
Effect of Intra-Coronary (IC) Tirofiban Following Aspiration Thrombectomy on Infarct Size, in Patients with Large Anterior STEMI undergoing Primary PCI
*Corresponding Author(s):
Ahmed BasuoniCardiology Department, Dar Al-Fouad Hospital, Cairo, Egypt
Tel:+20 111234582,
Email:ahmedmos179@gmail.com
Abstract
Thrombus embolization during Percutaneous Coronary Intervention (PCI) in STEMI results in sub-optimal myocardial perfusion and increased infarct size. This study aimed to evaluate effect of Intra-Coronary (IC) delivery of bolus Tirofiban following aspiration thrombectomy on reduction of infarct size using cardiac Magnetic Resonance (cMR) in patients with large anterior STEMI undergoing primary PCI.
Patients and Methods
A Prospective single-blinded randomized controlled trial of 100 patients with large anterior STEMI was screened at 2 sites in one country (Egypt). Aspiration thrombectomy was performed in all patients using a 6 F aspiration catheter. Patients were randomized to IC Tirofiban (Study group) and no IC Tirofiban (control group). To ensure high intra-thrombus drug concentrations, Tirofiban was administered locally at the site of the infarct lesion via the aspiration catheter after flushing of the aspiration catheter well.
Results
Patients randomized to IC Tirofiban compared with no IC Tirofiban had a significant reduction of infarct size at 30 days (median, 15.451 gm - IQR, 17.404 gm - n = 50) vs (median, 43.828 gm - IQR, 49.599 gm - n = 50) P value = 0.002. There is no significant difference in MACCE at 90 days between patients received bolus IC Tirofiban and patients who did not receive (P value = 0.723).
Conclusion
In patients with large anterior STEMI presenting early after symptom onset and undergoing primary PCI, infarct size at 30 days was significantly reduced by bolus intracoronary Tirofiban delivered to the infarct lesion site followed aspiration thrombectomy but not by manual aspiration thrombectomy only.
Keywords
INTRODUCTION
PATIENTS AND METHODS
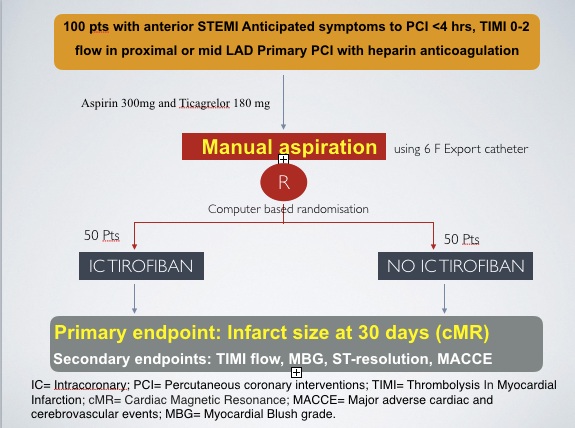 Figure 1
Figure 1The study was approved by the institutional review board at each participating center, and all eligible patients signed informed and written consent. Patients 18 years and older of both genders with symptoms consistent with STEMI longer than 30 minutes duration and 1 mm or greater of ST-segment elevation in 2 or more contiguous leads in V1-V4, or new left bundle-branch block, with anticipated symptom-onset-to-device time of 6 hours or less (i.e., symptom-to-presentation time, 4 hours) were eligible for enrolment. Large acute Anterior STEMI defined by ECG showing at least 1 mm of ST- segment elevation in 2 or more contiguous leads in V1-V4, or new or presumably new) left bundle branch block. Infarct artery located in the proximal or mid Left Anterior Descending Artery (LAD), with TIMI 0/1/2 flow at the time of initial diagnostic angiography and based on coronary anatomy, PCI is indicated for revascularization.
Principal exclusion criteria included prior myocardial infarction, prior systolic dysfunction (ejection fraction < 40%), prior Coronary Artery Bypass Graft (CABG), previously stent implantation in LAD and in whom CA demonstrates stent thrombosis to be the cause of the AMI. As well as severe vessel tortuosity, diffuse disease or severe calcification is present which may impede successful delivery of aspiration device. Finally patients with Contraindication to cardiac Magnetic Resonance (cMR) were excluded.
Patients were loaded with dual antiplatelet regimen at time of presentation (Aspirin 300mg and Ticagrelor 180 mg). Adequate anticoagulation was done using IV un-fractionated heparin guided by ACT. All patients did diagnostic angiography to determine the culprit lesion, TIMI flow and any angiographic exclusion criteria. Guiding Catheter was placed then PTCA wire was placed distal to culprit lesion. Aspiration thrombectomy was performed in all patients using a 6 F Export Catheter. Patients were randamozied (computer based) to IC Tirofiban (Study group) and no IC Tirofiban (control group). The protocol specified actively aspirating whenever crossing the lesion or withdrawing the catheter, making several passes until no further thrombus or debris was retrieved. To ensure high intra-thrombus drug concentrations, a 25 mcq/kg bolus of Tirofiban was administered locally at the site of the infarct lesion via the aspiration device after flushing of the aspiration device well. Percutaneous coronary intervention was performed using standard techniques, with drug-eluting stents implantation. Assessment of final TIMI flow and myocardial blush were done by blinded observer. After PCI, all patients were treated with aspirin indefinitely and with Ticagrelor for at least 1 year. cMR and clinical follow up were scheduled for all patients at 30 days. The MRI studies have been read blindly to treatment allocation. The cardiac MRI studies (by blind operator) were performed using 1.5 Tesla MRI machine with dedicated cardiac software, phased-array surface receiver coil, and electrocardiogram triggering. Breath-hold steady-state free-precession cine Cardiac MRI, T2-weighted imaging (edema imaging), 1st pass of contrast (perfusion study) and delayed myocardial enhancement using gadolinium (Gd-DTPA 0.2 mmol/kg) were performed. Off-line assessment of infarct mass as well as LVEF was done.
End Points and Definitions
Statistical analysis
RESULTS
|
Variables |
Aspiration + IC Tirofiban |
Aspiration + no IC Tirofiban |
P -value |
|
Age |
52.2 ± 6.9 |
47.32 ± 7.4 |
0.02 |
|
Sex |
38 (76%) |
42 (84%) |
0.48 |
|
Smoking |
36 (72%) |
34 (68%) |
0.76 |
|
Diabetes Mellitus |
22 (44%) |
20 (40%) |
0.77 |
|
Hypertension |
8 (16%) |
16 (32%) |
0.19 |
|
Dyslipidemia |
6 (12%) |
6 (12%) |
1 |
|
FH of CAD |
4 (16%) |
4 (16%) |
1 |
|
Obesity |
10 (20%) |
4 (8%) |
0.42 |
|
CKD |
0 |
0 |
|
|
LEAD |
0 |
0 |
|
|
Kilip class II |
6 (12%) |
6 (12%) |
1 |
|
Prior MI or CABG |
0 |
0 |
|
|
Anterior MI |
50 (100%) |
50 (100%) |
|
|
Pain to door (hours) |
median 4, IQR 2 |
median 4, IQR 2 |
0.99* |
|
Door to balloon (minutes |
median 30, IQR 30 |
median 30, IQR 15 |
0.75* |
Procedural data for the patients assigned to the study group who received Intracoronary Tirofiban vs the control group who did not receive Tirofiban appeared in (Table 2). Manual aspiration was performed in all patients. Discharge medications included Aspirin, Ticagrelor, statins, Beta blockers and ACEIs in 100% of patients with no difference between the 2 groups.
|
Variables |
Aspiration + IC Tirofiban n = 50 |
Aspiration + no IC Tirofiban n = 50 |
p- value |
|
Proximal LAD artery |
30 (60%) |
28 (56%) |
0.77 |
|
Mid LAD artery |
20 (41.7%) |
22 (45.8%) |
0.77 |
|
Thrombus grade III |
8 (16%) |
4 (8%) |
0.42 |
|
Thrombus grade IV |
0 |
0 |
|
|
Thrombus grade V |
40 (80%) |
46 (92%) |
|
|
Drug Eluting Stents |
50 (100%) |
50 (100%) |
1 |
|
Number of stents |
median 1, IQR 0 |
median 1, IQR 0 |
0.5* |
|
stent length >30 mm |
10 (41.7%) |
7 (29.2%) |
0.37 |
|
Radial approach |
30 (60%) |
29 (58%) |
Infarct Size
|
Variables |
Aspiration + IC |
Aspiration + no IC |
p- value |
|
Cardiac enzymes CKMB (TIME TO PEAK) |
median 13.5, IQR 7 |
median 12, IQR 8 |
0.58* |
|
ECG st. segment resolution post PCI |
50 (100%) |
50 (100%) |
|
|
ECHO (EF%) |
median 46, IQR 13 |
median 40.5, IQR 16 |
0.13* |
|
TIMI FLOW 3 |
44 (88%) |
46 (92%) |
1 |
|
Myocardial Blush grade 2/3 |
42 (84%) |
46 (92%) |
0.67 |
|
cMR infarction size gram (gm) |
Median 15.451, IQR 17.404 |
Median 43.828, IQR 49.599 |
0.002 * |
|
% Reduction of infarct size |
Median 13.3, IQR 8.7 |
Median 25.45, IQR 24.4 |
0.002 |
Myocardial Perfusion and ST-Segment Resolution
Clinical outcomes
|
Variables |
Aspiration + IC Tirofiban |
Aspiration + no IC Tirofiban |
p- value |
|
MACCE |
4 (8 %) |
6 (12 %) |
0.723 |
|
Heart failure |
3 |
5 |
|
|
Stroke |
0 |
0 |
|
|
Reinfarction |
1 |
1 |
|
|
Death |
0 |
0 |
|
|
Bleeding |
|||
|
TIMI major |
0 |
0 |
|
|
TIMI minor |
6 (12 %) |
4 (8 %) |
0.48 |
|
Thrombocytopenia |
0 |
0 |
DISCUSSION
We therefore limited enrolment of patients with proximal or mid LAD occlusion (and without prior MI) and operator assessed baseline TIMI 0-2 flow. We also restricted enrolment of patients who could be treated early, in whom the time window for effective myocardial salvage had not closed [9]. Indeed, the median time from the onset of symptoms to hospital arrival was 4 hours, as well as the median door-to-device time was 30 minutes. Thus, the study population represents a highly selected cohort of patients with large anterior MI (those with the greatest clinical need), in whom infarct size reduction should be feasible given early presentation and rapid treatment.
We assessed infarct size by cMR, which strongly consistent with subsequent mortality [2,10]. To decrease sample size, prior studies using cMR have typically measured infarct size early after reperfusion (2-7 days), a period during which substantial myocardial edema is present that may be mischaracterised as non-viable myocardium [11,12]. We therefore powered the present trial for assessment of the primary infarct size end point at 30 days (when much of the myocardial edema has resolved), a time more specific for identification of truly infarcted myocardium [11].
Myocardial reperfusion was assessed by several complementary parameters, including post-PCI TIMI flow, MBG, and STR [13]. Despite of using heparin as the procedural anticoagulant in addition to intracoronary Tirofiban, there was no significance in the major and minor bleeding risks. These results need to be placed in the context of previous studies. Two earlier randomized trials demonstrated infarct size reductions with intracoronary compared with intravenous glycoprotein IIb/IIIa receptor antagonist (despite enrolment of patients with nonanterior MI presenting up to 12 hours after symptoms) [14,15]. And a meta-analysis of 6 randomized trials (1246 patients) reported enhanced survival with intracoronary abciximab [16]. However, the recently completed AIDA STEMI trial, which with 2065 randomised patients was powered for clinical outcomes, found nearly identical rates of MACE (and biomarker-assessed infarct size) with bolus intracoronary and intravenous abciximab [17]. In contrast to INFUSE AMI trail which used bivilurdin as the procedural anticoagulant without routine intravenous glycoprotein IIb/IIIa receptor antagonist [18], we used heparin in our trial as many studies have suggested that infarct size might be reduced by adding intravenous glycoprotein IIb/IIIa receptor antagonist and heparin [19]. However, in addition to enrolling only anterior STEMI patients presenting early, except for INFUSE AMI trial, all prior trials (including AIDA-STEMI), intracoronary glycoprotein IIb/IIIa receptor antagonist was infused proximally through the guide catheter, limiting its penetration into occlusive thrombus and allowing preferential drug flow to lower resistance pathways (such as the left circumflex artery) and blowback into the aorta. In contrast, the local drug delivery through the aspiration device used in our present study directly achieves high intraclot concentrations of glycoprotein IIb/IIIa receptor antagonist at the site of LAD occlusion, which may enhance platelet disaggregation and thrombus resolution [20,21]. In the present study, glycoprotein IIb/IIIa receptor antagonist bolus delivered directly to the infarct lesion site reduced infarct size at 30 days (the primary end point of the study) in patients with anterior STEMI reperfused early. The local drug delivery through the aspiration device after thrombus aspiration might decrease the chance of mechanically dislodged thrombus downstream while using clear way catheter in INFUSE AMI trial, perhaps explaining why infarct size was lowest in the combined aspiration/abciximab group of INFUSE AMI trial.
Regarding aspiration thrombectomy, in TAPAS, 1071 patients with anterior and nonanterior STEMI who presented within 12 hours of symptom onset at a single center were randomized to manual aspiration vs no aspiration before primary PCI; aspiration resulted in modest improvements in MBG and STR but a marked reduction in 1-year mortality [7,22]. Other trials of manual aspiration thrombectomy have reported conflicting results [23,24]. And in contrast to single-center studies, multicenter aspiration trials (TASTE 2013 and TOTAL 2015) have largely been negative [25]. Moreover, in TAPAS, aspiration did not reduce infarct size as measured by cardiac biomarkers [7], calling into question the mechanism underlying the survival benefit. In the present trial, in which only patients presenting early with anterior MI and coronary anatomy optimal for aspiration were enrolled, and in which cMRI was used to assess infarct size at 30 days, was specifically designed to overcome many of the limitations from these earlier studies. The fact that manual thrombus aspiration did not reduce infarct size in our study makes a substantial clinical benefit unlikely, questioning its routine use in STEMI.
Although infarct size at 30 days was reduced with intracoronary tirofiban group, early markers of microcirculatory reperfusion (MBG and STR) were not improved. This discordance may reflect different ascertainment times give infarct evolution over 30 days (especially as edema is substantially reduced during this time) and variable accuracy of different biomarkers. The comparable 90-day clinical event rates between groups is consistent with the early MBG and STR results [13,26].
Our study has several limitations. First, the trial was single-blind, with the operator knowing the randomisation assignment. Thus, while some bias cannot be excluded, the patient and follow-up personnel were unaware of the treatments provided, and the study used numerous core laboratories and a clinical events committee blinded to treatment assignment. Second, using of aspiration device as local drug delivery may have a risk of distal embolisation but there was no information about its hazardous before. Third, manual aspiration catheters with a larger internal diameter than the one used in the present trial are now available. However, studies have not shown greater thrombus retrieval or improved myocardial perfusion with larger bore devices [23]. Fourth, larger trials are required to determine whether the degree of infarct size reduction at 30 days achieved with intracoronary tirofiban with aspiration thrombectomy in the present study translates into improved late clinical outcomes without increasing bleeding.
CONCLUSION
ACKNOWLEDGEMENT
REFERENCES
- Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, et al. (2016) Relationship between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J Am Coll Cardiol 67: 1674-1683.
- Wu E, Ortiz JT, Tejedor P, Lee DC, Bucciarelli-Ducci C, et al. (2008) Infarct size by contrast enhanced cardiac magnetic resonance is a stronger predictor of outcomes than left ventricular ejection fraction or end-systolic volume index: pro- spective cohort study. Heart 94: 730-736.
- Mayr A, Mair J, Klug G, Schocke M, Pedarnig K, et al (2011) Cardiac troponin T and creatine kinase predict mid-term infarct size and left ventricular function after acute myocardial infarction: A cardiac MR study. J Magn Reson Imaging 33: 847-854.
- Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, et al. (2000) Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation 101: 125-130.
- Stone GW, Witzenbichler B, Godlewski J, Dambrink JH, Ochala A, et al. (2013) Intralesional abciximab and thrombus aspiration in patients with large anterior myocardial infarction: one-year results from the INFUSE-AMI trial. Circ Cardiovasc Interv 6: 527-534.
- Kunichika H, Ben-Yehuda O, Lafitte S, Kunichika N, Peters B, et al. (2004) Effects of glycoprotein IIb/IIIa inhibition on microvascular flow after coronary reperfusion: A quantitative myocardial contrast echocardiography study. J Am Coll Cardiol 43: 276-283.
- Svilaas T, Vlaar PJ, van der Horst IC, Diercks GF, van den Heuvel AF, et al. (2009) Thrombus Aspiration during Primary Percutaneous Coronary Intervention (TAPAS trial). N Engl J Med 358: 557-567.
- Stone GW, Dixon SR, Grines CL, Cox DA, Webb JG, et al. (2007) Predictors of infarct size after primary coronary angioplasty in acute myocardial infarction from pooled analysis from four contemporary trials. Am J Cardiol 100: 1370-1375.
- Gersh BJ, Stone GW, White HD, Holmes DR (2005) Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future? JAMA 293: 979-986.
- Cheong BY, Muthupillai R, Wilson JM, Sung A, Huber S, et al. (2009) Prognostic significance of delayed-enhancement magnetic resonance imaging: survival of 857 patients with and without left ventricular dysfunction. Circulation 120: 2069-2076.
- Ripa RS, Nilsson JC, Wang Y, Søndergaard L, Jørgensen E, et al. (2007) Short- and long-term changes in myocardial function, morphology, edema, and infarct mass after ST-segment elevation myocardial in- farction evaluated by serial magnetic resonance imaging. Am Heart J 154: 929-936.
- Abdel-Aty H, Cocker M, Meek C, Tyberg JV, Friedrich MG (2009) Edema as a very early marker for acute myocardial ischemia: a cardiovascular magnetic resonance study. J Am Coll Cardiol 53: 1194-1201.
- Brener SJ, Cristea E, Mehran R, Dressler O, Lansky AJ, et al. (2011) Relationship between angiographic dynamic and densitometric assessment of myocardial reperfusion and survival in patients with acute myocardial infarction treated with primary percutaneous coronary intervention: the harmonizing out- comes with revascularization and stents in AMI (HORIZONS-AMI) trial. Am Heart J 162: 1044-1051.
- Thiele H, Schindler K, Friedenberger J, Eitel I, Fürnau G, et al. (2008) Intracoronary compared with intravenous bolus abciximab application in patients with ST-elevation myo-cardial infarction undergoing primary percutaneous coronary intervention: the randomized Leipzig immediate percutaneous coronary intervention abciximab IV versus IC in ST-elevation myocardial infarction trial. Circulation 118: 49-57.
- Gu YL, Kampinga MA, Wieringa WG, Fokkema ML, Nijsten MW, et al. (2010) Intracoronary versus intravenous administration of abciximab in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention with thrombus aspiration: the comparison of intracoronary versus intravenous abciximab administration during emergency reperfusion of ST-segment elevation myocardial infarction (CICERO) trial. Circulation 122: 2709- 2717.
- Navarese EP, Kozinski M, Obonska K, Margheri M, Gurbel PA, et al. (2012) Clinical efficacy and safety of intracoronary vs intravenous abciximab administration in STEMI patients undergoing primary percutaneous coronary intervention: a meta-analysis of randomized trials. Platelets 23: 274-281.
- Alexander W (2012) AIDA STEMI: No advantage for intracoronary vs. intravenous Abciximab.
- Stone GW, Maehara A, Witzenbichler B, Godlewski J, Parise H, et al. (2012) Intracoronary Abciximab and Aspiration Thrombectomy in Patients With Large Anterior Myocardial Infarction: the INFUSE-AMI randomized trial. JAMA 307: 1817-1826.
- Montalescot G, Barragan P, Wittenberg O, Ecollan P, Elhadad S, et al. (2001) Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med 344: 1895-1903.
- Marciniak SJ Jr, Mascelli MA, Furman MI, Michelson AD, Jakubowski JA, et al. (2002) An additional mechanism of action of abciximab: dispersal of newly formed platelet aggregates. Thromb Haemost 87: 1020-1025.
- Moser M, Bertram U, Peter K, Bode C, Ruef J (2003) Abciximab, eptifibatide, and tirofiban exhibit dose-dependent potencies to dissolve platelet aggregates. J Cardiovasc Pharmacol 41: 586-592.
- Vlaar PJ, Svilaas T, Vogelzang M, Diercks GF, de Smet BJ, et al. (2008) A comparison of 2 thrombus aspiration devices with histopathological analysis of retrieved material in patients presenting with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 1: 258-264.
- Kaltoft A, Bøttcher M, Nielsen SS, Hansen HH, Terkelsen C, et al. (2006) Routine thrombectomy in percutaneous coronary intervention for acute ST-segment elevation myocardial infarction: a randomized, controlled trial. Circulation 114: 40-47.
- De Luca G, Dudek D, Sardella G, Marino P, Chevalier B, et al. (2008) Adjunctive manual thrombectomy improves myocardial perfusion and mortality in patients undergoing primary percutaneous coronary intervention for ST-elevation myocardial infarction: a meta-analysis of randomized trials. Eur Heart J 29: 3002-3010.
- Inaba Y, Chen JA, Mehta N, Bergmann SR (2009) Impact of single or multicentre study design on the results of trials examining the efficacy of adjunctive devices to prevent distal embolisation during acute myocardial infarction. EuroIntervention 5: 375-383.
- McLaughlin MG, Stone GW, Aymong E, Gardner G, Mehran R, et al. (2004) Prognostic utility of comparative methods for assessment of ST- segment resolution after primary angioplasty for acute myocardial infarction: the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Com- plications (CADILLAC) trial. J Am Coll Cardiol 44: 1215-1223.
Citation: Basuoni A, El-Naggar W, El-Mahdy M, El-Kaffas S (2019) Effect of Intra-Coronary (IC) Tirofiban Following Aspiration Thrombectomy on Infarct Size, in Patients with Large Anterior STEMI undergoing Primary PCI. J Cardiol Stud Res 5: 015.
Copyright: © 2019 Ahmed Basuoni, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

