
Effect of Gonadotropin Therapies in Adolescent Males with Hypogonadotropic Hypogonadism: Meta-Analysis
*Corresponding Author(s):
Swashti AgarwalDepartment Of Pediatric Endocrinology, Texas Children’s Hospital, Baylor College Of Medicine, United States
Tel:+13473427255,
Email:swashti@gmail.com
Abstract
Objective: Gonadotropins are a recent strategy for inducing puberty inadolescent males with Hypogonadotropic Hypogonadism (HH). Thebenefits of combination gonadotropin therapies (hCG+) over humanchorionic gonadotropin (hCG) monotherapy are not clear for the prepubertal adolescent population. We performed a meta-analysis to assesstherapeutic outcomes, in terms of virilization and testicular growth, inperipubertal boys with HH treated with hCG vs. hCG+ therapy.
Method: A comprehensive search of the PubMed andGoogle Scholar databases was conducted. Seven studies met ourinclusion criteria. We calculated pooled means for the post-treatmentmean testicular volume, testosterone levels, and penile length for hCGmonotherapy and for hCG+ therapy. A meta-regression analysis wasperformed to examine the contribution of various factors to post treatment outcomes, including baseline age, testosterone level,treatment duration, and study quality.
Results: Participants were prepubertal (13.3–25.9 years) with weightedmeantreatment durations of 10.95 and 28.2 months for hCG monotherapy and hCG+, respectively. Baseline age and testosteronelevels were significantly different between groups. The post-treatment mean testicular volume and penile length were not significantly different between treatment groups. The post-treatment testosterone levels were101.89 ng/dL and 424.10 ng/dL for hCG monotherapy and hCG+, respectively (P<0.0001). Treatment duration explained 3.04% of the difference (P<0.0001). However, the difference remained significant after adjusting for treatment duration.
Conclusion: hCG+ provided potential benefits over hCG monotherapy for pubertal induction in male HH. Large prospective studies are needed to establish guidelines for gonadotropin therapy in the adolescent population.
Keywords
Gonadotropin therapies; Hypogonadotropic hypogonadism, Pubertal induction; Testicular volume; Testosterone
INTRODUCTION
Hypogonadotropic Hypogonadism (HH) is one of theetiologiesofdelayed puberty. Congenital HH occurs in about 1-10 infantsper 100,000 live births [1]. Although patients with HH have normal testiclular tissue,the lackof pituitary gonadotropins causes failure of testicular stimulation and testosterone production, leading to delayed puberty.
For pubertal induction, testosterone is currently the recommended standard of care among pediatric endocrinologists [2,3]. Monthly testosteroneinjections can successfully induce puberty in patients with HH. However, testosterone therapy does not inducetesticular growth [4], and additionally reduces testicular volume in normal, unaffected male patientsthat receive androgen supplementation [5]. Changes in testicular volume are correlated with sperm countchanges in adults [4]. Although theeffects of testosterone on testicular volume were thought to be fully reversible [5], recent reports have shown that theduration of androgen use was independently and inversely associated with thelikelihood of achieving sperm output thresholds and conception [6-8]. Despite cessation of androgen treatment, patients experienced delays in achieving sperm output thresholds, decreased rates of natural conception, and increased requirements for artificial reproductive technologies [7]. Therefore, testosteroneclearly exerts several negative effects that reduce fertility and natural conceptionrates, when used for extensive time periods.Its use should be minimized when possible, in adolescentswith HH that desire to preserve future fertility.
Sertoli cell proliferation occurs during late fetal, early neonatal, and peri-pubertal phases [9,10]. Lack of gonadotropin exposure during the peri-pubertal phase in males with earlyhypothalamic-pituitary-gonadal axis impairments, causes greater impact on Sertoli cell proliferation, compared to thosethatacquire HH after puberty [10].This preludes to the benefit of using gonadotropin therapy inperi-pubertal males with HH.
Testicular size has been positively correlated with sperm counts, normal sperm morphology, spermatogenesis induction, and unassisted pregnancy in hypogonadal males [7,11]. Therefore, testicular size couldpotentiallyserve as a surrogate marker for future fertility outcomes. Treatinghypogonadal adult males with Human Chorionic Gonadotropin (hCG) and testosterone restored and maintained normal sperm counts, motility, and morphology [12,13]. In addition, males that underwentpubertal induction with gonadotropin therapy achieved adequate sperm counts in lesser time than those that received androgen therapy [7,14]. Thus, gonadotropin therapies offer prepubescent males with HH, an alternative treatment that might improve future fertility. Additionally, many males desire to have testicular growth in addition to other virilizing effects.
For inducing pubertal development and preserving fertilityin peripubertal boys with HH, the American Association of Clinical Endocrinologists guidelines (2002) recommended hCG therapy or Gonadotropin Releasing Hormone (GnRH) therapy [2]; the European Consensus Statement (2015) recommended hCG monotherapy, or hCG plus recombinant Follicle Stimulating Hormone (rFSH), or GnRH [3]; and the Japanese Society for Pediatric Endocrinology (2015) recommended hCG+Rfsh [15]. The inclusion of rFSHin the latter guidelines were based on studies that showed significant increases in testicular size, inhibin B levels, and spermatogenesis with independent rFSH administration prior to initiating other gonadotropins, in males with HH [16-18].
Recently, therapeutic strategies have shifted towards an increase in the use of gonadotropins, particularly hCG monotherapy,for pubertal induction. hCG primarily targets the Luteinizing Hormone (LH) receptor, whichleads to leydig cell stimulation and proliferation. However, both leydig and sertoli cells can be targeted with combination gonadotropin regimens, like hCG+rFSH, GnRH, and hCG + Human Menopausal Gonadotropin (HMG). Although these combination therapies are recommended in several guidelines,they are not extensively used in clinical practice for inducing puberty, largely due to the lack of evidence in the pediatric population. Consequently, pediatric endocrinologists have begun to participate in research focused onhow various gonadotropin regimens affect pubertal induction and fertility parameters.One study conducted inpre-pubescent adolescent males showed that hCG+Rfsh treatment maintained larger testicular volumes than hCG Monotherapy [19]. Another study found no significant difference in testicular volumes between the two treatment groups [20]. Currently, convincing data haveshown that gonadotropin therapies could inducepuberty in males with HH without hampering future fertility. Nevertheless, no study has systematically compared hCG monotherapy to combination gonadotropin therapies and evaluatedpubertal outcomes in prepubescent tmales with HH.
The present meta-analysis was aimedto investigate whethercombination gonadotropin therapies, targeting LH and FSH receptors (withcomplex administration regimens),were superiorto LH receptor targeted hCGmonotherapy, in terms of pubertal induction and testicular growth.
METHODS
This meta-analysis followed the Preferred Reporting Items for Systemic Reviews and Meta-Analyses (PRISMA) guidelines for design, implementation, and reporting [21].
Search strategy and study eligibility
The PubMed and Google Scholar databaseswere searched through November 2018 with the following search terms: [(“Hypogonadotropic Hypogonadism”) AND (“Males”) AND (“Treatment”)]; [(“Pre-pubertal adolescent males”) AND (“Hypogonadotropic Hypogonadism”)]; and [(“Puberty Induction”) AND (“Gonadotropin therapy”)].We also manually searched article references to identify other relevant articles.
We considered all prospective or retrospective studies that involved males with HH, with/without other pituitary deficiencies, that received gonadotropin treatment. We included all studies that assessed gonadotropin treatment outcomes, including testicular volume, testosterone, and/or virilization. The search was not restricted by country of origin. We excluded case reports, articles in languages other than English, studies involving adult males, and studies that reported mixed data on HH acquired after puberty. We also excluded studies that included patients with prior androgen or other alternative therapies, and studies within adequate data on outcomes relevant to our review.
Study selection and data extraction
Two reviewers independently evaluated study eligibility,based on the title and abstract. Potentially eligible articles were retrieved, and both reviewers assessed the full-text version for eligibility. Conflicts between reviewers were resolved through discussion. Data were extracted onthe study method, sample selection, and relevant outcomes. Data were then tabulated for analysis by a statistician.
Risk of bias assessment and study grading
Two reviewers conducted independent critical appraisalsof the studies,based on guidance and suggestions from the PRISMA statement, the Newcastle-Ottawa Scale for assessing the quality of non-randomized studies in meta-analyses and the reviewers’ knowledge of this research area (e.g., the quality of measurements for testicular volume) [22].
Study grading (Table 1) was based on three overarching topics:(1) Study population (3 criteria):tests used for confirmation of HH; significant number of post-pubertal males in sample; and attrition £20% vs.>20%; (2) study design (3 criteria): outcome of interest was/was notincluded inthe hypothesis; retrospective/prospective study; and <15vs.>15 patients per treatment group;and (3) quality of the outcome assessment (6 criteria): blinded/non-blinded investigators;testicular volume measured with Praderorchidometer/sonogram; follow-up period>6 months vs. <6 months; radioimmunoassay/chemiluminescence for measuring testosterone; selective reporting of outcomes; and single/multiple investigator study.
|
RISK OF BIAS |
STUDIES |
||||||
|
|
Aydogdu 2013 |
Balducci 1997 |
Bistritzer 1989 |
Gong 2015 |
Rohayem 2016 |
Shiraishi 2014 |
Bouvattier 1999 |
|
STUDY POPULATION |
|||||||
|
a. Standard tests for diagnosing HH in pre-pubertal males |
1 |
1 |
0 |
0 |
1 |
1 |
1 |
|
b. Reported baseline/outcome data separately for pre-pubertal males |
1 |
1 |
1 |
1 |
1 |
0 |
1 |
|
c. Attrition bias (<20% vs. <20%) |
1 |
1 |
0 |
1 |
1 |
1 |
1 |
|
STUDY DESIGN |
|||||||
|
a. Outcome of interest included in hypothesis |
1 |
1 |
1 |
1 |
1 |
0 |
1 |
|
b. Retrospective/Prospective design |
0 |
1 |
0 |
1 |
1 |
1 |
1 |
|
c. Adequate sample size (>15 vs. <15 patients per group) |
1 |
0 |
0 |
1 |
1 |
1 |
1 |
|
QUALITY OF THE OUTCOME ASSESSMENT |
|||||||
|
a. Investigator blinding for clinical outcome assessment |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
|
b. Method of measuring TV |
1 |
0 |
0 |
0 |
1 |
1 |
0 |
|
c. Adequate follow-up time |
0 |
1 |
1 |
1 |
1 |
1 |
1 |
|
d. Type of testosterone assay |
1 |
0 |
0 |
1 |
NE |
NE |
NE |
|
e. Selective reporting and publication bias |
1 |
1 |
0 |
1 |
0 |
0 |
1 |
|
f. Single or multiple investigators |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
|
TOTAL |
8 |
7 |
3 |
9 |
8 |
6 |
8 |
|
GRADEa |
Moderate |
Moderate |
Very Low |
Moderate |
Moderate |
Low |
Moderate |
|
HH: Hypogonadotropic Hypogonadism; TV: Testicular volume; NE: Not evaluable aGrades: 1-3: Very low; 4-6: Low; 7-9: Moderate; 10-12: High |
|||||||
Table 1: Study grading based on risk of bias.
Statistical analysis
Analyses were performed withR v.3.5.1, usingthe‘meta’ package, as described by Harrer, et al. [23,24]. We compared three outcomes (testosterone levels, Mean Testicular Volume [MTV], and penile length) between patients treated with hCGmonotherapyand patients treated witheither a combination ofgonadotropin therapies or GnRH (hCG+). We calculated the overall pooled means separately for the two treatment groups.
We estimated heterogeneity in baseline age, FSH levels, LH levels, testosterone levels, Tanner stages, penile lengths, and MTVs across all included studies and between the pooled studieswithin eachtreatment group (hCGmonotherapy vs. hCG+). Heterogeneity was presented usingtheDerSimonian and Laird Q-statistic.
Pooled means for the two treatment groups were calculated using arandom effects modeldue to heterogeneity in baseline factors within each group. The treatment effect was assessed via a significant estimate of heterogeneity in the pooled effects between treatment groups. When a treatment effect was significant (P<0.05), we performed meta-regression to examine whether potential confounders could account for the differences between treatment groups.Confoundersincluded baseline testosteronelevels, age, treatment duration, and study quality. We performed this step in univariable analyses (separate models for each potential confounder). When a meta-regression suggested that a given factor significantlyinfluenced the treatment effect (P<0.05),the intercept from a meta-regression using the centered factor was used to estimate theadjusted pooled mean.
RESULTS
Included studies
Of3227 articlesidentified,1474 were duplicates, and 1699 were excluded based on abstract and title screening (Figure 1).We retrieved the full text for54 articles; of these, 17 were pediatric studies. Ten studies were excluded either becausethe outcome of interestwas not clearly reportedor because a significant number of patientshadhistory of prior treatments.The final analysis included seven studies [25-31], with five hCG monotherapy and four hCG+ treatment groups.
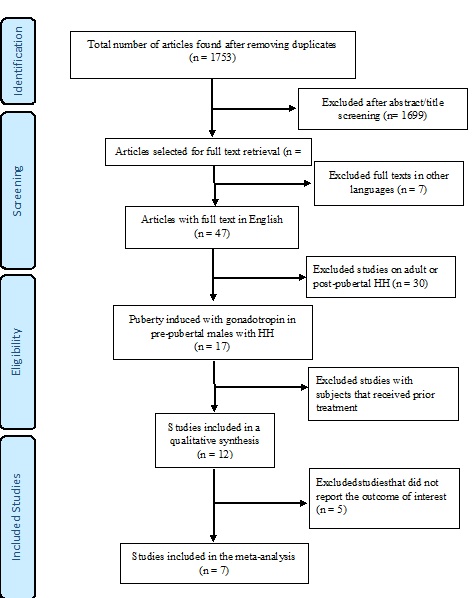 Figure 1. Flow chart of the study selection process.
Figure 1. Flow chart of the study selection process.
Study characteristics
In all participants, HH was confirmed withstandard diagnostic criteria defined in each study. None of the studies had evidence of including patients with other diagnoses like constitutional delay of puberty. No participant had prior androgen treatment. The mean participant agesranged from 13.3 to 25.9 years (Table 2). Treatmentsin the hCG+ group includedGnRH alone, hCG+rFSH, or hCG+HMG. The weighted-mean treatment durations were 10.95 months for the hCG monotherapy groupand 28.2months for the hCG+ group. Two studies also included participants treated withtestosterone [25,27]; these participants were not included in our meta-analysis, but their mean outcome data were included on forest plots as reference.This meta-analysis did not include testosterone treatment, because our search criteriadid not capture all studies that employed testosterone treatments.
|
Author (year, country) [subgroup] |
Study design (% attrition) |
Diagnostic criteria |
Cause of HH (N) |
Baseline status; prior androgen use |
Dosage
(Treatment duration) |
Other hormone deficiencies |
|
Aydogdu (2013, Turkey) [hCG] |
Retrospective (no attrition) |
1. Absent pubertyat 18 y 2. T 3. SubnormalFSH/LH 4. TS 1-2 5. Brain MRI |
IHH (25) |
Pre-pubertal; None |
5000 units twice weekly
(6 months) |
None |
|
Balducci (1997, Italy) [hCG] |
Prospective (no attrition) |
1. Pre-pubertal MTV 2. Subnormal T 3. Subnormalresponse to GnRH |
IHH (2) aCraniopharyngioma (3) Idiopathic MAPD (1) |
Pre-pubertal; None |
1500 units every 6 days
(12 months) |
GH (3) LT4 (4) Cortisone (2) |
|
Bistritzer (1989, Israel) [hCG] |
Retrospective (45% attrition) |
1. Pre-pubertal MTV 2. T 3. Subnormal FSH/LH |
IHH (22) |
Pre-pubertal; None |
5000 units weekly
(30 months) |
None |
|
Gong (2015, China) [hCG] |
Prospective (no attrition) |
1. Subnormal T 2. Subnormal FSH/LH 3. Brain MRI |
KS (15) Normosmic IHH (7) |
Pre-pubertal; None |
4-step ramp-up regimen every 3 months. Initial: 1000 units twice weekly; Goal: 2000 units every other day
(12 months) |
None |
|
Rohayem (2016, Germany) [hCG] |
Multi-center prospective (20% attrition) |
1. Absent pubertyat 14 y 2. MTV 3. SubnormalFSH/LH and T levels 4. Subnormal response to GnRH (peak LH |
KS (11) Normosmic HH (10) Pubertal arrest HH (2) Congenital MAPD (5) a Brain tumor (4) CHARGE Syn. (2)
|
Pre-pubertal; None |
Ramp-up regimen. Initial: 250-500 units twice weekly; Goal: 2500 units three times/week
(6 months) |
Replacement was provided for other hormone deficiencies |
|
Gong (2015, China) [GnRH] or [hCG+] |
Prospective (no attrition) |
1. Subnormal T 2. Subnormal FSH/LH 3. Brain MRI |
KS (8) Normosmic IHH (4) |
Pre-pubertal; None |
8-10μg of GnRH SC every 90 min
(12 months) |
None |
|
Rohayem (2016, Germany) b [hCG+rFSH] or [hCG+] |
Multi-center prospective (20% attrition) |
1. Absent pubertyat 14 y 2. MTV 3. SubnormalFSH/LH and T levels 4. Subnormalresponse to GnRH (peak LH <4U/L) |
KS (11) Normosmic HH (10) Pubertal arrest HH (2) Congenital MAPD (5) a Brain tumor (4) CHARGE Syn. (2) |
Pre-pubertal; None |
hCG: Ramp-up regimen for 6 months. Added rFSH 75-150 IU, 3 times/week
(36 months) |
Replacement was provided for other hormone deficiencies |
|
Shiraishi (2014, Japan) [hCG+rFSH] or [hCG+] |
Prospectivec (no attrition in population of interest) |
1. Subnormal morning LH/FSH and T levels 2. Pre-pubertal MTV 3. hCG stimulation test |
IHH (10) Pituitary surgery (4) KS (3) Pituitary ischemia (2) |
Pre-pubertal; None |
hCG for 6 months. Added rFSH 75 IU, 3 times/week
(24 months) |
Not reported |
|
Bouvattier (1999, France) [hCG+HMG] or [hCG+] |
Prospective (no attrition) |
1. Absent puberty 2. Bone age >13 y 3. T 4. Subnormal response to GnRH 5. Brain MRI |
KS (7) PWS (1) Congenital MAPD (7) aCraniopharyngioma (6) PSIS (4) IHH (12) |
Pre-pubertal; None |
hCG: 1500 units twice weekly for 6 months. Added HMG 75 mg, 3 times/week
(30 months) |
LT4 Corticosteroids Vasopressin |
|
Gong (2015, China) [hCG] |
Prospective (no attrition) |
4. Subnormal T 5. Subnormal FSH/LH 6. Brain MRI |
KS (15) Normosmic IHH (7) |
Pre-pubertal; None |
4-step ramp-up regimen every 3 months. Initial: 1000 units twice weekly; Goal: 2000 units every other day
(12 months) |
None |
Table 2: Characteristics of the included treatment groups.
Outcomes of interest
Our primary outcomes of interest werethe MTV, as a surrogate marker offuture fertility, and the total testosterone level. Other outcomes included the penile length and reported adverse events.
Quality of evidence
Of the seven studies included, five had a low risk of bias and a moderate grade of quality (Table1, Figure 2). Two studies had a high risk of bias with low and very low grades of quality, primarily due to small sample size, attrition bias, lack of detail about investigator blinding, and suboptimal methods for measuring outcomes (Table1).
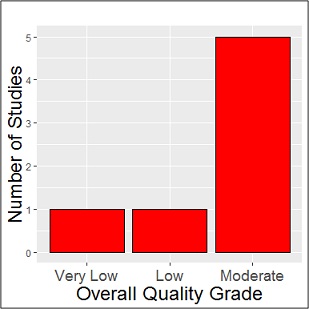 Figure 2: Quality assessment of the studies.
Figure 2: Quality assessment of the studies.
Heterogeneity in Baseline Characteristics
Across the studies,we found significant heterogeneity(Supplemental Table 1) in:baseline age (Q=147.2;df=2, P<0.001), FSH (Q=13.46;df=5;P=0.02), LH (Q=29.27;df=5;P<0.001), testosterone (Q=491.34;df=6;P<0.001), penile length (Q=39.34;df=2;P<0.001), and MTV (Q=35.52;df=8;P<0.001),but notTanner stage(Q=1.73;df=1; P=0.19). Then we pooled baseline data across studies within each treatment group and examined potential confounders of treatment effects (Supplemental Table 1). Between the two treatment groups, significant heterogeneity was found only in baseline age (Q=121.71; df=1;P<0.001) and baseline testosterone (Q=436.74;df=1;P<0.001)
|
Characteristic |
Pooled Estimates |
Tests of Heterogeneity |
||||||||
|
hCG |
hCG+ |
Between studies |
Between treatment groups |
|||||||
|
N |
Mean (95% CI) |
N |
Mean (95% CI) |
Q |
df |
P |
Q |
df |
P |
|
|
Age, years |
1 | 21.10(20.6- 21.7) | 2 | 16.63(16.1- 17.2) | 147.20 | 2
|
<0.001 |
121.71 | 1
|
< 0.001 |
|
FSH, mIU/mL |
3 |
0.79(0.7- 0.9) |
3 |
0.96(0.8- 1.1) |
13.46 |
5 |
0.02 |
2.47 |
1 |
0.12 |
|
LH, mIU/mL |
3 |
0.40(0.3- 0.5) |
3 |
0.49(0.4- 0.6) |
29.27 |
5 |
<0.001 |
1.17 |
1 |
0.28 |
|
Testosterone, ng/dL |
4 |
7.59(6.5- 8.7) |
3 |
24.82(23.6- 26.0) |
491.34 |
6 |
<0.001 |
436.74 |
1 |
<0.001 |
|
Subset of studies |
2 |
12.06(8.4- 15.8) |
2 |
15.66(12.1- 19.3) | 19.37
|
3 |
<0.001 |
1.88
|
1 |
0.12 |
|
Tanner stage |
1 |
1.30(1.1- 1.5) |
1 |
1.50 (1.3- 1.7) |
1.73 |
1 |
0.19 |
1.73 |
1 |
0.19 |
|
Penile length, cm |
3 |
5.09(4.9- 5.3) |
2 |
4.90(4.8- 5.0) |
39.34 |
2 |
<0.001 |
2.24 |
1 |
0.13 |
|
Mean testicular volume, mL |
5 |
2.03(1.8- 2.2) |
4 |
1.98(1.7- 2.3) |
35.52 |
8 |
<0.001 |
0.07 |
1 |
0.79 |
|
Note: Insufficient number of groups for subset analysis FSH: Follicle stimulating hormone; LH: luteinizing hormone; hCG: human chorionic gonadotropin groups; hCG+: combination gonadotropin therapy groups |
||||||||||
Supplemental Table 1: Heterogeneity in baseline characteristics.
Meta-analysis of outcomes
MTV: We evaluated post-treatment MTVs infivehCG monotherapygroups (n=92) andfourhCG+ groups (n=95). In addition, three testosterone groups (2 testosterone injections and 1 testosterone gel; n=68) were included in the forest-plots as reference [25,27].
Pooled mean baseline MTV (95% CI) was 2.03 mL (1.83- 2.24) and 1.98 mL (1.66- 2.30) for the hCG monotherapy and hCG+ groups, respectively. The pooled meanpost-treatment MTVs (95%CI) for the hCG monotherapy and hCG+ groups were6.60 mL (3.18–10.02) and 10.02 mL (8.30-11.75), respectively (Figure 3), but the difference was not significant (Q= 3.07;df= 1;P=0.0799).However, within-group heterogeneity in MTV was significant in bothgroups (hCG monotherapy:Q=334.49;τ2=15.10;I2=98.8%;P<0.01; hCG+: Q=15.03; τ2=2.32;I2=80.0%;P<0.01). It was unclear which factors contributed to heterogeneity in the hCG+ group. However,in the hCG monotherapy group,one study had a 95%CI for MTV that fell outside the 95%CI of the overall pooled mean [27], suggesting a strong source of heterogeneity. After excluding that study, a re-analysisrevealed a significant treatment effect (Q=34.17;df=2;P<0.001).With this exclusion, the pooledpost-treatment MTV means (95%CIs) were 4.79 (3.29-6.28) and 10.02 (8.30-11.75) for the hCG monotherapy and hCG+ groups, respectively. This post hoc analysis suggested that hCG+ therapies provided a better MTV outcome than hCG monotherapy. However, there was stillnotable heterogeneity in the hCG+ group (Q=107.96; τ2=2.26; I2=97.2%; P<0.01) that precluded drawing any firm inferences from these results.
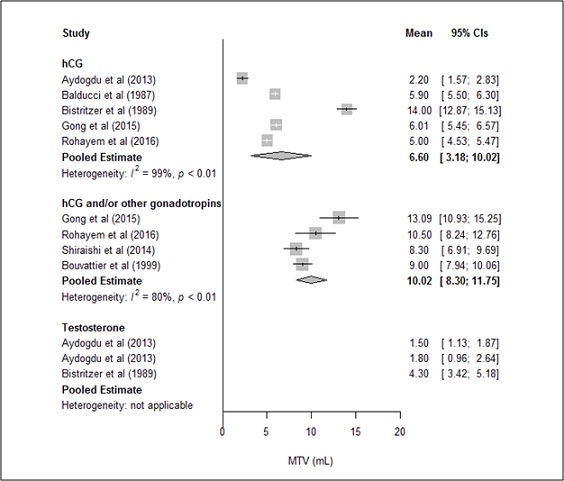
*Bold values are pooled means
Data for testosterone groups were included as a reference.
The 2013 study included two testosterone groups: testosterone gels and injections
Figure 3. Post-treatment mean testicular volumes (MTVs)in the hCG monotherapygroup versus hCG+ group.
Testosterone levels
Post-treatment testosterone levels were available forthreehCG monotherapy groups(n=53)and three hCG+groups (n=68). Two testosterone groups (1 testosterone injection and 1 testosterone gel; n=52) were included in the forest-plotsas reference [25].
The pooled mean (95%CI) testosterone levels were 101.89 ng/dL (50.7–153.08) and 424.10 ng/dL (304.59-543.62) for the hCG monotherapy and hCG+ groups, respectively. Thelack of overlapping 95%CIs indicated that post-treatment testosterone levels were significantly lower with hCG monotherapy than with hCG+, consistent with a significant difference in treatment effect between groups(Q=23.59; df=1; P<0.0001; Figure 4).
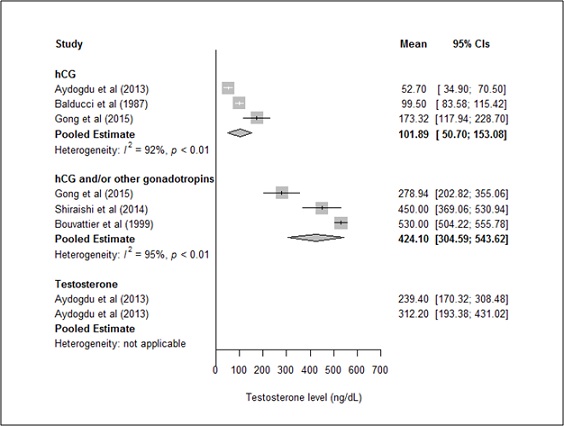
*Bold values are pooled means
Data for testosterone groups were included as a reference.
The 2013 study included two testosterone groups: testosterone gels and injections
Figure 4. Post-treatment testosterone levelsin hCG monotherapy group versus hCG+group.
A meta-regression analysis of testosterone levels showed that the treatment duration significantly contributed tothe between-group difference in treatment effects (β=20.48; Standard Eror [SE]=2.36; P<0.0001).Treatment duration explained 3.04% of the difference between groups.After adjusting for treatment duration, the pooled mean (95%CI) testosterone levels were 151.02 ng/dL (118.37-183.67) and 243.53 ng/dL (216.53–270.53) for the hCG monotherapy and hCG+ groups, respectively. The non-overlapping 95%CIs suggested that,after adjusting for treatment durations, post-treatment testosterone levels remained significantly higher in the hCG+ groupthan in the hCGmonotherapygroup. The treatment effect was notsignificantly influenced by the mean baseline participant age (β=9.44; SE=24.97; P=0.71), the mean baseline testosterone level (β=3.48; SE=6.82; P=0.61),or the study grade (β=32.89; SE=104.27; P=0.75).
Both treatment groups had significant within-group heterogeneity in post-treatment testosterone levels (hCG group: Q=25.48; τ2=1780.50; I2=92.2%; P<0.01; hCG+ group: Q=39.07; τ2=10069.69; I2=94.9%; P<0.01). The 95% CIin the Gonget al. study did notoverlap with thatof the estimated pooled mean testosterone levels [28]; suggesting thatthis study might have contributed to the heterogeneity, even though the treatment duration in the Gong, et al. study was not very different from other studies (Table 2). After excluding the Gong, et al. study from the hCG monotherapy group [28], a re-analysis (Supplemental Figure 1) showed pooled mean (95%CI) testosterone levels of 76.45 ng/dL (44.03-108.87) and 424.10 ng/dL (304.59-543.62), for the hCG monotherapy and hCG+ groups, respectively. Thus, post-treatment testosterone levels remained significantly different between the two groups (Q=30.28; df=1; P<0.0001).
Even though baseline testosterone levels did not significantly contribute to the estimated treatment effect, we re-ran the analysis after excluding the Gong and the Bouvattier studies that contributed to baseline testosterone heterogeneity (Supplemental Figure 2) [28,31]. However, thedifference between the two groupsremained significant (Q=20.94;df=1; P<0.0001).
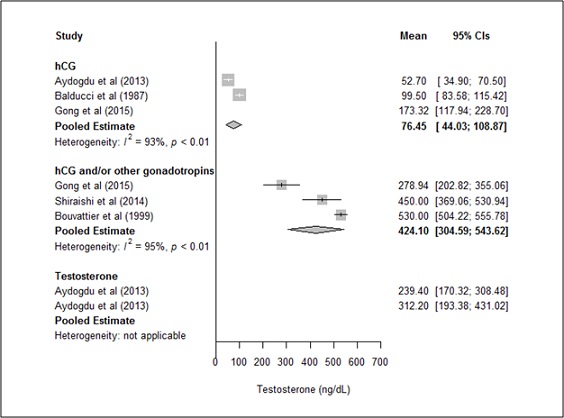
*Bold values are pooled means
Data for testosterone groups were included as a reference.
The 2013 study included two testosterone groups: testosterone gels and injections
Supplemental Figure 1: Post-treatment testosterone levels in hCG monotherapygroup (excluding the Gong et al. hCG subgroup) versus hCG+ group.
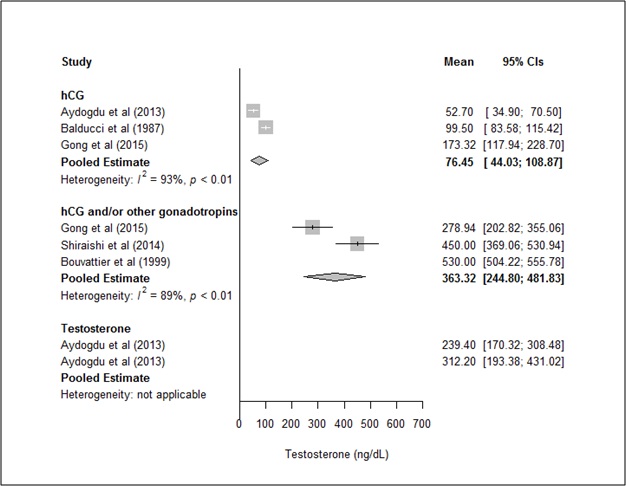
*Bold values are pooled means
Data for testosterone groups were included as a reference.
The 2013 study included two testosterone groups: testosterone gels and injections
Supplemental Figure 2. Post-treatment testosterone levels in hCG monotherapygroup (excluding the Gong et al. hCG subgroup) versus hCG+ group (excluding the Bouvattier, et al. hCG+ subgroup).
Penile length
Penile length was reported in three studies, includingtwohCG monotherapy groups (n=28) and one hCG+ group (n=12). The pooled mean (95%CI) post-treatment penile lengths were 6.09 cm (5.71–6.46) and 8.03 cm (7.33–8.73) forthe hCG monotherapy andhCG+ groups, respectively (Figure 5). The non-overlapping 95% CIs suggested that the hCG+ therapies provided a better post-treatment penile length than hCG monotherapy.However, the small number of studies was insufficient to drawfirm conclusions.
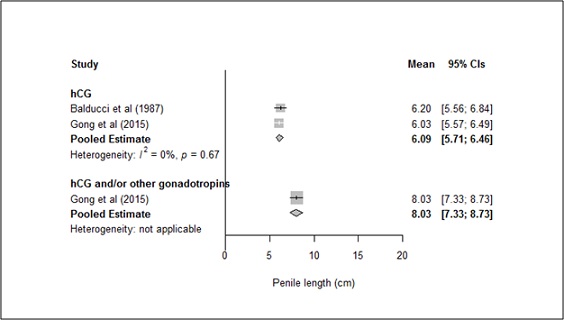
*Bold values are pooled means
Figure 5: Post-treatment penile length in hCG monotherapy group versus hCG+ group.
Ameta-regression analysis with penile length as the dependent outcome showed that treatment duration significantly contributed to the estimated treatment effect (β=19.44; SE=2.65; P<0.0001). Indeed, treatment durationexplained >99% of the treatment effect. However, we had insufficientdata points to adjust the pooled means for treatment duration. In contrast, baseline age (β=9.33; SE=25.54; P=0.71) and study grade (β=37.97; SE=97.16; P=0.70) did not significantly contribute to the estimated treatment effect.
Adverse events
Nolocal injection sitereactions were reported.Although few adverse events occurred, the hCG+ group reported them more often than the hCG monotherapy group. Pulsatile GnRH therapy (hCG+ group) was associated with increased frequencies of erections and testicular pain in two patients; these events required a reduction in the GnRH dose [28]. Gynecomastiawas reported by five patients in the hCG+ group [28,29]. In the hCG monotherapy group, two patients reported gynecomastia, and another patient reported increased acne [28].
DISCUSSION
Our results showed that post-treatment MTVs, in both treatment groups, were higher than the baseline values, but were not significantly differentbetween the two groups. Otherpediatric studies that were excluded from our analysis also found significant increases in MTV over baseline with bothhCG monotherapy and hCG+ therapies,but there was no comparisonbetween regimens [16,32-34].
Comparison between gonadotropin regimens in adults also found no difference in post-treatment MTVs between hCG monotherapy and hCG+ treatment groups [20,35,36]. Comparison in pediatrics, albeit few, have shown variedtesticular growthwith these therapies. In a study by Zacharin, et al. the final MTVs, in pre-pubescent males (some received prior androgen treatments), were similar after 9 months of therapy with hCG and hCG+Rfsh [20]. This finding was similar to our findings, but they contrasted withresults from Sinisi, et al. who found significantly different final MTVs between these therapeutic groupsafter 18 months of treatment in prepubertal males [19]. The discrepancy between these studies might be explained by different treatment durations. However, the weighted-mean treatment durationwas 19.7 months for all studies analyzed in our meta-analysis, which was comparable to the treatment duration in the Sinisi study.
We found that testosterone levels were significantly lower withhCG monotherapythan with hCG+ therapy suggesting that hCG+ therapies were superior to hCG monotherapy, in terms of androgenic activity. However,there were no reports of decreased libido or fewer erections in the hCG monotherapy group. Our results were not consistent with other pediatric studies [19,20], that foundno differencesin total testosterone levels between hCG monotherapy and hCG+rFSH groups. This discrepancy mightbe explained by differences in assays and dose regimens. However, it is difficult to overlook the significant difference that we observed between the treatment groups.
In deed, hCG+ therapies would have an added stimulative effect on Sertoli cells, but since Leydig cells are primarily responsible for testosterone production, we could not explainthe significantly higher levels of testosterone in the hCG+ group. Animal studies have suggested thatthe addition of FSH improves the androgenic activity of Leydig cells.Previous animal studies had indicated that FSH directly stimulated androgenesis in Leydig cells [37,38]. However, those studies were conducted before the availability of rFSH; therefore, results were likely confounded by contamination with LH components. More recently, the effect of rFSH on testosterone levels were studied in vivo (FSH-receptor knockout mice vs. wild-type mice) and in vitro (Leydig cellsexposed vs. unexposed to FSH anti-serum). Increased androgenesis was not seen inresponse to rFSH alone. However, androgenesis was increased in Leydig cells treated with both LH and rFSH, compared to those treated with LHalone [39,40]. It was therefore suggested that FSH plays a role in the post-LH receptor signaling promoting steroidogenesis in Leydig cells.
We found significantly higher post-treatment penile lengthsin the hCG+ groupthan in the hCG group. However, it was difficult to draw conclusions,because these findings were based on data from only three studies.
Strengths and limitations
This meta-analysis was the first to comparethe effects of different gonadotropin therapies in prepubescent adolescent males with HH. Despite the lack of large pediatric studies on gonadotropin therapies, by pooling study findings, we could evaluate outcomes formore than 100 pediatric patients. We found that hCG monotherapy and hCG+ therapies had similar effects on the final MTV, but significantly different effects on the final testosterone levels. This result was highly novel.
With strict inclusion/exclusion criteria, we avoided intermixing treatment groups with adult or post-pubertal patients or with patients that had received prior androgen therapies. Amajor limitation of this meta-analysiswas the limited number of studiesthat met our inclusion and exclusion criteria. This small numberprevented a full sensitivity analysis within subgroups to reduce the effects of heterogeneity. However, we could evaluate heterogeneity across studies and between treatment groups, andadjusted for it,when there was a significant difference in outcomes.Another limitation was the lack of studies that directly compared outcomes between treatment groups. This limitation made it difficult to draw firm conclusions on the differences in post-treatment outcomes between groups.Finally, we could not fully rule out the possibility that population differences might have occluded some true effects. This possibility remains to be tested in more rigorous study designs.
Clinical implications and future directions
As we shifttowards the use of gonadotropin therapy forpubertal induction, it is important to understand the different outcomes and adverse events associated with different therapies. Our approach was an exploratory comparison of the effects ofdifferent gonadotropin therapies in pre-pubescent adolescents. Larger prospective pediatric trials are needed to compare different dose regimens and evaluate pubertal outcomes, fertility outcomes, and adverse effects. Ultimately, this knowledge will provide better understanding and a basis for establishing guidelines for gonadotropin therapy adinistration in adolescents with HH.
CONCLUSION
Our results demonstrated thatinducing puberty with either hCG monotherapy or hCG+ therapiesin adolescent males with HH was associated with increases in post-treatment MTVs compared to baseline MTVs. However, post-treatment MTVs were not significantly different between the two treatment groups. Our findings also implied that,compared to hCG monotherapy, hCG+ therapies might result in high ertestosterone levels and penile growth, when inducing puberty in males with HH. Our results should increase awareness among pediatric endocrinologists about the use of gonadotropins. This knowledge will aid clinicians in explaining the expected outcomes of different gonadotropin regimensto patients and families.
ACKNOWLEDGEMENT
Dr. Wood was funded, in part, by a USDA/ARS cooperative agreement #58-3092-5-001. The contents of this publication do not necessarily reflect the views or policies of the U.S. Department of Agriculture, nor does any mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
REFERENCES
- Fraietta R, Zylberstejn DS, Esteves SC (2013) Hypogonadotropic hypogonadism revisited. Clinics (Sao Paulo) 68: 81-88.
- Petak SM, Nankin HR, Spark RF, Swerdloff RS, Rodriguez-Rigau LJ, et al. (2002) American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients--2002 update. Endocr Pract 8: 440-456.
- Boehm U, Bouloux P-M, Dattani MT, de Roux N, Dodé C, et al. (2015) European Consensus Statement on congenital hypogonadotropic hypogonadism--pathogenesis, diagnosis and treatment. Nat Rev Endocrinol 11: 547.
- Palacios A, McClure RD, Campfield A, Swerdloff RS (1981) Effect of testosterone enanthate on testis size. J Urol 126: 46-48.
- Zachmann M, Ferrandez A, Murset G, Gnehm HE, Prader A (1976) Testosterone treatment of excessively tall boys. J Pediatr 88:116-123.
- Kohn TP, Louis MR, Pickett SM, Lindgren MC, Kohn JR, et al. (2017) Age and duration of testosterone therapy predict time to return of sperm count after human chorionic gonadotropin therapy. Fertility and sterility 107: 351-357.
- Liu PY, Baker HW, Jayadev V, Zacharin M, Conway AJ, et al. (2009) Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab 94: 801-808.
- Coward RM, Rajanahally S, Kovac JR, Smith RP, Pastuszak AW et al. (2013) Anabolic steroid induced hypogonadism in young men. J Urol 190: 2200-2205.
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS (2003) Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125: 769-784.
- Rohayem J, Sinthofen N, Nieschlag E, Kliesch S, Zitzmann M (2016) Causes of hypogonadotropic hypogonadism predict response to gonadotropin substitution in adults. Andrology 4: 87-94.
- Bujan L, Mieusset R, Mansat A, Moatti JP, Mondinat C, et al. (1989) Testicular size in infertile men: relationship to semen characteristics and hormonal blood levels. British Journal of Urology 64: 632-637.
- Chen YW, Niu YH, Xu H, Wang D-Q, Jianget H-Y, al. (2019) Testosterone undecanoate supplementation together with human chorionic gonadotropin does not impair spermatogenesis in males with isolated hypogonadotropic hypogonadism: a retrospective study. Asian J Androl 21: 413-418.
- Hsieh TC, Pastuszak AW, Hwang K, Lipshultz LI (2013) Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol 189: 647-650.
- Liu PY, Gebski VJ, Turner L, Conway AJ, Wishart SM, et al. (2002) Predicting pregnancy and spermatogenesis by survival analysis during gonadotrophin treatment of gonadotrophin-deficient infertile men. Hum Reprod 17: 625-633.
- Sato N, Hasegawa T, Hasegawa Y, Arisaka O, Ozono K, et al. (2015) Treatment situation of male hypogonadotropic hypogonadism in pediatrics and proposal of testosterone and gonadotropins replacement therapy protocols. Clin Pediatr Endocrinol 24: 37-49.
- Raivio T, Wikstrom AM, Dunkel L (2007) Treatment of gonadotropin-deficient boys with recombinant human FSH: long-term observation and outcome. Eur J Endocrinol 156: 105-111.
- Dwyer AA, Sykiotis GP, Hayes FJ, Boepple PA, Lee H, et al. (2013) Trial of recombinant follicle-stimulating hormone pretreatment for GnRH-induced fertility in patients with congenital hypogonadotropic hypogonadism. J Clin Endocrinol Metab 98: 1790-1795.
- Young J, Chanson P, Salenave S, Noël M, Brailly S, et al. (2005) Testicular anti-mullerian hormone secretion is stimulated by recombinant human FSH in patients with congenital hypogonadotropic hypogonadism. J Clin Endocrinol Metab 90: 724-728.
- Sinisi AA, Esposito D, Maione L, Quinto MC, Visconti D, et al. (2008) Seminal anti-Mullerian hormone level is a marker of spermatogenic response during long-term gonadotropin therapy in male hypogonadotropic hypogonadism. Human Reproduction 23: 1029-1034.
- Zacharin M, Sabin MA, Nair VV, Dabadghao P (2012) Addition of recombinant follicle-stimulating hormone to human chorionic gonadotropin treatment in adolescents and young adults with hypogonadotropic hypogonadism promotes normal testicular growth and may promote early spermatogenesis. Fertility and sterility 98: 836-842.
- Moher D, Shamseer L, Clarke M, Ghersi, D, Liberati A, et al. (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4: 1.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, et al. (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62: 1-34.
- Guido Schwarzer JRC, Gerta Rücker (2015) Meta-Analysis with R 1ed: Springer International Publishing.
- Harrer M, Cuijpers P, Furukawa TA, Ebert DD (2019) Doing Meta-Analysis in R: A Hands-on Guide. 2019: https://bookdown.org/MathiasHarrer/Doing_Meta_Analysis_in_R/.
- Aydogdu A, Bolu E, Sonmez A, Tasci I, Haymana C, et al. (2013) Effects of three different medications on metabolic parameters and testicular volume in patients with hypogonadotropic hypogonadism: 3-year experience. Clin Endocrinol (Oxf) 79: 243-251.
- Balducci R, Toscano V, Casilli D, Maroder M, Sciarra F, et al. (1987) Testicular responsiveness following chronic administration of hCG (1500 IU every six days) in untreated hypogonadotropic hypogonadism. Horm Metab Res 19: 216-221.
- Bistritzer T, Lunenfeld B, Passwell JH, Theodor R (1989) Hormonal therapy and pubertal development in boys with selective hypogonadotropic hypogonadism. Fertil Steril 52: 302-306.
- Gong C, Liu Y, Qin M, Wu D, Wang X (2015) Pulsatile GnRH Is Superior to hCG in Therapeutic Efficacy in Adolescent Boys With Hypogonadotropic Hypogonadodism. J Clin Endocrinol Metab 100: 2793-2799.
- Rohayem J, Hauffa BP, Zacharin M, Kliesch S, Zitzmann M (2017) Testicular growth and spermatogenesis: new goals for pubertal hormone replacement in boys with hypogonadotropic hypogonadism? -a multicentre prospective study of hCG/rFSH treatment outcomes during adolescence. Clin Endocrinol (Oxf) 86: 75-87.
- Shiraishi K, Oka S, Matsuyama H (2014) Assessment of quality of life during gonadotrophin treatment for male hypogonadotrophic hypogonadism. Clin Endocrinol 81: 259-265.
- Bouvattier C, Tauber M, Jouret B, Chaussain JL, Rochiccioli P (1999) Gonadotropin treatment of hypogonadotropic hypogonadal adolescents. J Pediatr Endocrinol Metab 1: 339-344.
- Barrio R, de Luis D, Alonso M, Lamas A, Moreno JC (1999) Induction of puberty with human chorionic gonadotropin and follicle-stimulating hormone in adolescent males with hypogonadotropic hypogonadism. Fertil Steril 71: 244-248.
- Wang Q, Jiang W, Li G, Tang L, Hu Y (2014) Comparison of therapeutic response to gonadotropin therapy between chinese male adolescents and young adults with hypogonadotropic hypogonadism caused by pituitary stalk interruption. Horm Metab Res 46: 668-673.
- Kim S-O, Ryu KH, Hwang IS, Jung SI, Oh KJ, et al. (2011) Penile growth in response to human chorionic gonadotropin (HCG) treatment in patients with idiopathic hypogonadotrophic hypogonadism. Chonnam Med J 47: 39-42.
- Yang L, Zhang SX, Dong Q, Xiong ZB, Li X (2012) Application of hormonal treatment in hypogonadotropic hypogonadism: more than ten years experience. Clinical Trial 44: 393-399.
- Vicari E, Mongioi A, Calogero AE, Moncada ML, Sidoti G, et al. (1992) Therapy with human chorionic gonadotrophin alone induces spermatogenesis in men with isolated hypogonadotrophic hypogonadism--long-term follow-up. Int J Androl 15: 320-329.
- Chen YI, Payne AH, Kelch RP (1976) FSH stimulation of Leydig cell function in the hypophysectomized immature rat. Proc Soc Exp Biol Med 153: 473-475.
- van Beurden WM, Roodnat B, de Jong FH, Mulder E, van der Molen HJ (1976) Hormonal regulation of lh stimulation of testosterone production in isolated leydig cells of immature rats: The effect of hypophysectomy, fsh, and estradiol-17β. Steroids 28: 847-866.
- Baker PJ, Pakarinen P, Huhtaniemi IT, Abel MH, Charlton HM, et al. (2003) Failure of normal Leydig cell development in follicle-stimulating hormone (FSH) receptor-deficient mice, but not FSHbeta-deficient mice: role for constitutive FSH receptor activity. Endocrinology 144:138-145.
- Sriraman V, Jagannadha Rao A (2004) Evaluation of the role of FSH in regulation of Leydig cell function during different stages of its differentiation. Molecular and Cellular Endocrinology 224: 73-82.
Citation: Agarwal S, Austin P, Wood AC, Karaviti LP (2020) Effect of Gonadotropin Therapies in Adolescent Males with Hypogonadotropic Hypogonadism: Meta-Analysis. Arch Urol 3: 010.
Copyright: © 2020 Swashti Agarwal, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

