
Effect of Nhexane Extract of Caryota no Seed on Markers of Neurodegeneration and Fecundity in Drosophila Melanogaster
*Corresponding Author(s):
Chinonye A MaduagwunaDepartment Of Pharmacology And Toxicology, University Of Jos, Jos, Nigeria
Tel:+234 8037114483,
Email:elchinonye@gmail.com
Abstract
Study background: Chronic neuroinflammation is a common emerging hallmark of several neurodegenerative diseases. Alzheimer’s Disease (AD) is the most common cause of dementia among the elderly and is characterized by loss of memory and other cognitive functions. The purpose of this study is to investigate the nhexane extract of Caryota no (CN) for effect on fecundity, negative geotaxis assay, a marker of neurodegeneration and in vivo reactive oxygen species scavenging activity in D. Melanogaster (DM). Experimental design was employed.
Place and duration: Sample: African Centre of Excellence for Phytomedicine Research and Development, University of Jos, Jos Plateau State Nigeria between June 2018 and February 2019
Methodology: 50 flies were exposed in each vial to the following concentrations: 300 mg, 350 mg, 400 mg, 500 mg and 600 mg of nhexane extracts in 5 replicates for 7 days with daily recording of mortality. Total protein assays were carried out by Randox method from the supernatant from homogenized whole flies. In vivo antioxidant activity study was conducted by measuring level of acetylcholinesterase (AChE) enzyme activity from supernatants of whole fly homogenates using a spectrophotometer at specific wavelengths over 3 minute duration. The values were derived as part of the total protein value. Negative geotaxis was done by the climbing assay and fecundity was examined by rate of emergence of larva after exposure of the flies to treatment. The statistical difference among test groups was presumed at P < 0.05.
Results: The nhexane extract of CN caused nonsignificant decreases in AChE (P = 0.54) activity, nonsignificant increase in fecundity (P = 0.70) and nonsignificant observable change in negative geotactic (P = 0.84) behaviour in the nhexane extract-treated flies compared to the controls. The 7 days survival plot revealed significant (P = 0.0002) improvement in survival by the doses of the extract in comparison to the control.
Conclusion: It can therefore be concluded that the nhexane extract of Caryota no significantly improved survival and nonsignificantly increased fertility and AChE activity but produced no observable change in negative geotaxis in DM.
Keywords
Acetylcholinesterase; Caryota no; Drosophila melanogaster; Fecundity; Geotaxis; In vivo
BACKGROUND
Human neurodegenerative diseases largely affect the elderly and it is associated with devastating illnesses due to the limitation of human genetics, it is necessary to use model systems to investigate the affected genes. For several years now, genetically amenable DM has been used as suitable model system in the study of human neurodegenerative diseases such as Alzheimer’s, Huntington’s and Parkinson’s diseases, Sleep, Seizure and Cognitive disorders [1]. In the early 21st century, Drosophila melanogaster had been established as a model system for immune studies after analysis of its genome revealed unsuspected sophistication and similarity to the mammalian innate immune system [2]. For more than a century, the low cost, rapid generation time, and excellent genetic tools have made the drosophila fly indispensable for basic research [3].
Chronic neuroinflammation is a common emerging hallmark of several neurodegenerative diseases [4]. Alzheimer’s Disease (AD) is the most common cause of dementia among the elderly and is characterized by loss of memory and other cognitive functions. The major pathological hallmarks include extensive synaptic and neuronal loss, astrogliosis, and accumulation of proteinaceous deposits [5]. Parkinson’s Disease (PD) is an age-related neurodegenerative disorder primarily caused by the progressive loss of Dopaminergic (DA) neurons from the Substantia nigra in the brain [6]. The clinical motor symptoms include rest tremor, rigidity, progressive bradykinesia and postural instability and several non-motor symptoms like sleep disorder, depression, constipation, anxiety, impaired reaction time, that often manifest during the early pre-clinical stages of PD [7]. Oxidative stress plays a major role in the loss of dopaminergic neurons due to excessive production of Reactive Oxygen Species (ROS) [8]. For example, increase in GST activities has been reported in the brains of human PD patients, possibly due to increased levels of oxidative stress in PD pathogenesis [9]. RNA seq data demonstrate that Paraquat (PQ) exposure triggers transcriptional changes in genes involved in innate immune response pathways in Drosophila. PQ treatment activates genes associated with Drosophila hemocytes, which are macrophage-like cells involved in phagocytosis and inflammation. This finding is in agreement with previous data showing neuroinflammatory responses through the induction of nitric oxide synthase in response to PQ treatment [10].
The ROS can be produced from either endogenous or exogenous sources. The endogenous sources of ROS include different cellular organs such as mitochondria, peroxisomes and endoplasmic reticulum, where the oxygen consumption is high [11]. Meanwhile, antioxidant molecules could also be derived endogenously or from exogenous sources. Choline could be obtained from food sources like bananas, whole wheat, bread, broccoli, shrimps and other animal sources. This is converted into acetylcholine in vivo. Acetylcholinesterase (AChE) is a key enzyme in the cholinergic nervous system. During the progression of AD, many different types of neurons deteriorate, although there is a profound loss of forebrain cholinergic neurons, which is accompanied by a progressive decline in acetylcholine [5]. In neurodegeneration, both the acetylcholine-synthesizing enzyme Choline Acetyltransferase (ChAT), as well as the acetylcholine-hydrolyzing enzyme, AChE is affected [5].
The endocrine glands of DM are derived from epithelial tissues and, at molecular and cellular levels, they function in a similar way to those of vertebrate glands. For instance, DM possesses circulating hormones, plasma membrane receptors and nuclear receptors governed by the same chemical and biological mechanisms found in the hormonal system of vertebrates [12] and DM also possesses two major hormonal systems: Ecdysone (Ecy) and Juvenile Hormone (JH). Ecdysone is a steroid hormone produced in the prothoracic glands, and it is homologous to the steroid hormones such as estradiol synthesised from cholesterol. Juvenile Hormone is a sesquiterpinoid produced in the Corpora allata in the brain of DM, and it shares some traits with retinoic acid [13]. The moult in DM is preceded and controlled by Ecy. The production of JH ceases at the end of the third instar larva to allow for the peak in Ecy to initiate pupation, cell death and the development of new cellular structure. JH later returns in the adult stage and regulates spermatogenesis, lifespan, locomotor behaviour, feeding, secondary sexual differentiation and courtship. It also interacts with Ecdysone to promote fertility [14]. Caryota no palm is reported to be one of the largest species of the genus found in Borneo rain forests of the family Arecaceae (Palmae). The common name is the Giant Fishtail Palm [15]. In habitat, this palm can reach a height of 75 inches and the stems measure 18-20 inches in diameter [15]. Caryota species are mostly found in Asia, and are used traditionally in the treatment of gastric ulcer, migraine headaches, snakebite envenomation and also rheumatic swellings by preparing porridge from the flowers [16]. Additionally, in Ayurveda, C. urens, another closely related member, is suggested for treatment of seminal weakness and urinary disorders [17].
Infertility and age -related neurodegeneration have for decades been linked to various sources of endogenous and exogenous stressors. Agents which can become useful in ameliorating these oxidative states have been found useful in the clinical management of these conditions. The aim of this work is to screen nhexane extract of CN for effect on fecundity, negative geotaxis assay, a marker of neurodegeneration and in vivo reactive oxygen species scavenging activity in D. melanogaster.
METHODS
Study design and population
Experimental design was used and sample of population used was fifty (50) for each experimental group in a vial.
Chemicals and reagents
All chemicals used were of analytical grade. nHexane and distilled water were obtained from Africa Centre of Excellence in Phytomedicine Research and Development, Jos, Plateau State, Nigeria. Randox Protein kit was purchased from Medicom, Jos Plateau State. 1-chloro-2, 4-dinitrobenzene (CDNB), 5,5'-dithiobis (2-nitro-benzoic acid) (DTNB) and acetylthiocholine iodide were purchased from Sigma Aldrich (St Louis, MO).
Plant collection and preparation
The plant material was collected from Games Village (9.0166 0N, 7.4475 0E), Abuja, Nigeria. The plant was identified by a taxonomist in the herbarium of the Federal college of Forestry Jos. The seeds were sorted, air-dried for several days and then pulverized to powder using a commercial grinding machine. The soxhlet extractor was used for extraction of the seed powder using analytical grade nhexane as a solvent following a method described by Virot, et al., [18]. A rotary evaporator was employed to recover the solvent. The extract was further dried in a water bath regulated at 400C and further kept in a fume cupboard. This yielded the nhexane extract from the CN seeds (2%), which was used in the biological tests.
Fly strains and diet
melanogaster Harwich strain was obtained from Africa Center of Excellence in Phytomedicine Research and Development, University of Jos and maintained at constant temperature and humidity (23°C; 60% relative humidity, respectively) under 12 hour dark/light cycle. The flies were cultured by feeding them with a standard medium of the following compositions; 1700 ml of water, 16 g agar, 20 g of baker’s yeast, 100 g of corn flour, and 1 g of methyl paraben dissolved in 5 ml of absolute ethanol, 1700 ml of water [19]. The duration (days) of fly treatment for biochemical assays were pre-determined based on information from the literature, pilot studies and/or survival assays. Young flies 1-4 days old were preferred. To obtain the young flies of known age the culture bottles or vials with pupa were strictly emptied of all flies and the date noted and labelled accordingly. Adult flies of known age were then harvested from the newly hatched population.
7 Days survival assay of nHexane seed extract of CN -treated flies
50 flies of both genders (1-3 days old) were exposed to selected concentrations of nhexane extracts of CN seeds (300mg 350mg, 400mg, 500mg and 600mg prepared in distilled water) in five replicates for 7 days [20,21].
The flies were divided into six groups containing 50 flies each. Control group was placed on normal diet alone while groups II-IV were placed on basal diet containing nhexane seed extract of CN at various concentrations of diet as shown thus;
Control group Basal diet
300 mg group Basal diet + 300mg CN nhexane seed extract/10g fly food
350 mg group Basal diet + 350mg CN nhexane seed extract/10g fly food
400 mg group Basal diet + 400mg CN nhexane seed extract/10g fly food
500 mg group Basal diet + 500mg CN nhexane seed extract/10g fly food
600 mg group Basal diet + 600mg CN nhexane seed extract/10g fly food
During the experimental period, flies were transferred onto new vials containing fresh food every 2 days. The flies were exposed to these treatments for 7 days, and the vials containing flies were maintained at room temperature. All experiments were carried out in triplicate (each experimental group was carried out in five independent vials). Mortality of flies was scored every 24 hours for 7 days and the survival rate was expressed as percentage of live flies. The data were subsequently analyzed and plotted as cumulative mortality and percentage survival after the treatment period. Survival analyses were calculated based on the number of deaths recorded and evaluated by the log-rank Mantel-Cox test.
In vivo antioxidant activities of nHexane seed extract of CN -treated flies
50 flies were treated with 350 mg, 400 mg and 500 mg nhexane seed extract of CN for 7 days. Control flies were only treated with distilled water; each concentration was replicated five times. At the end of 7 days, the treated flies were anaesthetized in ice, weighed, homogenized in 0.1 M phosphate buffer, pH 7.0 (1 mg: 10 µL), and centrifuged for 10 min at 4000 rpm (temperature, 4°C). The supernatant obtained was used to determine the level of total protein and hence the activities of total protein and acetylcholinesterase activity. From the total protein values, the levels of other antioxidants were derived by calculations after the assay.
Determination of acetylcholinesterase activity
The seven days nhexane extract- treated flies and the control were anesthetized on ice, homogenized in 1:10 volumes 100 mM phosphate buffer saline (pH7.4), and centrifuged using cold centrifuge (Eppendorf AG, 5227 R, Germany) at 4 min at 4000 rpm. The supernatant was collected and used for the determination of AChE activity following the method described by Ellman et al., [22] with slight modification. To the reaction mixture containing 285 µl of distilled water, 180 µl of 100 mM potassium phosphate buffer (pH 7.4), 60 µl of 10 mM DTNB, and 15 µl of sample, 60 µl of 8 mM acetylthiocholine was added. The change in absorbance was monitored at 412 nm for 3 min at 10 s intervals, using a UV Spectrophotometer (Jenway 7315). The protein concentration of the whole fly homogenates was determined using total protein kit (Randox) according to the manufacturer’s instructions. The data was calculated against blank and sample blank, and the results were corrected by the protein content. The enzyme activity was expressed as micromole/min/mg of protein.
Negative geotaxis trial
The locomotor (climbing) performance of treated and control flies were evaluated using the negative geotaxis assay. Briefly, ten (10) CN nhexane extract exposed and unexposed flies were immobilized under mild ice anaesthesia and placed separately in labelled vertical glass columns (length, 15 cm; diameter 1.5 cm). After the recovery period (about 20 min), the flies were gently tapped to the bottom of the column. Following 6 s, the numbers of flies that climbed up to the 6 cm mark of the column, as well as those that remain below this mark were recorded. Data were expressed as the percentage of flies that escaped beyond the 6 cm mark in 6 s. The score of each group is an average of three trials for each group of treated and controlled flies [23,24].
Fecundity assay
The fertility of the flies, after exposure to nhexane extract of CN, was assessed using the reproductive ability with slight modifications [24,25]. Briefly, virgin flies (both sexes) were isolated (within 8 hours after eclosion) from their normal fly food, and treated with series of concentrations (300 mg, 350 mg, 400 mg 500 mg and 600 mg each per 10 g fly food) for seven days. Thereafter, the treated flies were pair mated in vials containing normal food. Five (5) pairs of flies were taken in each treatment group including the control. All the flies in each treatment group were transferred into fresh vials with normal food for 24 hours, and the number of eggs laid in each vial during this period was monitored for 14 days for the emergence of adult flies. The mean number of flies emerged gives a measure reproductive ability.
STATISTICAL ANALYSIS
The data was expressed as mean ± SD (standard Deviation), and the statistical analysis was carried out using one-way Analysis of Variance (ANOVA) followed by Turkey's post-hoc test followed by Turkey's post-hoc test and two-way ANOVA in cases of comparisons with the software, Graphpad prism version 7.0 (GraphPad Software, San Diego, CA, USA). The results were considered statistically significant at P < .05.
RESULTS
7 days survival assay
By the 7th day, the survival proportions for the control, 300, 350, 400, 500, and 600 mg/10g diet groups were 87.2, 94.8, 92.4, 96.0, 96.0 and 93.3 percent respectively. There is evidence that the extract doses improved survival better than the control. The one way ANOVA resulted in P = 0.0002 *** while the Log Rank test gave P = 0.0012 **. It can therefore be inferred that 7 days exposure to nhexane extract of CN seeds significantly increased survival in DM. This survival assay helped in the choice of the 3 doses eventually used for other assays and it also agrees with an earlier work on toxicity of this nhexane extract (unpublished).
In vivo antioxidant activity
Acetylcholinesterase assay
The results of the kinetics of the enzyme, acetylcholinesterase revealed nonsignificant increase (P> 0.05) between the groups and the control. The one-way ANOVA summary revealed P = 0.54. It can therefore be inferred that exposure to nhexane extract of CN seeds nonsignificantly increased AChE levels in D. melanogaster.
Negative geotaxis
This was also carried out on both extracts after a seven-day exposure of the flies to the extracts. This result revealed nonsignificant difference (P> 0.05) between the groups and the control. There was no particular observable effect (positive or negative) caused by the administration of the nhexane extract on flies in comparison to the control. The one-way ANOVA summary revealed P = 0.84. It can be inferred that the nhexane extract produced no change in geotactic behavior in DM.
Fecundity
The rate of emergence of the larva, pupa and the adult fly was observed for the nhexane extract after seven days treatment plus one day mating grace as shown graphically below. This result revealed nonsignificant (P> 0.05) increase of emergence above the control. The One-way ANOVA summary revealed P = 0.70. On the whole, it can be inferred that the nhexane extract produced an improvement in fertility above basal level.
DISCUSSION
The different doses of nhexane extract of CN improved survival better than the control (Figure 1). It is worthy of note that several additives and dietary constituents modulate survival in drosophila [21,26]. 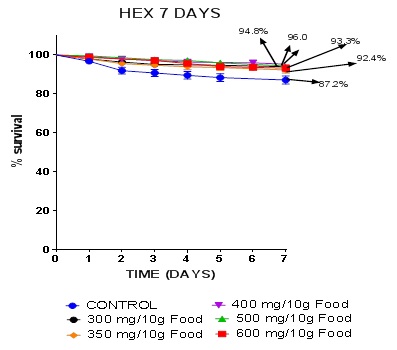 Figure 1: Kaplan-Meier 7 days survival curves.
Figure 1: Kaplan-Meier 7 days survival curves.
Data presented as Mean ± S.E.M = Mean values ± Standard error of means of five independent biological replicates of for each extract concentration (n = 50). Extracts: significant from normal control * P < 0.05; ** P < 0.001; *** P = 0.0002 - 0.0004; **** P < 0.0001
The nhexane extract of CN generally caused a rise in levels of AChE above basal levels in a nearly regular pattern (Figure 2). The highest extract dose caused the greatest increase of this enzyme activity. This appears to suggest that the extract may be an exogenous indirect source of the Neurotransmitter (NT). The NT, Acetylcholine (Ach) is produced at the Neuromuscular Junction (NMJ) and nerve synapse of the central nervous system and the periphery. This may also imply that this extract also causes slight increase in acetylcholine levels because the release of the enzyme at for instance, the NMJ or at the synapse is usually in response to the release of the NT so that the enzyme would metabolize excess circulating Ach and ensure its return to basal physiological level. AChE hydrolyses the NT- Ach that regulates locomotion, motor function and memory. Higher dietary inclusions of GK seeds (0.5% and 1.0%) showed a significant decrease in survival rate and also induced a decrease in AChE activity [23]. An opposite constellation of effects were produced by the nhexane CN extract on drosophila which included improved survival, longevity (unpublished) and increased AChE. A group of researchers reported that the RNA seq screen revealed upregulation of genes involved in redox reactions upon paraquat (PQ) exposure [10]. In measuring markers of neurodegeneration, physiological levels of AChE and butyrylcholinesterase are monitored and are often used for prognosis of disease process. AChE decreased in Huntington’s chorea and in multiple sclerosis [27].
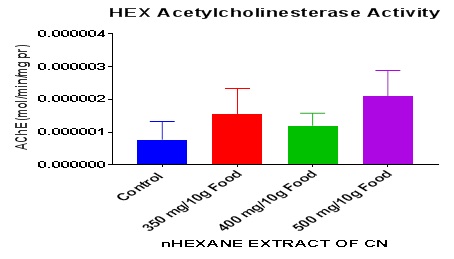
Figure 2: Acetylcholinesterase activity in whole fly homogenate of nHexane Extract of CN-treated flies after 7 days.
Data presented as Mean ± S.E.M = Mean values ± Standard error of means of five independent biological replicates of for each extract concentration (n = 50). Extracts: significant from normal control.
On negative geotaxis, it was observed that the nhexane extract of CN caused no observable changes in that parameter in DM (Figure 3). Neurodegenerative diseases are frequently associated with a progressive loss of movement ability, reduced life span, and age-dependent neurodegeneration [28]. PQ exposure has been previously linked to altered movement phenotypes in Drosophila PD models [29]. To evaluate whether reduction of Relish levels in Dopaminergic (DA) neurons could rescue the mobility defects induced by PQ, negative geotaxis assay was employed, which is an indicator of the onset of PD pathogenesis-linked movement dysfunction. After ingestion of 5 mM PQ for 24 h, both the control groups exhibited resting tremors and bradykinesia, which are characteristic clinical symptoms associated with PD in human patients [10]. There is also accumulating evidence for increased innate immune activation in PD [30] and a recent study in humans links the immune system to PD by demonstrating a heightened immune response to α-synuclein [31] with additional evidence suggesting a critical role of chronic intestinal inflammation to PD pathogenesis [32].
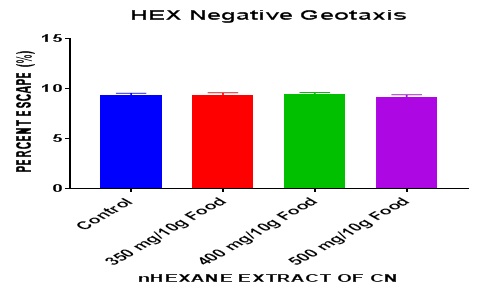 Figure 3: Effect of nhexane extract of CN on negative geotaxis in DM after 7 days exposure.
Figure 3: Effect of nhexane extract of CN on negative geotaxis in DM after 7 days exposure.
Data presented as Mean ± S.E.M = Mean values ± Standard error of means of five independent biological replicates of for each extract concentration (n = 50). Extracts: significant from normal control
Involvement of hemocytes which are major phagocytes, has also been implicated in the progression of AD in the fly model [33]. Similarly, increased NF-κB activation has been linked to age-related neurodegeneration in Drosophila [34] and reports suggest a link between increased expression of Antimicrobial Peptides (AMPs) and neurodegeneration [34]. Therefore, tight regulation of the IMD pathway is crucial to avoid uncontrolled chronic inflammation. It has also been hypothesized that activation of innate immune genes could play a neuroprotective role [35]. Growth Arrest and DNA-Damage-inducible protein 45 (Gadd45) transcript functions in DNA repair in response to environmental and physiological stress, and overexpression of its drosophila ortholog in the nervous system has been shown to extend life span, delay age dependent neurodegeneration and increase resistance to oxidative stress [36]. Since the nhexane does not produce an abnormality in locomotor behavior, it may imply that it may actually play a neuroprotective role in DM as it also has proven to prolong life span in DM (unpublished).
The nhexane extract has proved to improve fertility in DM in comparison with the control (Figure 4). Opposite effects on fertility was observed in DM by a research [37] which shows that aqueous extract of Mangifera indica at high doses greatly inhibited fertility. Some researchers showed that dolutegravir-based HAART [24] and EFV-based HAART [38] reduce AChE, negative geotaxis (a measure of neurotoxicity), survival and fertility but in summary of the index study, the nhexane extract of CN increased AChE, survival, fertility, longevity and had no change in locomotion in DM.
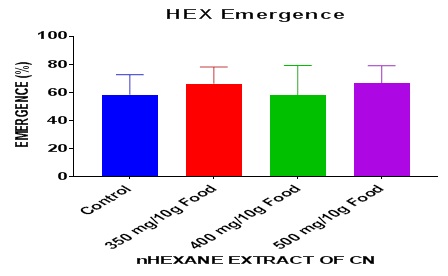 Figure 4: Effect of nhexane extract of CN on fly emergence in DM after 7 days exposure.
Figure 4: Effect of nhexane extract of CN on fly emergence in DM after 7 days exposure.
Data presented as Mean ± S.E.M = Mean values ± Standard error of means of five independent biological replicates of for each extract concentration (n = 50). Extracts: significant from normal control
CONCLUSION
From the findings it can be concluded that the nhexane extract of Caryota no nonsignificantly improved fertitity and survival, increased AChE activity but produced no observable change in negative geotaxis in DM. This implies that there are biologically active substances present in this extract requiring further pharmacological evaluation of the extract which would help to validate or abrogate the claims of the folkloric usage of the plant. It is recommended that detailed studies of drosophila fly neuronal, locomotor and endocrine systems at the molecular levels after treatment with nhexane extract of C. no seeds should be carried out to fully understand the dynamics of oxidative stress in relation to this plant extract. This nhexane extract may be developed for the clinical management of infertility and neurodegenerative illnesses in the future after advanced studies.
ACKNOWLEDGEMENT
The authors are grateful to African Centre of Excellence for Phytomedicine Research and Development, University of Jos, Jos Plateau State Nigeria for the technical support obtained for this work.
COMPETING INTERESTS
All authors have none to declare.
AUTHORS’ CONTRIBUTIONS
This work was carried out in collaboration among all authors. ‘Author A’ (Maduagwuna CA) designed the study, performed the statistical analysis, wrote the protocol, and wrote the first draft of the manuscript. ‘Author B’ (Omale S) and ‘Author C’ (Etuh MA) managed the analyses of the study. ‘Author D’ (Gyang SS) managed the literature searches. All authors read and approved the final manuscript.
REFERENCES
- Hirth F (2010) Drosophila melanogaster in the Study of Human Neurodegeneration. CNS Neurol Disord Drug Targets 9: 504-523.
- Alarco A-M, Marcil A, Chen J, Suter B, Thomas D, et al. (2004) Immune-deficient Drosophila melanogaster: A model for the innate immune response to human fungal pathogens. J Immunol 172: 5622-5628.
- Tolwinski NS (2017) Introduction: Drosophila-A Model System for Developmental Biology. J Dev Biol 5: 9.
- Chen W-W, Zhang X, Huang W-J (2016) Role of neuroinflammation in neurodegenerative diseases (Review). Mol Med Rep 13: 3391-3396.
- García-Ayllón MS, Small DH, Avila J, Sáez-Valero J (2011) Revisiting the role of acetylcholinesterase in Alzheimer’s disease: Cross-talk with P-tau and β-amy Frontiers in Molecular Neuroscience 4: 1-9.
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, et al. (2017) Parkinson disease. Nat Rev Dis Primers 3: 17013.
- Barnum CJ, Tansey MG (2012) Neuroinflammation and Non-motor Symptoms: The Dark Passenger of Parkinson’s Disease? Curr Neurol Neurosci Rep 12: 350-358.
- Blesa J, Trigo-Damas I, Quiroga-Varela A, Jackson-Lewis VR (2015) Oxidative stress and Parkinson's disease. Front Neuroanat 9: 91.
- Smeyne M, Smeyne RJ (2013) Glutathione Metabolism and Parkinson’s Disease. Free Radic Biol Med 62: 13-25.
- Maitra U, Scaglione MN, Chtarbanova S, O’Donnell JM (2019) Innate immune responses to paraquat exposure in a Drosophila model of Parkinson's disease. Sci Rep 9: 12714.
- Phaniendra A, Jestad DB, Periyasamy L (2015) Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Indian Journal of Clinical Biochemistry 30: 11-26.
- Riddiford LM (1993) Hormones and Drosophila development. In: Bate M, Arias MA (eds.). The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, USA.
- King-Jones K, Thummel CS (2005) Nuclear receptors--a perspective from Drosophila. Nat Rev Genet 6: 311-323.
- Abolaji A, Kamdem JP, Farombi O, da Rocha JBT (2013) Drosophila melanogaster as a Promising Model Organism in Toxicological Studies: A Mini Review. Arch Bas App Med 1: 33-38.
- Beck C (2010) Caryota no in Palm Beach County Submitted by Charlie Beck.
- Kavitha S (2017) A study on the bioefficacy of Caryota urens World journal of pharmaceutical research.
- Antony A, Mahimaidoss J, Ramani VA (2011) Quantitative estimation of primary and secondary metabolites on flowers of L. International Journal of Applied Biology and Pharmaceutical Technology 3: 431–435.
- Virot M, Tomao V, Ginies C, Chemat F (2008) Total Lipid Extraction of Food Using d -Limonene as an Alternative to n -Hexane. Chromatographia 68: 311-313.
- Etuh MA, Aguiyi JC, Ogwu OS, Omale S, Oyeniran O, et al. (2019) The In vivo Antioxidant Protective Activity of Mangifera indica Cold Aqueous Leaf Extract in Drosophila Melanogaster. J Adv Biol Biotechnol 22: 1-7.
- Adedara IA, Abolaji AO, Rocha JBT, Farombi EO (2016) Diphenyl Diselenide Protects Against Mortality, Locomotor Deficits and Oxidative Stress in Drosophila melanogaster Model of Manganese-Induced Neurotoxicity. Neurochem Res 41: 1430-1438.
- Oboh G, Ogunsuyi OB, Ojelade MT, Akomolafe SF (2018) Effect of dietary inclusions of bitter kola seed on geotactic behavior and oxidative stress markers in Drosophila melanogaster. Food Sci Nutr 6: 2177-2187.
- Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7: 88-95.
- Abolaji AO, Kamdem JP, Lugokenski TH, Nascimento TK, Waczuk EP, et al. (2014) Involvement of oxidative stress in 4-vinylcyclohexene-induced toxicity in Drosophila melanogaster. Free Radic Biol Med 71: 99-108.
- Iorjim WM, Omale S, Bagu GD, Gyang SS, Alemika ET (2020) Reproductive and Oxidative Stress Toxicity of Dolutegravir-Based Combination Antiretroviral Therapy in Drosophila melanogaster. J Ad Med Pharm Sc 22: 26-40.
- Graham BH, Li Z, Alesii EP, Versteken P, Lee C, et al. (2010) Neurologic dysfunction and male infertility in Drosophila porin mutants: A new model for mitochondrial dysfunction and disease. J Biol Chem 285: 11143-11153.
- Miquel J, Fleming J, Economos AC (1982) Antioxidants, metabolic rate and aging in Drosophila. Arch Gerontol Geriatr 1: 159-165.
- Ruberg M, Villgeois A, Bonnet AM, Pillon B, Rieger F, et al. (1987) Acetylcholinesterase and butyrylcholinesterase activity in the cerebrospinal fluid of patients with neurodegenerative diseases involving cholinergic systems.. J Neurol Neurosurg Psychiatry 50: 538-543.
- Cao W, Song L, Cheng J, Yi N, Cai L, et al. (2017) An Automated Rapid Iterative Negative Geotaxis Assay for Analyzing Adult Climbing Behavior in a Drosophila Model of Neurodegeneration. J Vis Exp 127: 56507.
- Inamdar AA, Chaudhuri A, O’Donnell J (2012) The Protective Effect of Minocycline in a Paraquat-Induced Parkinson's Disease Model in Drosophila is Modified in Altered Genetic Backgrounds. Parkinsons Dis 2012: 938528.
- Schlachetzki JCM, Winkler J (2015) The innate immune system in Parkinson's disease: A novel target promoting endogenous neuroregeneration. Neural Regen Res 10: 704-706.
- Sulzer D, Alcalay RN, GarrettiF, Cote L, Kanter E, et al. (2017) T cells from patients with Parkinson's disease recognize α-synuclein peptides. Nature 546: 656-661.
- Houser MC, Tansey MG (2017) The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis 3: 3.
- Wu SC, Cao Z-S, Chang K-M, Juang J-L (2017) Intestinal microbial dysbiosis aggravates the progression of Alzheimer's disease in Drosophila. Nat Commun 8: 24.
- Kounatidis I, Chtarbanova S, Cao Y, Hayne M, Jayanth D, et al. (2017) NF-κB Immunity in the Brain Determines Fly Lifespan in Healthy Aging and Age-Related Neurodegeneration. Cell Rep 19: 836-848.
- Cantera R, Barrio R (2015) Do the Genes of the Innate Immune Response Contribute to Neuroprotection in Drosophila? J Innate Immun 7: 3-10.
- Bgatova N, Dubatolova T, Omelyanchuk L, Plyusnina E, Shaposhnikov M, et al. (2015) Gadd45 expression correlates with age dependent neurodegeneration in Drosophila melanogaster. Biogerontology 16: 53-61.
- Alexander EM, Aguiyi JC, Mdekera IW, Ogwu OS, Imoleayo OO, et al. (2019) The Climbing Performance, Neuromuscular Transmitter (ACHE) Activity, Reproductive Performance and Survival of Drosophila melanogaster Fed Diet with Mangifera indica Cold Aqueous Leaf Extract. Journal of Applied Life Sciences International 22: 1-11.
- Walter I, Omale S, Etuh MA, Bagu GD, Ogwu OS, et al. (2020) EFV b -HAART Increases Mortality, Locomotor Deficits and Reduces Reproductive Capacity in Drosophila melanogaster. JABB 23: 26-38.
Citation: Maduagwuna CA, Omale S, Etuh Ma, Gyang SS (2020) Effect of Nhexane Extract of Caryotano Seed on Markers of Neurodegeneration and Fecundity in Drosophila Melanogaster. J Gerontol Geriatr Med 6: 073.
Copyright: © 2020 Chinonye A Maduagwuna, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

