
Effect of Obesity on Serum Maternal and Fetal Magnesium Level in Preeclamptic Recipients of Magnesium Sulphate
*Corresponding Author(s):
Ahmed ShehataDepartment Of Obstetrics And Gynecology, Faculty Of Medicine, Assiut University, Assiut, Egypt
Email:ahmed111@aun.edu.eg
Abstract
- Background
Obese women with preeclampsia have risky outcomes. This study assessed the efficacy and safety of the standard dose of intravenous MgSo4 infusion in obese vs. non-obese women with preeclampsia.
- Materials and methods
This Randomized control trial was conducted in Women's Health Hospital, Obstetrics and Gynecology Department, Assiut University from January 2020 to August 2021. A total of 200 women with severe preeclampsia were enrolled and randomly subdivided based on body mass index into two equal groups: non-obese and obese. Both groups received a loading dose of 4 gm of MgSo4 infusion as 4 grams of magnesium sulphate, then a maintenance dose of 1 gm MgSo4 /hour for 24 hours. The primary outcome was assessment of maternal serum magnesium level at 30 minutes (after the end of the loading dose) and 2,4 and 8 hours after the start. Also, adverse events and maternal and fetal outcomes were recorded.
- Results
Based on classes of BMI, both groups had insignificant differences regarding the majority of baseline data. The non-obese group had significantly higher serum magnesium at different assessment times during follow-up. Body mass index negatively correlated with serum magnesium at four hours (r=-0.30, p= 0.01) and eight hours (r=-0.31, p= 0.02) assessment. Maternal and fetal outcomes showed no significant differences between both groups.
- Conclusion
It seems that obese women with preeclampsia tend to have lower serum magnesium levels during intravenous magnesium sulphate infusion, with a low chance of achieving a therapeutic level.
Keywords
Magnesium Sulphate; Obesity; Preeclampsia; Therapeutic Level Fits
Introduction
Pre-eclampsia is defined after 20 weeks of pregnancy as new-onset hypertension, proteinuria, or other end-organ damage. In contrast, eclampsia is the development of grand mal seizures in a woman with pre-eclampsia [1]. In modern obstetrics practice, magnesium sulphate (Mgso4) is administered to patients with severe pre- or eclampsia. It is also used as a tocolytic to prevent preterm birth. The use of magnesium sulphate in patients with mild pre-eclampsia is still debatable [2]. Even when BMI is within the normal range, the risk of pre-eclampsia increases. The risk of late or moderate pre-eclampsia and early and severe pre-eclampsia, associated with increased fetal morbidity and mortality, is increased. It is unknown how pathologic pregnancy conditions such as pre-eclampsia, bleeding and a higher BMI affect serum magnesium levels following magnesium treatment in severe pre-eclampsia [3,4]. Because of the importance of pre-eclampsia, numerous studies have been conducted to assess its determinants, which include parity, maternal age, race, hereditary factors, environmental factors, obesity, poverty, pre-existing hypertension, multiple pregnancies and others, all of which are considered causative [5-7]. Therefore, this study was conducted to investigate the adverse effect of high BMI on maternal serum levels of MgSO4in women with preeclampsia.
Patients and methods
- Trial registration
The Ethical Committee and Institution Review Board of the Faculty of Medicine, Assiut University, Egypt (IRB NO.17100781), approved the study protocol.
- Study settings
- This Randomized control trial was conducted in Women's Health Hospital, Assiut University from January 2020 to August 2021.
- Study participants
We included all pregnant women complicated with severe pre-eclampsia according to the recent ACOG practice bulletin 2019 [2]. We excluded any woman with one or more of the following conditions: known seizure disorder, already had eclamptic fits before hospital admission, gestational hypertension but without severe features, severe renal impairment and/or other medical conditionsas myasthenia gravis, myocardial damage, diabetic coma, heart block.
- Study Outcomes
- Primary outcome: mean difference in serum magnesium level.
- Secondary Outcomes: Maternal and fetal outcomes in relation to body mass index Sample size calculation.
The sample size was calculated Using the PASS Program for sample size calculation. A previous study reported that the therapeutic serum level of magnesium sulphate is 4,2- 8,4 mg/dl. Using a two-sided Chi-square (x2) test with an alpha error of 0.05, a total sample size of at least 200 patients (100 in each arm), 100 0bese and 100 nonobesehad80%powertodetecta 50%decreasein the percentage of women reachingthetherapeuticdoseofmagnesiumsulphaterate(oddsratio=0.48) assuming a rate of losstofollowup10%.
Method of randomization
Permuted blocked randomization was carried out online to generate the randomization list(https://www.sealedenvelope.comhttps://www.sealedenvelope.com/simple.randomiser/v1/lists).Blocked
randomization method was used to balance treatment arms. Then enrolled women were categorized into two groups; Group A (non-obese group) included underweight and normal weight, while Group B (obese group) included overweight and obese.
- Interventions
All women were subjected to detailed history and complete clinical examination. The following investigations were ordered: kidney function tests, liver function tests, total blood count and coagulation profile.
- Magnesium Sulphate Regimens
All patients received an IV loading dose of magnesium sulphate infusion as 4 grams of magnesium sulphate diluted in 150 cc saline infused within 30 minutes. Then, all patients received a 1 gm MgSo4 /hour maintenance dose for 24 hours. The maintenance dose was kept for 24 hours in both groups.
- Serum Magnesium Level Measurements
Baseline serum Mg before the start of IV MgSO4 loading dose therapy was evaluated, then follow-up serum Mg was done at 30 minutes (after the end of loading dose) and at 2, 4 and 8 hours after the start if applicable unless delivery was imminent.
- Fetal and Maternal Monitoring
CTG and Ultrasound, including Doppler assessment, were done to evaluate the fetal biometry, estimated fetal weight, amniotic fluid index, placental location and biophysical profile. Also, ultrasound was used to exclude congenital anomalies. If the patient had fits in the trial, she received an additional 2 grams of IV magnesium sulfate bolus to control the fits. She was transferred immediately to the intensive care unit.
- Statistical Analysis
Statistical analysis was performed using SPSS version 20 (SPSS Inc., Chicago, Illinois, USA). Patient characteristics and outcomes were reported using standard descriptive statistics: frequency (percentage) for categorical variables and mean (SD) or median (Interquartile Range [IQR]) for continuous variables. Shapiro-Wilk test was used as a test of normality for continuous variables. Parametric variables were expressed as mean ± Standard Deviation. (SD) while skewed variables were described using the median and the interquartile range (IQR). Comparisons between treatment groups were evaluated using the χ2 test for categorical variables and the 2-sample t-test or Wilcoxon rank sum test for continuous variables. All calculated P values were 2-sided and P values less than 0.05 were considered statistically significant.
Results
As shown in tables 1 and 2, Both groups of the study based on the frequency of chronic hypertension among obese patients (20 (20%) vs. 9 (9%); P= 0.02). At baseline, both cohort groups had insignificant differences regarding serum magnesium, while after 30 minutes, 2, 3 and 4 hours, serum magnesium was significantly higher among the non-obese group. (Table 3 & Figure 1).
|
|
Category of BMI |
P-value |
|
|
Non-obese(n=100) |
Obese(n=100) |
||
|
Age (years) |
28(18-44) |
30(18-43) |
0.09 |
|
Gravidity |
3(1-9) |
4(1-11) |
0.38 |
|
Parity |
2(0-8) |
2(0-7) |
0.62 |
|
Gestational age (week) |
37(31-40) |
37(30-39) |
0.94 |
|
SBP (mmHg) |
160(140-210) |
160(130-210) |
0.85 |
|
DBP(mmHg) |
110(90-130) |
100(90-130) |
0.14 |
|
Multiple pregnancy |
8 (8%) |
6 (6%) |
0.39 |
|
Prior preeclampsia |
11 (11%) |
8 (8%) |
0.31 |
|
Fetal growth restriction |
8 (8%) |
3 (3%) |
0.10 |
|
Abnormal doppler |
9 (9%) |
12 (12%) |
0.32 |
|
Chronic hypertension |
9 (9%) |
20 (20%) |
0.02 |
|
Symptoms |
|
|
|
|
CNS |
95 (95%) |
97 (97%) |
|
|
CNS/GIT |
1 (1%) |
1 (1%) |
0.28 |
|
CNS/GIT/abdominal pain |
0 |
1 (1%) |
|
|
None |
4 (4%) |
1 (1%) |
|
Table 1: Baseline characteristics of the cohort base do the category of BMI. Data expressed as frequency (percentage), median (range). N: number; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; CNS: central nervous system; GIT: gastrointestinal tract.
|
|
Category of BMI |
P value |
|
|
Non-obese(n=00) |
Obese(n=100) |
||
|
Urea (mmol/l) |
3.7(1.6-0.5) |
3.5(1-25) |
0.69 |
|
Creatinine(umol/l) |
53.20(31-98) |
53(18-266) |
0.60 |
|
Hemoglobin (gm/dl) |
11.50 ± 1.80 |
11.66 ± 1.83 |
0.95 |
|
Platelets count(103/ul) |
239(397-414.7) |
238.50(82-397) |
0.24 |
|
Mean platelets volume (fl) |
8.60(6.20-16.10) |
8.65(5.70-16.40) |
0.69 |
|
PC/MPV |
27.30 ± 12.31 |
26.50 ± 11.12 |
0.52 |
|
Platelets distribution width (%) |
15.45(9.10-23) |
15.35(9.10-54.40) |
0.66 |
|
Platelets crit |
0.21(0.09-0.35) |
0.33(0.13-0.45) |
0.47 |
|
Hematocrit value (%) |
34.10(21-46)) |
33.70(13-45) |
0.96 |
|
RDW (%) |
14.05(10-47) |
14.15(10-41) |
0.66 |
|
Leucocytes(103/ul) |
9.3(2.6-24.59) |
9.83(10.21-74.50) |
0.54 |
|
Neutrophil/lymphocyte ratio |
3.55(0.79-12.50) |
3.25(1.10-9.20) |
0.70 |
|
Neutrophil/leucocyte ratio(10-3) |
0.80(0.40-0.80) |
0.70(0.50-0.80) |
0.15 |
|
Bilirubin (mmol/l) |
8.97(3.45-56) |
10.34(4.55-100) |
0.12 |
|
Alanine transaminase(u/l) |
18.50(7-285) |
17.5(6-1054) |
0.49 |
|
Aspartate transaminase(u/l) |
30(9-644) |
28(10-889) |
0.78 |
|
Prothrombin time (%) |
12 |
12 |
--- |
|
Prothrombin concentration (%) |
100 |
100 |
--- |
|
International randomized ratio |
1 |
1 |
--- |
|
Dipstick albumin in urine Plus-2 Plus-3 Plus-4 |
2 (2%) 25 (25%) 73 (73%) |
2 (2%) 23 (23%) 75 (75%) |
0.78 |
Table 2: Baselinelaboratorydataof the cohort based on the category of BMI. Data expressed as frequency (percentage), median (range). N: number; BMI: bodymass index; RDW: red cell distribution width; PC/MPV: platelets count/mean platelets volume.
|
|
Category of BMI |
P-value |
|
|
Non-obese(n=100) |
Obese(n=100) |
||
|
At baseline (mmo/l) |
2(1.60-2.70) |
2(1.40-2.50) |
0.26 |
|
At 30 minutes (mmo/l) |
2.50(2.10-6.30) |
2.60(2-3.40) |
<0.001 |
|
At two hours (mmo/l) |
3.75(2.40-5.80) |
3.05(2.10-4.30) |
<0.001 |
|
At four hours (mmo/l) |
4.30(2.40-5.70) |
3.50(2.40-5.90) |
<0.001 |
|
At eight hours (mmo/l) |
5(2.50-8.90) |
3.90(2.80-8.60) |
<0.001 |
Table 3: Serummagnesium of the cohort based on the category of BMI. Data expressed as median (range). N: number; BMI: body mass index.
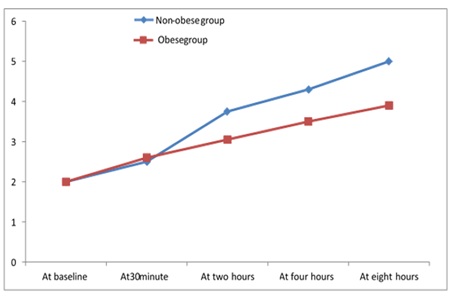 Figure 1: Serum magnesium in the cohort based on category of body mass index.
Figure 1: Serum magnesium in the cohort based on category of body mass index.
As shown in Table 4, none of the studied patients developed hyporeflexia/loss of knee jerk, vomiting, or magnesium stoppage/lowering due to near toxicity level. Five non-obese and seven obese patients had fits, while confusion occurred in 4 (4%) and 2 (2%) of the non-obese and obese groups, respectively.
|
|
Category of BMI |
P-value |
|
|
Non-obese(n=100) |
Obese(n=100) |
||
|
Fits |
5 (5%) |
7 (7%) |
0.38 |
|
Loss of knee jerk/ hyperreflexia |
0 |
0 |
--- |
|
Pulmonary edema/dyspnea |
1 (1%) |
3 (3%) |
0.31 |
|
Oliguria |
5 (5%) |
3 (3%) |
0.36 |
|
Flushing/hotness |
1 (1%) |
2 (2%) |
0.50 |
|
Headache |
1 (1%) |
1 (1%) |
0.71 |
|
Dizziness |
3 (3%) |
1 (1%) |
0.31 |
|
Shivering |
0 |
1 (1%) |
0.50 |
|
Vomiting |
0 |
0 |
--- |
|
Confusion |
4 (4%) |
2 (2%) |
0.34 |
|
Magnesium stoppage/lowering |
0 |
0 |
--- |
Table 4: Maternal adverse events in the cohort based on category of BMI. Data expressed as frequency (percentage).N: number; BMI: body mass index.
As regards the Maternal and fetal outcomes in the cohort based on the category of body mass index, HELLP syndrome and post-partum hemorrhage occurred in 3 (3%) and 2 (2%) of non-obese, respectively and appeared in 1 (1%) and 3 (3%) of obese patients, respectively. Neonatal mortality occurred in three cases of each group. Also, NICU admission was required in 27 (27%) of the non-obese group and 25 (25%) of the obese. Serum magnesium had a negative correlation with BMI at four and eight hours, as shown in Figure 2-3.
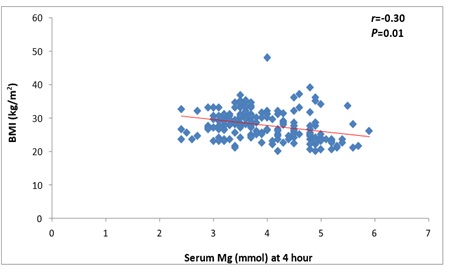 Figure 2: Correlation between serum magnesium at 4 hours with body mass index.
Figure 2: Correlation between serum magnesium at 4 hours with body mass index.
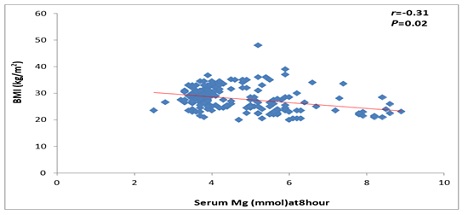 Figure 3: Correlation between serum magnesium at 8 hours with body mass index.
Figure 3: Correlation between serum magnesium at 8 hours with body mass index.
Discussion
The enrolled patients in the current study were subdivided based on BMI, where 100 (50%) patients were non-obese (BMI ≤ 24.9 kg/m2 and the other 100 (50%) patients were obese (BMI ≥25 kg/m2). All baseline characteristics showed no significant differences between both groups (P> 0.05). The non-obese group had significantly higher serum magnesium at different assessment times during follow-up. Body mass index negatively correlated with serum magnesium at four hours (r=-0.30, p= 0.01) and eight hours (r=-0.31, p= 0.02) assessment. Maternal and fetal outcomes showed no significant differences between both groups. In line with the current study, Schneider et al. (2012) identified shared risk factors between the two diseases, such as increasing mother age, null parity, multiple gestation pregnancies and a higher pre-pregnancy BMI. The disease's underlying pathophysiology is thought to be vascular endothelial dysfunction [8]. Also, Duckitt & Harrington(2005) reported that age greater than 40 years of age, a previous history of pre-eclampsia, pre-pregnancy obesity and women who become pregnant with donor eggs, embryo donation, or donor insemination are considered significant risk factors for pre-eclampsia [9]. In the current study, 29 (14.5%) patients had chronic hypertension. Also, it was found that most (96%) of patients had only central nervous system symptoms in the form of headache and visual symptoms. Boriboonhirunsarn et al. (2017) stated that superimposed pre-eclampsia was 43.3% among pregnant women with chronic hypertension, with increased adverse neonatal outcomes [10].
In agreement with the current study, a recently published systematic review stated that visual disturbances, epigastric pain and headache were most frequently- reported across the studies. These symptoms moderately increased the likelihood of eclampsia when present [11]. In agreement with this study, Choi et al. (2020) studied 153women treated with antenatal magnesium sulfate at less than 32 weeks of gestation. They divided their participants into three groups: Group I, 18.5–22.9 kg/m2 (normal); group II, 23.0–24.9 kg/m2 (overweight); and group III, ≥25.0 kg/m2 (obese). The authors found no significant differences between the groups as regards baseline data [12]. In the current work, it was found that both groups of the study had insignificant differences as regards baseline serum magnesium. In contrast, serum magnesium was significantly higher among the non-obese group after 30 minutes, 2nd, 3rd and 4th hour. In line with these results, Dayicioglu et al. (2003) stated the same results [4]. A further study revealed that normal-BMI pregnant women are more likely than high-BMI pregnant women to have therapeutic serum magnesium levels. This could be due to the pregnant woman's weight, which affects the distribution and buffering of serum magnesium. Overweight patients with more muscle volume, bone, soft tissue, fats and extracellular space, as well as those with severe pre-eclampsia and a higher BMI, are more likely to have sub therapeutic serum magnesium levels [13].
In contrast, Choi et al. (2020) concluded maternal BMI did not affect serum magnesium [12]. An explanation of such discrepancy is the indication for magnesium sulfate, where all our patients had severe pre-eclampsia. Choi et al. (2020) included patients treated with antenatal magnesium sulfate for fetal neuroprotection and severe pre-eclampsia [12].
In the current study, seizures (6%) and oliguria (4%) were the most frequent adverse events. It was reported that eclamptic seizure so occurred in four women with low BMI; three had therapeutic serum magnesium levels [4]. Both groups of our cohort had insignificant differences regarding maternal and fetal outcomes. This was consistent with many reported previous studies [12,13]. The strengths of our study are that it was done in a tertiary center with good randomization of severe pre-eclampsia cases with the availability of mgso4 infusion protocols, ICU and lap. Our study measures maternal outcomes of severe pre-eclampsia, including complications and perinatal outcomes regarding mode of delivery, mortality, APGAR score at 0, 1 and 5 minutes of birth and NICU admission. The main limitations of the current study are that it was a single-center study (confounding factors could have influenced the results), the sample size was insufficient to demonstrate a difference in the outcomes and finally, we were unable to display a difference in clinical outcomes due to differences in serum magnesium concentrations, particularly the long-term effects of the babies. Further studies are needed to determine more appropriate magnesium sulphate infusion protocols, especially for women with higher BMI and to be performed in multiple centers with a large sample size. In conclusion, we found that the serum magnesium level in pregnant women with high BMI is lower than in pregnant women with normal BMI.
Consent for Publication
NA
Availability and Data Material
The data sets used and /or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing Interests
The authors report there are no competing interests to declare.
Funding
No fund
References
- Moussa HN, Leon MG, Marti A, Chediak A, Pedroza C, et al. (2017) Pregnancy outcomes in women with preeclampsia superimposed on chronic hypertension with and without severe features. American J of perinatology 34: 403-408.
- Khooshideh M, Ghaffarpour M, Bitarafan S (2017) The comparison of anti-seizure and tocolytic effects of phenytoin and magnesium sulphate in the treatment of eclampsia and preeclampsia: a randomised clinical trial. Iran J Neurol 16: 125-129.
- Lopez-Jaramillo P, Barajas J, Rueda-Quijano SM, Lopez-Lopez C, Felix C (2018) Obesity and preeclampsia: common pathophysiological mechanisms. Front Physiol 9: 1838.
- Dayicioglu V, Sahinoglu Z, Kol E, Kucukbas M (2003) The use of standard dose of magnesium sulphate in prophylaxis of eclamptic seizures: do body mass index alterations have any effect on success? Hypertens Pregnancy 22: 257-265.
- Gathiram P, Moodley J (2016) Pre-eclampsia: its pathogenesis and pathophysiolgy. Cardiovasc J Afr 27: 71-78.
- Al-Tairi ANQ, Isa ZM, Ghazi HF (2017) Risk factors of preeclampsia: a case control study among mothers in Sana’a, Yemen. J of Public Health 25: 573-80.
- Sugawara J, Oe Y, Wagata M (2018) Genetic background of Preeclampsia. Preeclampsia: Springer 29-43.
- Schneider S, Freerksen N, Röhrig S, Hoeft B, Maul H (2012) Gestational diabetes and preeclampsia–similar risk factor profiles. Early human development 88: 179-84.
- Duckitt K, Harrington D (2005) Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. Bmj 330: 565.
- Boriboonhirunsarn D, Pradyachaipimol A, Viriyapak B (2017) Incidence of superimposed preeclampsia among pregnant Asian women with chronic hypertension. Hypertension in pregnancy. 206: 226-231.
- Hastie R, Brownfoot FC, Cluver CA, Walker SP, Hesselman S, et al. (2019_Predictive value of the signs and symptoms preceding eclampsia: a systematic review. Obstetrics & Gynecology 134: 677-684.
- Choi YS, Hong JY, Hong JY, Kim YM, Sung JH, et al. (2020) The effects of maternal body mass index and plurality on maternal and umbilical cord serum magnesium levels in preterm birth at less than 32 weeks of gestation. Obste Gynecol Sci 64: 62-72.
- Jaisamut P, Kitiyodom S (2017) Effect of Maternal Body Mass Index on Serum Magnesium Level in Pregnant Women with Preeclampsia at Maharat Nakhon Ratchasima Hospital. Thai J of Obst and Gynaecol 159-66.
Citation: Ahmed Shehata Ramadan MF, Youssef MA (2022) Effect of Obesity on Serum Maternal and Fetal Magnesium Level in Preeclamptic Recipients of Magnesium Sulphate. J Reprod Med Gynecol Obstet 8: 98.
Copyright: © 2022 Mohamed F Ramadan, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

