex PCR [2]. Ribo Nucleic Acid (RNA) PCR could not used due to logistic and financial constraints as RNA PCR was very expensive.
AMH (Anti-Müllerian Hormone) assay
On the same day (2nd day) venous blood samples were collected from those subjects for the measurement of AMH level. Serum and plasma samples were centrifuged at 1700g within 2 hours from the collection time and stored at -80°C until assayed [12].
Multiplex-PCR
DNA was extracted from already collected subject’s endometrial tissue/menstrual blood samples by using QIAamp Bacterial DNA Mini Kit (Qiagen, Hilden, Germany) and eluted in 200µl of elution buffer and was stored in -80°C for future study. A multiplex PCR based on amplification of 165, 365 and 541 bp target fragments of unrelated genes, hsp 65 coding for 65 kDa antigen, dnaJ gene of mycobacteria and insertion element IS 6110 of Mycobacterium tuberculosis, respectively was performed for all the samples as collected above [13]. Three sets of primers were used in multiplex PCR and details of primers sequence are described in elsewhere and here we used two type of primers for identification of mycobacteria strain because IS6110 insertion element was absent in the genome of mycobacteria. Most of these strains appeared to be isolated from Vietnamese people as well as north east origin [10]. So IS 6110 insertion element may be limited some certain population but our multiplex system is capable to identify the mycobacteria strain. Mastermix and condition of multiplex PCR was performed accordingly [2]. From the obtained PCR products, 15µl of each product was run on 2% agarose gel stained with ethidium bromide and visualized under Gel Documentation System (Bio-Rad, USA). The length of the PCR products was estimated by pGEM or ΦX174/Hae markers (Promega Corporation, Madison, Wisconsin, USA).
AMH assay
AMH is estimated by the AMH Gen II Enzyme-Linked Immunosorbent Assay (ELISA) from Beckman Coulter Inc., USA. AMH Gen II Elisa is an enzymatically amplified 2-site immunoassay in which the serum samples to be tested were incubated at room temperature along with calibrators and controls in microtitre wells, coated with anti-AMH antibody. Serial incubation & washing with anti-AMH detection antibody, enzyme and substrate enables to determine the enzymatic turnover of the substrate that is directly proportional to the concentration of AMH in the serum. According to our reference interval, median AMH concentration for ages 20-24 was 2.42 ng/ml, ages 25-29 was 2.87 ng/ml, ages 30-34 was 2.49 ng/ml, ages 35-39 was 1.51 ng/ml [12].
Statistical analysis
The mean, median of minimum and maximum of AMH concentration in PCR positive and negative as well as fertile control group is presented as Interquartile Range Formula (IQR) and histograms. The latter shows none of them follow normal distribution. Non-parametric test of Mann-Whitney was performed on AMH value of above 3 groups to find the difference between population averages. A nested ANOVA was performed to find out the AMH concentration in the above groups. In our study, sensitivity of AMH concentration in diagnosis of pregnancy is 36.3% and specificity of AMH concentration in diagnosis of pregnancy is 96.1%. Positive predictive value of AMH concentration in diagnosis of pregnancy is 90.7% and negative predictive value of AMH concentration in diagnosis of pregnancy is 58.6%. All the statistical analyses were done using MINITAB 17.
Ethical approval
A specific written consent was designed according to the ethical guidelines of Helsinki declaration, 1975 and received the ethical clearance from Institute of post graduate medical education & research from 03.05.2015 (Registration no- IPGME&R/IEC/2015/423) and Calcutta fertility mission(Registration no: CFM/ETHICS/008).
All infertile subjects undergoing IVF and those of normal fertile group were comparable by age (31.25 years for infertile group and 29.26 year for fertile groups), mean body mass index was 24.32 kg/m2 for former versus 22.79 kg/m2 for latter group and mean menstrual cycle length 31.14 days and 29.68 days respectively (Table 1). 230 (55.55%) subjects were DNA PCR positive both from menstrual blood and endometrial aspirate and 184 (44.44%) subjects were PCR negative (Table 2). 108 subjects did not have any PCR performed. Figures 1-3 represent the AMH concentration with PCR positive and negative and fertile group. The AMH concentration of PCR positive is found to be much less than PCR negative and fertile group. The median AMH concentration of PCR negative subjects was almost similar to that of fertile subjects (Table 2). With age stratified distribution of AMH concentration of PCR positive was much lower than PCR negative group and fertile group and value as percent and 95% CI of AMH was showed in table 3. There were significantly differences in AMH level was found in PCR positive infertile vs fertile group and PCR negative vs PCR positive group. But not significant difference between PCR negative vs fertile group. The AMH concentration among the fertile groups were significantly better than PCR positive groups (p<0.05) (Table 3). In Table 4 age wise patient percentage of PCR positive group, PCR negative group and fertile group were described.
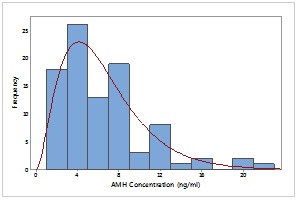 Figure 1:
Figure 1: Distribution of AMH concentration of participants with infertile PCR negative.
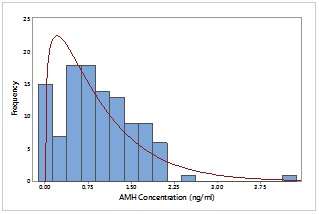 Figure 2:
Figure 2: Distribution of AMH concentration of participants with infertile PCR positive.
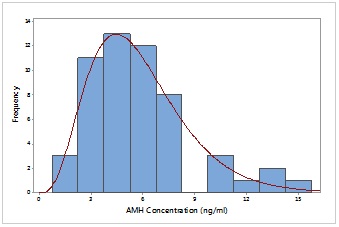 Figure 3:
Figure 3: Distribution of AMH concentration of participants with normal control group.
|
|
Infertile
|
Fertile
|
|
Characteristics
|
Range
|
Mean
|
Range
|
Mean
|
|
Age (Years)
|
20-39
|
31.25
|
24-38
|
29.26
|
|
Body mass index (kg/m2)
|
18.3-32.4
|
24.32
|
18.3-32.4
|
22.79
|
|
Normal cycle (day)
|
25-35
|
31.14
|
25-35
|
29.68
|
Table 1: Characteristics pattern of infertile and fertile group (n=414), (n= total number).
|
|
Mean
|
Median
|
Min
|
Max
|
IQR
|
|
PCR negative (n=184, 44.44%)
|
6.531
|
5.400
|
1.980
|
22.850
|
4.625
|
|
PCR positive (n=230, 55.55%)
|
0.963
|
0.880
|
0.010
|
0.880
|
0.890
|
|
Fertile group (n=108)
|
5.795
|
5.190
|
1.250
|
15.360
|
3.137
|
Table 2: Descriptive statistics of AMH concentration (IQR- Interquartile Range Formula).
|
Age (years)
|
Infertile
|
Normal control group
|
P-value of Mann-Whitney test
|
|
Median AMH concentration (ng/ml) in percent with 95% CI
|
Median AMH concentration (ng/ml) in percent with 95% CI
|
Infertile PCR negative - normal
|
Infertile PCR positive - normal
|
Infertile PCR negative - Infertile PCR positive
|
|
PCR positive
|
PCR negative
|
|
20-24
|
114.0 (91.5-134)
|
466.5 (211-772)
|
659.0 (578-970)
|
0.515
|
0.000
|
0.000
|
|
25-29
|
115.5 (84.5-152)
|
536.0 (479-792)
|
630.0 (459-790)
|
|
30-34
|
100.0 (82.5-119.5)
|
646.5 (543-804)
|
413.5 (350-538)
|
|
35-39
|
65.0 (43.5-76.5)
|
375.0 (277-550)
|
491.0 (390-620)
|
Table 3: Descriptive statistics of AMH concentration and value as percent and 95% CI of AMH.
|
Age (years)
|
Infertile
|
Normal control or Fertile group
|
|
No. of Patients (%)
|
No. of Patients (%)
|
|
PCR - Positive
|
PCR - Negative
|
|
20 – 24
|
4 (4)
|
10 (11)
|
9 (17)
|
|
25 – 29
|
20 (18)
|
31 (33)
|
21 (39)
|
|
30 – 34
|
47 (42)
|
40 (43)
|
18 (33)
|
|
35 – 39
|
40 (36)
|
12 (13)
|
6 (11)
|
Table 4: Age wise patient percentage of PCR positive group, PCR negative group and fertile group. Figure in parenthesis indicate no of patients.
Tubercular affection in latent form is gradually becoming more and more important because it affects reproductive process significantly. GTBI exerts its deleterious effects by mere presence of bacilli and due to harmful cytokine secretion [14]. The same mechanism may play a role in the ovary as harmful inflammatory cytokine might diminish ovarian function indicated by low AMH. The retrograde menstruation is a normal phenomenon of most women. In PCR positive patient, this blood may come in contact with ovary, influencing the ill effects of inflammatory cytokine. It has been evidenced in this study that AMH level significantly lowered in TB-PCR positive group, that is, patients with tubercular infestation of the genital tract. The ovarian reserve which controls the ovulatory process is usually determined by AMH level along with early follicular FSH and antral follicular. AMH maintains static level throughout the cycle without showing much variation in follicular or luteal phases. Due to low AMH level, ovary responds sub-optimally during folliculogenesis, which becomes a feature for ovulatory failure in TB-PCR positive cases. The comparison of AMH level in PCR positive and negative cases also showed significant difference even where age-stratified values were studied. Statistically significant diminished ovarian reserve was found in affected cases. The fertile controls in whom AMH was estimated has been found that their level is better than infertile group, but much better than TBPCR positive and little better than TB-PCR negative cases. We have estimated the AMH level after complete of anti tubercular treatment. We got the AMH level which was 15% higher than previous value but that rise was not exceed 25%. It is very interesting that this rise was not age specific [15]. We have estimated the AMH level after 3 months and 6 months of treatment. After 6 months of treatment, AMH value was better than 3 months value. As the fertility potential of a woman depends on its ovarian reserve vis-à-vis the AMH level, the latent tubercular involvement imparts a negative impact in female reproductive potential.
Tubercular infestation on human genital organs imparts ill effect on reproduction not only influence in endometrium or fallopian tube negatively but lowering ovarian function indicated by low AMH level in affected individuals.
No bodies have not any conflicts of interest that are directly relevant to the content of this study.
The study was funded by an intramural grant from Calcutta fertility mission.

 Figure 1: Distribution of AMH concentration of participants with infertile PCR negative.
Figure 1: Distribution of AMH concentration of participants with infertile PCR negative. Figure 2: Distribution of AMH concentration of participants with infertile PCR positive.
Figure 2: Distribution of AMH concentration of participants with infertile PCR positive. Figure 3: Distribution of AMH concentration of participants with normal control group.
Figure 3: Distribution of AMH concentration of participants with normal control group.
