
Effect of PH on Gelation Properties of Protein Flours and Isolate from Two Varieties (DAS & BS) Of Nigerian Cultivated Solojo Cowpea (Vigna Unguiculata L. Walp)
*Corresponding Author(s):
Henry O ChibudikeDepartment Of Chemical, Fiber And Environmental Technology, Federal Institute Of Industrial Research, Oshodi, Lagos, Nigeria
Email:henrychibudike@gmail.com
Abstract
Effect of pH on Gelation Property of legume flours and isolate from the two varieties (DAS & BS) of Nigeria cultivated solojo cowpeas were investigated. Results show that hydrogen ion concentration exercises its influence on gelling of protein through its control on charge and balance of ions (electrostatic balance) and amide protein molecules. As the hydrogen ion concentration of the protein is shifted away from the isoelectric point, the influence of electrostatic bonds on gel formation was greatly felt. The protein isolates of DAS and BS also had improved LGC with germination, going from 14%w/v to 6%w/v and 16%w/v to 8%w/v respectively. Gelation property of raw (native/ control) and germinated Dark-ash Solojo Cowpea (FFDAS, DFDAS, FFBS and DFBS flours; DAS and BS protein isolate) was influenced by germination and physico-functional characteristic. Utilizing the LGC as evidence of gelling capability, it was observed that the LGC for all the samples both flours and isolates was generally at pH 4 except for the FFDAS and FFBS, Raw and 6h, which both had their LGC at pH 10. The four flour samples, FFDAS, DFDAS, FFBS and DFBS as well as the two protein isolates exhibited some variations in gelation property. The protein isolates gave stiff paste instead of regular gel, going from, DAS, 18 – 4% and BS, 18-6%. Disparity in gelling properties observed, may be as a result of the proportion of diverse components for example proteins, fat and carbohydrate in the various legume flours. The prevalent charges on the exterior of the proteins, which is affected by pH, causes variation in the balance between the polar and non-polar residues, thereby bringing about differences in gelation.
Keywords
BS; DA; Essential amino acids; Gelation Property LGC; Nutraceuticals; Solojo Cowpea; Under-utilized legumes
Introduction
Gel is a dispersed system with a minimum of two constituents which forms a cohesive structure. It is distinguished by the absence of fluency and flexible deformation. Globular proteins like legume proteins, under definite conditions (such as heating and denaturation), forms gel. Ordinarily, this sort of gel is described as collective dispersion [1]. Gelation of protein is very crucial in the build-up and approval of several food products. Gelling mechanism and gel presentation are basically regulated by the equilibrium between attractive electrostatic forces and repulsive hydrophobic interaction. (Adebowale and Lawal, 2004; Lawal et al., 2007) the repelling forces are as a result of exposed outer charges and attractive forces which are as a result of varied functional groups uncovered by the heat induced uncoiling of the protein (Lawal et al., 2007).
The LGC is a quality specification that indicates the lowest quantity of protein required to form gel that doesn’t slip/slide along the walls of test tube when placed up-side-down [2]. A gel can also be defined as a system of uncoiled molecules cross-linked to produce an assemblage comprising of large quantities of en-captured water [3,4]. The mechanism of forming gels is dependent upon numerous factors such as, protein quantity, salt concentration, H+ concentration and inter-action with other content. The nature of the protein, carbohydrate and lipids has an influence on gel forming ability of flour.
Legume seeds have different storage proteins, but the major storage proteins are the globulins, which can disintegrate and integrate in various shapes forming gels by heating [5]. The capability of protein in gel formation, providing a structure matrix to hold food ingredients such as water, flavours, sugars and others, is of uttermost importance in food operations and in the evolvement of new product [2]. Protein ability to form gel is the outcome of a process involving two-step, the incomplete unfolding of respective proteins in order to access additional responsive sideways groups inside the protein molecules and the gathering of these proteins through the sensitive side groups to a three-dimensional arrangement of structures able of keep substantial quantity of H2O. This occurrence is of great essence in the nutrition business because it is partly responsible for the texture and conformational changes in several foods. Reduced gelation capacity conveys an improved gelling ability [3,4,6] also reported LGC of 12% for hulled Vigna unguiculata seed and Lupinus albus L. seed flours just like the observed result for BS 6 h isolate.
Proteins having high concentration of hydrophobic amino acids form gels that are clot-like, while proteins with high prevalence of hydrophilic amino acids, form gels that are transparent [5] Legumes are made up of globular protein, especially the globulins which are soluble in NaCl and albumins which are soluble in water. During the production of protein isolate, there is the likelihood of portions of the water-soluble albumin to go into solution, making the isolates richer in salt-soluble portion [7]. This could result in the production of better gels for legumes higher in globulins, because the globulins unfold easily especially in ionic environment. Gel strength has also been linked with the ability of the protein to unfold. When protein unfolds the buried functional groups are exposed, like, hydrophilic, hydrogen, sulfhydryl and electrostatic groups. When protein unfolds, protein gathering occur through non-polar interactions and are toughened the more by the creation of disulphide bridges [8]. The disulfide bridges in protein gelation play the role similar to their capability to raise the protein molecular mass by increasing the chain length, instead of acting as a preliminary network stabilizer [8].
Gelation property variation could be connected to the sizes of the various component in the protein which also plays an essential part in the functional characteristics [9]. The improvement of LGC with ionic strength could be due to improved protein solubility in the salt solution, which brought about an efficient overlying of the functional groups within adjacent protein molecules, a necessary precondition for gel network formation [10,11] observed an enhancement of the gelling quality with salt concentration elevation to 0.4 M, but beyond this value affected the gelation concentration of mucuna, the concentrate and flours in a negative manner. The formation of a gel is conditioned on the exposure of the specific substituents or moieties (functional groups) within molecules (e.g. non-polar groups) inside the protein, which leads to easier interaction of the groups and the formation of a three-dimensional system. Gel creation is intricate and is influenced by duration and temperature in addition to H+ ion concentration and inter-reaction with additional constituents in the food matrix [12,13]. The path way of gelation is shown below;
Increased temperature induces the denaturation of native protein leading to the formation of disulphide bonds and the exposure of non-polar residues of the amino acid [13].
Materials And Methods
Two varieties of the underutilized cowpea (V. unguculata) found in South west region of Nigeria where it is called ‘Solojo’ were used. Seeds obtained from Bodija market in Ibadan, Western Nigeria, were screened to get rid of every irrelevant materials and unwholesome seeds. The beans were then portioned into six (6). The Solojo seeds for germination were sterilised by soaking in 0.07 % Sodium hypochlorite for 30 min, then, it was rinsed thoroughly. The Solojo seeds were then immersed for 6 h in distilled water at ambient temperature (1:10 w/v) (~25oC), then placed in a colander and germinated under subdued light in an open laboratory for, 24, 36, 48 and 72 h.
Preparation of flours
Raw flour: The grains were segregated to remove the spoilt ones; then dry dehulled with a mechanical dry dehuller (Fabricated in FIIRO), dried at 40oC and later milled dry to powder then sifted using 80 µm mesh. The flour was stored in flexible bags and preserved at 4oC preceding utilization in a refrigerator freezer.
6 h Soaked flour: The seeds were segregated to remove the unwholesome ones, then immersed for 6 h in the ratio (1:10 w/v) (seed/water). The grains were then frozen to prevent germination from setting in, then the hull was removed manually, dried for 48 h at 40oC later milled dry to smooth powder prior to sieving using 80 µm mesh screen. The resulting flour was packaged in plastic pack and preserved in a fridge- freezer at 4oC pending utilization.
Germination of seed: This was implemented by the method of Mubarak A.E. 2005 with minor adjustment. The seeds for germination were disinfected by soaking in 0.07 % Sodium hypochlorite [14] for 30 mins, then, it was rinsed painstakingly. The Solojo seeds were then immersed for 6 hours at ambient temperature in water in the ratio (1:10 w/v) (seed/water) (~25oC), then placed in a colander and germinated under subdued light in an open laboratory [15] for various hours such as 24, 36, 48 and 72 h. The process of germination was terminated by freezing, the seeds were manually dehulled (Figures 1 & 2), dried in a draught oven (Schutzart DIN EN 60529-IP 20. Memmert, Germany) at 40oC for 48 h, cooled, milled and packaged in an air tight plastic bag in the refrigerator pending analysis (Figure 3).
 Figure 1: Brown Solojo Cowpea.
Figure 1: Brown Solojo Cowpea.
 Figure 2: Dark-Ash Solojo Cowpea.
Figure 2: Dark-Ash Solojo Cowpea.
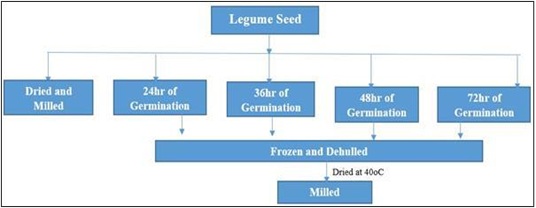 Figure 3: Preparation of Beans Flour/Schematic representation.
Figure 3: Preparation of Beans Flour/Schematic representation.
Results and Discussion
Hydrogen ion concentration exercises its influence on gelling of protein through its control on charge and balance of ions (electrostatic balance) and amide protein molecule. Also, as the hydrogen ion concentration of the protein is shifted away from the isoelectric point, the influence of electrostatic bonds on gel formation is greatly felt [16,17].
Effect of pH on gelation capability of legume flours and isolate is as enumerated in Tables 1-6. Utilizing the LGC as evidence of gelling capability, it was observed that the LGC for all the samples both flours and isolates was generally at pH 4 except for the FFDAS and FFBS, Raw and 6h, which both had their LGC at pH 10.
Table 1 showed that germination improved the gelation capacity of the FFDAS as it brought the Least Gelation Concentration (LGC) from 4%w/v to 2%w/v except for 6 h and 72 h germination; likewise, FFBS had its LGC improve with germination from 4%w/v to 2%w/v, except for 6 h. The protein isolates of DAS and BS also had improved LGC with germination, going from 14%w/v to 6%w/v and 16%w/v to 8%w/v respectively [18]. For the DFDAS and DFBS, it was observed that more of the flours were needed to effect gelation as germination progressed. This could be as a result of rise in attraction of protein for water, which reduced the reaction between proteins.
LGC for the Solojo flours ranged between 8%w/v to 2%w/v which is still better than the Least Gelation Concentration (LGC) of Lupin seed flour which gave 14%w/v, Jack bean flour 14%w/v, Bambara groundnut flour 16%w/v and even Mucuna beans 16% and germinated Ugba seed as reported in literature. The protein isolates had the LGC range for DAS between 14% - 6%; and for BS between 16% - 8%w/v, the isolates formed more of thick paste than gel (Table 1).
|
LGC/FFDAS(%w/v) |
pH2 |
pH4 |
pH7 |
pH8 |
pH10 |
|
Raw |
10 |
4 |
6 |
8 |
8 |
|
6 h |
12 |
4 |
8 |
8 |
8 |
|
24 h |
12 |
2 |
4 |
6 |
6 |
|
36 h |
14 |
4 |
6 |
6 |
6 |
|
48 h |
14 |
2 |
16 |
8 |
8 |
|
|
|
|
|
|
|
|
72 h |
8 |
4 |
8 |
6 |
8 |
Table 1: Effect of pH on Least Gelation Concentration (LGC) of FFDAS flour.
*LGC- Least gelation concentration
*FFDAS- Full Fat Dark Ash Solojo Cowpea
The nature of the protein, carbohydrate and lipids has an influence on gel forming ability of flour. This is what might have caused the difference in value of the Lowest Gelling Concentration (LGC) of the undefatted and defatted flours. These flours may be used for the formation of cords because of their low gelling concentration or as an additive in food products for other gel forming materials [19]. Gelling characteristics is also significant in the manufacture of and acceptance of several foods products, such as jellies, puddings, vegetables, many meat and dessert utilizations (Table 2).
|
LGC/DFDAS(%w/v) |
|
pH2 |
|
pH4 |
|
pH7 |
|
pH8 |
|
pH10 |
|
Raw |
|
16 |
|
6 |
|
8 |
|
10 |
|
8 |
|
6 h |
|
18 |
|
6 |
|
8 |
|
10 |
|
8 |
|
24 h |
|
18 |
|
6 |
|
8 |
|
10 |
|
6 |
|
36 h |
|
20 |
|
6 |
|
8 |
|
12 |
|
6 |
|
48 h |
|
18 |
|
6 |
|
8 |
|
12 |
|
6 |
|
72 h |
|
18 |
|
6 |
|
8 |
|
14 |
|
16 |
Table 2: Effect of pH on Least Gelation Concentration (LGC) of DFDAS Flours.
*LGC- Least gelation concentration
*DFDAS- Defatted Dark Ash Solojo Cowpea
Varietal difference was also observed in the result obtained, with DAS isolate having lower gelation than BS isolate. Gelation has also been found to be affected by the type of dominant amino acids in the protein. Proteins having high concentration of hydrophobic amino acids form gels that are clot-like, while proteins with high prevalence of hydrophilic amino acids, form gels that are transparent (Table 3).
|
LGC/ FFBS |
pH 2 |
pH 4 |
pH 7 |
pH 8 |
pH 10 |
|
|
Raw |
8 |
4 |
10 |
8 |
10 |
|
|
6 h |
8 |
4 |
12 |
8 |
10 |
|
|
24 h |
8 |
2 |
10 |
8 |
8 |
|
|
36 h |
18 |
2 |
2 |
10 |
10 |
|
|
48 h |
18 |
2 |
10 |
8 |
8 |
|
|
72 h |
20 |
2 |
10 |
8 |
8 |
|
Table 3: Effect of pH on Least Gelation Concentration (LGC) of FFBS.
*LGC- Least gelation concentration
*FFBS- Full fat brown solojo cowpea.
Effect of pH on gelation capability of legume flours and isolate is as enumerated in Tables. The Lowest Gelation Concentration (LGC) of the Solojo cowpea germinated flours was better than those of Phaseolus vulgaris flour, Black gram bean (Vigna mungo) flour, Jack bean flour, Bambara groundnut flour and Mucuna bean flour reported in literature (Tables 4 & 5). The LGC of FFDAS was found to be lower than that obtained for undefatted African locust bean at all the different pH values, 16 -2% as against 16 – 6% for the Parkia biglobosa. Likewise, the Albumin and globulin had variations in the pH range of 2-20% having values 18 -10% and 18-12% LGC respectively as against those of FFDAS, 16 -2%; DFDAS, 20-6%; FFBS, 20 – 2%; DFBS, 16-2 %; for all the treatments respectively. This is as a result of the electrostatic bond, having a great influence on gel creation as the pH of the protein shifts from the isoelectric point (Tables 6).
|
LGC/ DFBS |
pH 2 |
pH 4 |
pH 7 |
pH 8 |
pH 10 |
|
|
Raw |
14 |
2 |
6 |
6 |
6 |
|
|
6 h |
16 |
2 |
6 |
8 |
6 |
|
|
24 h |
16 |
2 |
8 |
12 |
6 |
|
|
36 h |
16 |
2 |
8 |
12 |
6 |
|
|
48 h |
16 |
2 |
8 |
12 |
6 |
|
|
72 h |
16 |
2 |
8 |
16 |
6 |
|
Table 4: Effect of pH on Least Gelation Concentration (LGC) OF DFBS Flour.
*LGC- Least Gelation Concentration
*DFBS- Defatted Brown Solojo Cowpea
|
LGC/DAS |
pH 2 |
pH 4 |
pH 7 |
pH 8 |
pH 10 |
|
|
Raw |
12 |
4 |
10 |
12 |
12 |
|
|
6 h |
12 |
6 |
14 |
14 |
14 |
|
|
24 h |
10 |
6 |
10 |
12 |
12 |
|
|
36 h |
14 |
6 |
14 |
14 |
14 |
|
|
48 h |
8 |
6 |
10 |
10 |
10 |
|
|
72 h |
10 |
8 |
12 |
12 |
12 |
|
Table 5: Effect of pH on LGC of DAS Isolate.
*LGC – Least Gelation Concentration
*DAS – Dark- ash solojo cowpea protein isolate
|
LGC/BS |
pH 2 |
pH 4 |
pH 7 |
pH 8 |
pH 10 |
|||
|
Raw |
14 |
6 |
12 |
12 |
12 |
|||
|
6 h |
14 |
8 |
16 |
14 |
14 |
|||
|
24 h |
12 |
8 |
10 |
10 |
||||
|
10 |
||||||||
|
36 h |
14 |
6 |
12 |
12 |
||||
|
12 |
||||||||
|
48 h |
10 |
6 |
12 |
12 |
||||
|
12 |
||||||||
|
72 h |
10 |
8 |
12 |
14 |
||||
|
|
12 |
|||||||
|
|
||||||||
Table 6: Effect of pH on LGC of BS Isolate.
*LGC – Least Gelation Concentration
*BS – Brown solojo cowpea protein isolate
It was observed that the two protein isolates formed better stiff gels when equated with each other in a concentration range of 100-140 g/L. The reason deduced for this observation was that the strength of the molecular interaction was too weak to overshadow the repulsive forces.
Addition of salt improved gelation for all treatments of the full fat DAS and BS, but for defatted samples, inclusion of salt did not improve the gelation as the control had a lower LGC than the germinated except for the 24 h germinated DFBS which had the same LGC as the control [20]. This could be because part of the starch and fat has been used for germination, and some have also been removed as a result of defatting.
This goes to show that gelling properties does not only depend on protein nature, other constituents invariably affect the properties (protein and non- protein). The low LGC observed in this work at higher concentration could be as a result of the germination, which brought about a reaction between internal enzymes and starch in the seed. Germination improved gelation for all treatments of both the full FFDAS and the DFDAS but the defatted samples had better gelation than the full fat. Varying LGC has been reported for different legumes, the range is between 8-16 % [21]. The least gelation concentration reduced in range between 14% LGC to 2% for the DAS samples, while those of BS samples reduced in range between 12% and 2%. The result shows improvement of gelation properties with increase in ionic strength.
Conclusion and Recommendation
Biochemical modification which involves the activation of the intrinsic enzymes of the Solojo cowpea seed itself by germination was carried out for different hours for the two varieties, i.e. the Dark-Ash and the Brown Solojo beans.
Gelling mechanism and gel presentation are basically regulated by the equilibrium between attractive electrostatic forces (non-polar interactions) and repulsive hydrophobic interaction (repelling Van der Waals interaction). The repelling forces are as a result of exposed outer charges and attractive forces which are as a result of varied functional groups uncovered by the heat induced uncoiling of the protein. Hydrogen ion concentration exercises its influence on gelling of protein through its control on charge and balance of ions (electrostatic balance) with and amid protein molecules. Also, as the hydrogen ion concentration of the protein is shifted away from the isoelectric point, the influence of electrostatic bonds on gel formation was be greatly felt.
Analysis revealed that better gelation at the isoelectric point was experienced as a result of improved interaction between protein molecules. Mean-while further away from isoelectric point pH, the charge on the protein gets bigger and therefore serious repelling forces hinder protein-protein inter-action. It was also observed that, pH influences distribution of charges within the amino acid side chains whether by reducing or improving the protein with protein interaction.
This research work shows that germination had a profound and significant (p<0.05) effect on the nutritional composition of Solojo beans, revealing great improvement and thus making Solojo a potential substitute to other important legumes such as soya beans.
This research work also shows that biochemical modification (Germination/Malting/ Sprouting) had an enourmous impact on the nutritional composition, functional properties, mineral bioavailability, anti-nutrient content and amino assay of Solojo bean, thus, it could be used as protein supplement in infant, young children and geriatric foods. Efforts should be increased to promote the cultivation, encourage the consumption and industrial application of this under-utilized legume by the Government, especially in the south-western region where it can survive the rain fall level. Large scale production of this legume which is gradually going into extinction should be encouraged in order to fight the menace of malnutrition in developing countries where animal protein price is exorbitant; This will ensure food security and also creation of jobs, because people can engage in different aspects of the production process and thereby reducing the rate of unemployment.
References
- Barac MB, Pešic MB, Stanojevic SP, Kostic AZ, Cabrilo SB (2015) Techno-Functional Properties of Pea (Pisum sativum) Protein Isolates- A Review. APTEFF 46: 1-18.
- Eltayeb ASM, Ali OA, Abou-Arab AA, Abu-Salem FM (2011) Chemical and functional properties of flour and protein isolate extracted from Bambara ground nut (Vigna subterranean). African journal of Food Science 5: 82- 90.
- Khalid II, Elharadallou SB (2013) Functional Properties of Cowpea (Vigna Ungiculata L. Walp), and Lupin (Lupinus Termis) Flour and Protein Isolates. Journal of Nutrition Food Science 3: 234.
- Raikos V, Neacsu M, Russell W, Duthie G (2014) Comparative Study of the Functional Properties of Lupin, Green pea, Fava bean, Hemp and Buckwheat Flours as affected by pH. Food Science and Nutrition 2: 802- 810.
- Saavedra JP, Guemez-Vera N, Montanez- soto JL, Fernandez-Martinez MC, Yenez-Fernandez J (2013) Comparative study of functional properties of protein isolates obtained from three lipinus species. Advances in Biorearch 4: 106-116.
- Boye JI, Aksay S, Roufik S, Ribéreau S, Mondor M, et al. (2010) Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Research International, 43: 537-546.
- Berghout JAM, Boom RM, Goot AJV (2014) The potential of aqueous fractionation of lupin seeds for high- protein foods. Food Chemistry 159: 64-70.
- Berghout JAM, Pelgrom PJM, Schutyser MAI, Boom RM, van der Goot AJ (2015) Sustainability assessment of lupin fractionation processes. Journal of Food Engineering 150: 117-e124.
- Kaur M, Singh N (2007) Characterization of protein isolates from different Indian Chickpea. (Cicer arietium L.). Cultivars. Food Chemistry 102: 366-374.
- Chaparro Acuña SP, Gil González JH, Aristizábal Torres ID (2012) Physicochemical characteristics and functional properties of Vitabosa (Mucuna deeringiana) and Soybean (Glycine max). Ciênc Tecnol Aliment 32: 98-105.
- Adebowale YA, Adebowale KO (2008) Evaluation of the gelation characteristics of Mucuna bean flour and protein isolate. Electronic journal of Environment Agriculture Food Chemistry 7: 3206- 3222.
- Raikos V, Campbell L, Euston SR (2007) Rheology and Texture of Hen’s Egg Protein Heat-set Gels as Affected by pH and the Addition of Sugar And/or Salt. Food Hydrocolloids 21: 237-244.
- Söderberg J (2013) Functional properties of legume proteins compared to egg proteins and their potential as egg replacers in vegan food. Sweden 43.
- Rumiyati AP, James VJ (2012) Effect of Germination on the Nutritional and Protein Profile of Australian Sweet Lupin (Lupinus angustifolius L.). Food and Nutrition Science 3: 621-626.
- Rusydi MR, Noraliza CW, Azrina A, Zulkhairi A (2011) Nutritional changes in germinated legumes and rice varieties. International food Research Journal 18: 705-713.
- Aremu MO, Olaofe O, Akintayo ET (2007) Functional properties of some Nigerian Varieties of legume seed flours and flour concentration effect on foaming and gelation properties. Journal of Food Technology 5: 109-115.
- Butt MS, Batool R (2010) Nutritional and Functional properties of some promising Legumes protein isolates. Pakistan Journal of Nutrition 9: 373-379.
- Horax R, Hettiarachchy N, Kannan A, Chen P (2011) Protein extraction optimization, characterisation and functionalities of protein extracted from bitter melon (Momordica charantia) seed. Food Chemistry 124: 445.
- Mizubuti IY, Biondo Júnior O, Souza LW, da Silva RS, Ida EI (2000) Functional properties of pigeon pea (Cajanus cajan (L.) Millsp.) flour and protein concentrate. Archivos Latinoamericanos De Nutrición 50: 274-280.
- Mwasaru MA, Muhammad K, Bakar J, Che Man YB (2000) Influence of altered Solvent environment on the functionality of pigeon pea (Cajanus cajan) and cowpea (Vigna unguiculata) protein isolate. Food Chemistry 71: 157-165.
- Onimawo IA, Akpojovwo (2006) Toasting (Dry heat) and Nutritient Composition. Functional properties and anti-nutritional factors of pigeon pea (Cajanus cajan) flour. Journal of Food Processing and Preservation 30: 742-753.
Citation: Adeyoju AA, Chibudike HO, Oluwole BO, Adebowale KO, Chibudike CE (2023) Effect of PH on Gelation Properties of Protein Flours and Isolate from Two Varieties (DAS & BS) Of Nigerian Cultivated Solojo Cowpea (Vigna Unguiculata L. Walp). J Plant Sci Curr Res 7: 023.
Copyright: © 2023 Kayode O Adebowale, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

