
Effect of Progesterone Levels on the Day of hCG Trigger on Quality of Embryos in ICSI Cycles
*Corresponding Author(s):
Mohammad AlghazawiBritish-Syrian IVF Centre, Alrasheed Hospital, Damascus, Syria
Email:mhamadalghazawi@gmail.com
Abstract
This study aimed to identify whether there is a correlation between the concentration of Progesterone and the number of Mature Oocytes (MII) and between the concentration of progesterone and the number of Fertilized Oocytes 2PN, in addition to identify the Categories of the progesterone concentration range which may negatively affect the embryo quality and fertilization rate. The study was a non-interventional, retrospective, included 1078 patients who underwent ICSI cycles, using Long Luteal Phase protocol. Transvaginal ultrasound-guided oocyte retrieval was schadualed 34-36h after HCG trigger. This study revealed that A)- elevated serum progesterone on the day of oocyte maturation trigger is associated with an elevated Top-Quality Embryo (TQE) Rate, and ideally the best result obtained in the progesterone level ranged (2.0-2.5ng/ml). B) - there is a statistically significant correlation between the progesterone concentration and the number of fertilized oocytes. As our study was a single centre study, results need to be validated across different centres.
Keywords
Human Chorionic Gonadotropin (hCG); Progesterone (P4); Top-Quality Embryo (TQE).
Introduction
In Vitro Fertilization (IVF) regimens of treatment, which are continuously being improved, have allowed the birth of over a million babies all over the world. One such improvement, which represents a major breakthrough in this area, is Intracytoplasmic Sperm Injection (ICSI). It is considered progesterone elevation on the day of Human Chorionic Gonadotropin (hCG) administration is critical in Long luteal agonist protocol. Most research has reported that elevated progesterone had an adverse impact on quality oocytes [1,2], and the endometrial environment of fresh cycles, leading to a decrease in pregnancy rates [3,4]. However, the possible effects of these subtle progesterone increases on pregnancy outcomes are controversial [5]. Several studies have indicated that some patients have Progesterone (P4) elevation on the day of Human Chorionic Gonadotropin (hCG) administration during Controlled Ovarian Stimulation (COS) of Assisted Reproductive Technology (ART), although, the exact mechanism is unclear [6-8]. Despite effective suppression with Gonadotropin-Releasing Hormone (GnRH) analogs in long protocol, P4 elevation is reported in 5-53% of stimulated cycles [3,9,10].
The introduction of GnRH agonists and antagonists for pituitary suppression during ICSI significantly decreases the incidence of premature LH surge [11]. Despite the use of GnRH analogs (GnRHa), however, several researchers have described a phenomenon reported as ‘‘premature luteinization’’ [9,12]. Premature elevation of progesterone level on the day of ovulation trigger prior to IVF is common and causes a decrease in endometrial receptivity, a freeze all strategy is then recommended [13]. The Top-Quality Embryo (TQE) has a direct correlation with the quality of oocyte and consequences ICSI cycle outcomes. Therefore, the study aimed of whether there is a correlation between the concentration of progesterone and the number of mature oocytes (MII) and between the concentration of progesterone and the number of fertilized oocytes 2PN. And in addition to Identify Categories where the concentration of progesterone results in low levels of embryo quality and a low number of fertilized oocytes.
Material and Methods
Patients
This was a non-interventional, retrospective, single-center cohort study of patients in routine clinical practice. To reflect the broad range of patients typically encountered in clinical practice, no inclusion/exclusion criteria were applied to baseline characteristics apart from sever oligoasthenozoospermia. Patients were treated at the British-Syrian Centre of AL-Rasheed Hospital between December 2018 and December 2021. A total of 1078 patients undergoing ICSI treatment were enrolled, and all patients gave written informed consent. Several causes of infertility were present, including tubal factor, endometriosis, PCOS, male factor, unexplained infertility and mixed factors.
Ovarian stimulation
A total of 1078 ICSI-ET cycles were performed. 150-225 IU daily of human gonadotropins (Menopur, Ferring, Germany or Gonal F, Serono) were administrated for 11-12 days (total gonadotrophin dose of 1800 IU - 2700 IU) using Long Luteal Phase agonist protocol ( Decapeptyl as GnRH analogue), while triggering by (Ovitrelle 250microgram/0.5ml - 500microgram/0.5ml, Merck) according to the BMI, as trigger ovulation and Oocyte maturation. Specialized ultrasound technicians monitored the ovarian response by vaginal ultrasonography. HCG triggering was given when the 3 leading follicles reached a mean diameter of 18mm. Transvaginal ultrasound-guided oocyte retrieval was scheduled 34-36h after hCG trigger.
Fertilization of the aspirated oocyte was performed in vitro by ICSI depending on the semen parameters. Embryo assessment was performed for the number and regularity of blastomeres and the degree of embryonic fragmentation on the cleavage stage; the criteria were described previously [14]. Grade given 4/4 an excellent embryo and grade 3/4 embryos were good-quality embryos. Fresh embryo transfers were performed on day 3 after oocyte retrieval. The additional good-quality embryos were cryopreserved for subsequent Frozen Embryo Transfer (FET) cycles. The number of embryos transferred ranged from one to three according to age, reproductive history, number of failed cycles and ovarian reserve. We started the luteal phase support with 90mg progesterone vaginal gel twice a day (Crinone 8%, Merck) on the evening of egg collection day and continued till the 8 gestational week if pregnancy occurred. A folic acid one tablet a day commenced prior to treatment cycles.
Hormone measurement
Serum P4 and E2 levels were measured on the day of hCG administration. Samples were tested with a microparticle enzyme immunoassay (Biomerieux - Vidas - France).
Statistical analysis
Use escriptive statistics for quantitative data, categorical data, cross tables and graphs.
- Kolmogorov_samirnov normal distribution test to study the normal distribution of quantitative variables in order to find out whether parametric or non-parametric correlation tests will be used.
- Spearman correlation coefficient to study the correlation between quantitative variables that are not a normal distribution, as according to this test, there is a statistically significant correlation between the two variables if the p-value is less than 5%
The categorical variables were presented as frequencies and percentages and continuous variables were presented as mean ± standard deviations. The correlation was studied between study variables (MII, 2PN, Quality embryos) and progesterone by Spearman tests. The analysis was performed in a 95% confidence interval using Statistical Package for Social Science (SPSS), version 25 (IBM, Armonk, NY, USA).
Results
The average number of mature oocytes out of the total number of oocytes is approximately 8 oocytes from each individual, which is approximately 67% of the number of oocytes withdrawn for each member of the sample. The average number of fertilized oocytes out of the total number of mature oocytes is approximately equal to 6, which is equivalent to 75% of the mature oocytes and 50% of the total oocytes, and these values are for each individual in the sample. 41.58% of the sample members have a progesterone level within the category (<=1) which is the highest frequency, while 18.81% of the individuals who have a progesterone level fall within the category (1 1.5) (Tables 1 and 2) (Figure 1).
|
Descriptive Statistics |
|||||
|
Variable |
N |
Min |
Max |
Mean |
Std. Deviation |
|
P4 |
1111 |
0.20 |
7.31 |
1.9393 |
1.72864 |
|
OOCYTES |
1111 |
1.00 |
40 |
12 |
7.64818 |
|
MII |
1111 |
1.00 |
30 |
8 |
5.48521 |
|
%MII |
1111 |
0.30 |
1.00 |
0.6745 |
0.15648 |
|
2PN |
1111 |
0.00 |
24 |
6 |
4.23636 |
|
%2PN |
1111 |
0.00 |
1 |
0.7353 |
0.22284 |
|
total embryos day 2 (TED2) |
726 |
0.00 |
23 |
5.56 ~ (6) |
4.2612 |
|
embryos grade 4 in day 2 (D2G4) |
1111 |
0.00 |
15 |
3.0792 |
2.89373 |
|
embryos grade 3 in day 2 (D2G3) |
1111 |
0.00 |
10 |
1.7327 |
1.80494 |
|
embryos grade 2 in day 2 (D2G2) |
1111 |
0.00 |
8 |
0.7327 |
1.39922 |
|
total embryos day 3 (TED3) |
1111 |
0.00 |
23 |
5.52 ~ (6) |
4.21804 |
|
embryos grade 4 in day 3 (D3G4) |
1111 |
0.00 |
15 |
3.0198 |
2.8565 |
|
embryos grade 3 in day 3 (D3G3) |
1111 |
0.00 |
8 |
1.604 |
1.66781 |
|
embryos grade 2 in day 3(D3G2) |
1111 |
0.00 |
11 |
0.901 |
1.68822 |
|
SPERMPRG |
1089 |
0.00 |
85 |
35.2121 |
24.5404 |
Table 1: Descriptive statistics for quantitative variables.
|
Categories P4 |
Categories |
<=1 |
1-1.5 |
1.5-2 |
2-2.5 |
>2.5 |
Grand Total |
|
Frequency |
462 |
209 |
110 |
11 |
319 |
1111 |
|
|
Percent |
41.58% |
18.81% |
9.90% |
0.99% |
28.71% |
100.00% |
Table 2: Frequencies and percentages corresponding to progesterone categories.
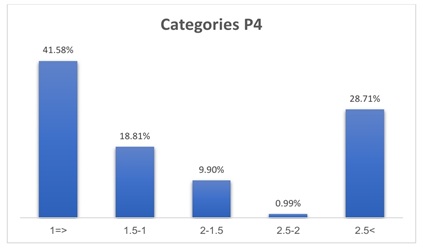 Figure 1: Progesterone categories.
Figure 1: Progesterone categories.
Study variables are not following the normal distribution, so the nonparametric Spearman test used as an alternative to the parametric Pearson test when the quantitative data do not follow the normal distribution. Individuals with a progesterone concentration level of the category (2-2.5) had an average of 6 fertilized oocytes that led to obtaining the highest number of embryos on the second and third days, as 6 embryos were obtained on the second and the third day (Table 3 and Figure 2).
|
P4 Categories |
<=1 |
1-1.5 |
1.5-2 |
2-2.5 |
>2.5 |
Total |
|
|
OOCYTES |
Freq |
4675 |
2277 |
1298 |
165 |
4741 |
13156 |
|
% |
35.54% |
17.31% |
9.87% |
1.25% |
36.04% |
100.00% |
|
|
Aver |
10.12 |
10.89 |
11.80 |
15.00 |
14.86 |
11.80 |
|
|
MII |
Freq |
3289 |
1551 |
858 |
110 |
3025 |
8833 |
|
% |
37.24% |
17.56% |
9.71% |
1.25% |
34.25% |
100.00% |
|
|
Aver |
7.12 |
7.42 |
7.80 |
10.00 |
9.48 |
7.95 |
|
|
2PN |
Freq |
2332 |
1100 |
572 |
66 |
2277 |
6347 |
|
% |
36.74% |
17.33% |
9.01% |
1.04% |
35.88% |
100.00% |
|
|
Aver |
5.05 |
5.26 |
5.20 |
6.00 |
7.14 |
5.71 |
|
|
TED2 |
Freq |
2266 |
1067 |
561 |
66 |
2200 |
6160 |
|
% |
36.79% |
17.32% |
9.11% |
1.07% |
35.71% |
100.00% |
|
|
Aver |
5.02 |
5.11 |
5.10 |
6.00 |
6.90 |
5.60 |
|
|
TED3 |
Freq |
2266 |
1067 |
561 |
66 |
2178 |
6138 |
|
% |
36.92% |
17.38% |
9.14% |
1.08% |
35.48% |
100.00% |
|
|
Aver |
4.90 |
5.11 |
5.10 |
6.00 |
6.83 |
5.52 |
|
Table 3: Average, frequencies, and percentages of the OOCYTES, MII, 2PN, TED2, and TED3.
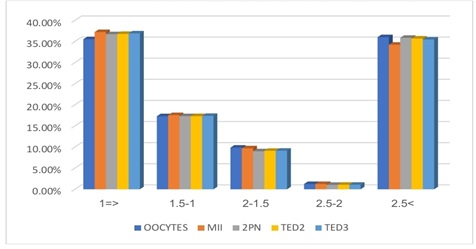 Figure 2: Distribution of the number of mature and fertilized oocytes and the total number of embryos on the second and third days according to progesterone categories.
Figure 2: Distribution of the number of mature and fertilized oocytes and the total number of embryos on the second and third days according to progesterone categories.
We note that the second category of progesterone hormone 1-1.5 gave the highest percentage of the number of poor-quality embryos Grade 2 (G2), while the fourth category 2-2.5 gave the highest percentage in the number of Grade 4 (G4), However, at most different levels of progesterone, we find that the majority of the quality of the embryos are of Grade 4(G4) (Table 4 and Figure 3).
|
Quality embryos (Day 2 + Day 3) *p4 |
|||||||
|
P4 categories |
G4 |
G3 |
G2 |
Total |
|||
|
Frequency |
Percent |
Frequency |
percent |
Frequency |
Percent |
||
|
<=1 |
1320 |
58.25% |
627 |
27.67% |
627 |
27.67% |
2266 |
|
1-1.5 |
495 |
46.39% |
407 |
38.14% |
407 |
38.14% |
1067 |
|
1.5-2 |
308 |
54.90% |
121 |
21.57% |
121 |
21.57% |
561 |
|
2-2.5 |
55 |
83.33% |
11 |
16.67% |
11 |
16.67% |
66 |
|
>2.5 |
1210 |
55.28% |
687.5 |
31.41% |
687.5 |
31.41% |
2189 |
|
Total |
3388 |
55.10% |
1853.5 |
30.14% |
1853.5 |
30.14% |
6149 |
Table 4: Average frequencies and percentages of embryo quality by progesterone categories.
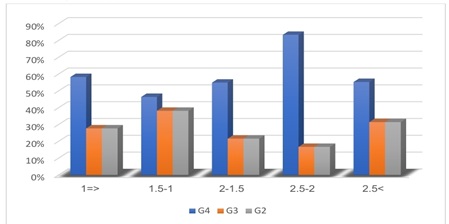 Figure 3: Distribution of oocyte quality according to progesterone categories.
Figure 3: Distribution of oocyte quality according to progesterone categories.
Embryos grade 4 (G4) - grade 3 (G3) - grade 2 (G2).
By studying the correlation matrix between the P4 values and other quantitative variables according to the Spearman coefficient, we found several results, the most important of which are:
- There is a statistically significant correlation between the level of progesterone concentration and the number of fertilized oocytes, and it is a weak positive correlation
- There is a statistically significant relationship between the level of progesterone concentration and the number of excellent embryos on the second and third days, and it is a weak positive correlation (Table 5)
|
Variable |
Spearman correlation with P4 |
Sig |
|
MII |
0.132 |
0.188 |
|
2PN |
0.210* |
0.035 |
|
D2G4 |
0.198* |
0.047 |
|
D2G3 |
0.187 |
0.061 |
|
D2G2 |
0.093 |
0.354 |
|
D3G4 |
0.197* |
0.048 |
|
D3G3 |
0.151 |
0.131 |
|
D3G2 |
0.115 |
0.254 |
Table 5: Study of the correlation between study variables and progesterone.
Discussion
This retrospective study including 1078 fresh ICSI cycles revealed that elevated serum progesterone on the day of oocyte maturation is associated with an elevated top-quality embryo TQE rate. We found that when serum progesterone levels were 2.0 - 2.5 ng/ml, the TQE rate was significantly elevated. Currently, most investigators have showed that elevated progesterone has a negative impact on the endometrial environment [15,16]. However, there is limited data available on the impact on the embryo quality, and the TQE rate associated with elevated progesterone.
Our study coincided with Singh et al where it was found that high levels of progesterone did not affect fertilization and cleavage rates [17]. Although some previous studies suggested that embryo quality is not affected by elevated progesterone [18], others postulated the opposite, namely that elevated serum progesterone on the day of hCG trigger may negatively influence the top embryo quality rate [19,20].
Oocyte capacitation or cytoplasmic maturation is critical for the oocyte to achieve developmental potential and involves numerous morphologic and biochemical processes. During oocyte maturation in vivo, P4 synthesis occurs in the granulosa cells due to stimulation by FSH; with progesterone production further increasing in preovulatory follicles due to differentiation of LH receptors, corresponding to the LH surge [21]. This means that during maturation the oocyte is subjected to increasing concentrations of P4. Researchers believe that preovulatory P4 elevation can be caused by several mechanisms: accumulation of P4 production from multiple developing follicles during COS [7], an excessive amount of exogenous gonadotropins [3], premature luteinization [22], poor ovarian response with increased Luteinizing Hormone (LH) sensitivity, the total dose of administered gonadotropins [23] and duration of ovarian stimulation [9]. A previous study in bovine demonstrated that in vitro maturation of oocytes in the absence of P4, resulted in decreased embryonic developmental competence [24]. Studies in other animal experiments showed that oocyte competence was regulated by progesterone-responsive genes [25]. Stanger and Yovich [26], also reported that raised serum progesterone reduced fertilization rate and proposed that this reduction may be the result of premature oocyte maturation or ageing. Accordingly, when oocytes were aged, resulting in disorganization of the spindle and cytoplasm and retention of the second polar body with resultant chromosomal anomalies, the fertilization rate decreased [27], Eichenlaub-Ritter et al., [28], revealed evidence of a link between abnormal mitochondrial function in oocytes and disturbances in chromosome cohesion and segregation, cell-cycle control and fertilization in aged oocytes. In addition, other studies have shown that elevated serum progesterone is associated with changes in the follicular microenvironment, including nitric oxide concentration [29], oxidative stress [30], and gene expression [31], which are critical mediators of oocyte fertilization.
Furthermore, the morphology of embryos from cycles with premature progesterone elevation was inspected to assess for any differences or abnormalities. Hofmann et al., [12] previously described a similar morphologic embryo grade between those with and without premature progesterone elevation, by evaluating blastomere size and the presence of cytoplasmic fragments or blebs. Similarly, Hill et al., [32] reported that good-quality embryos were obtained from cycles with premature progesterone elevation; however, they did not specify the criteria for a “good” -quality embryo [32]. Study found increase in progesterone levels acts as a messenger about the apoptosis rate of cumulus cells [33].
Conclusion
Our study showed that the rise of progesterone on the day of giving a hormone injection led to a rise in the number of mature eggs, a high rate of fertilization and a good quality of embryos in ICSI patients, we found that when serum progesterone levels were 2.0 - 2.5 ng/ml, the TQE rate was significantly elevated. Since the issue is still controversial, more studies and results are needed to make the evaluation more comprehensive. This was a single center study. Results need to be validated across different centers and high numbered different study population. Meta-analysis was not possible due to varying methodological heterogeneity which leads to Limitations, reasons for caution.
Trial Registration Number
Not applicable.
References
- Fanchin R, Righini C, Olivennes F, Ferreira AL, de Ziegler D, et al. (1997) Consequences of premature progesterone elevation on the outcome of in vitro fertilization: Insights into a controversy. Fertil Steril 68: 799-805.
- Bourgain C, Devroey P (2003) The endometrium in stimulated cycles for IVF. Hum Reprod Update 9: 515-522.
- Bosch E, Labarta E, Crespo J, Simón C, Remohí J, et al. (2010) Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: Analysis of over 4000 cycles. Hum Reprod 25: 2092-2100.
- Labarta E, Martínez-Conejero JA, Alamá P, Horcajadas JA, Pellicer A, et al. (2011) Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: A functional genomics analysis. Hum Reprod 26: 1813-1825.
- Wu Z, Dong Y, Ma Y, Li Y, Li L, et al. (2019) Progesterone elevation on the day of hCG trigger has detrimental effect on live birth rate in low and intermediate ovarian responders, but not in high responders. Scientific reports 9: 1-10.
- Al-Azemi M, Kyrou D, Kolibianakis EM, Humaidan P, Van Vaerenbergh I (2012) Elevated progesterone during ovarian stimulation for IVF. Reprod Biomed Online 24: 381-388.
- Kiliçdag EB, Haydardedeoglu B, Cok T, Hacivelioglu SO, Bagis T (2010) Premature progesterone elevation impairs implantation and live birth rates in GnRH-agonist IVF/ICSI cycles. Arch Gynecol Obstet 281: 747-752.
- Huang R, Fang C, Xu S, Yi Y, Liang X (2012) Premature progesterone rise negatively correlated with live birth rate in IVF cycles with GnRH agonist: an analysis of 2,566 cycles. Fertil Steril 98: 664-670.
- Bosch E, Valencia I, Escudero E, Crespo J, Simón C, et al. (2003) Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril 80: 1444-1449.
- Huang Y, Wang EY, Du QY, Xiong YJ, Guo XY, et al. (2015) Progesterone elevation on the day of human chorionic gonadotropin administration adversely affects the outcome of IVF with transferred embryos at different developmental stages. Reprod Biol Endocrinol 13: 82.
- Smitz J, Ron-El R, Tarlatzis BC (1992) The use of gonadotrophin releasing hormone agonists for in vitro fertilization and other assisted procreation techniques: experience from three centres. Hum Reprod 7: 49-66.
- Hofmann GE, Bentzien F, Bergh PA, Garrisi GJ, Williams MC, et al. (1993) Premature luteinization in controlled ovarian hyperstimulation has no adverse effect on oocyte and embryo quality. Fertil Steril 60: 675-679.
- Lefebvre T, Duval G, Loubersac S, Lammers J, Barriere P, et al. (2021). P–665 Influence of premature progesterone elevation on embryo development. Human Reproduction 36: 130-664.
- Balaban B, Urman B, Isiklar A, Alatas C, Aksoy S, et al. (2001) The effect of pronuclear morphology on embryo quality parameters and blastocyst transfer outcome. Hum Reprod 16: 2357-2361.
- Venetis CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, et al. (2007) Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A systematic review and meta-analysis. Hum Reprod Update 13: 343-355.
- Griesinger G, Mannaerts B, Andersen CY, Witjes H, Kolibianakis EM, et al. (2013). Progesterone elevation does not compromise pregnancy rates in high responders: A pooled analysis of in vitro fertilization patients treated with recombinant follicle-stimulating hormone/gonadotropin-releasing hormone antagonist in six trials. Fertil Steril 100: 1622-1628.
- Singh N, Malik N, Malhotra N, Vanamail P, Gupta M (2016) Impact of progesterone (on hCG day)/oocyte ratio on pregnancy outcome in long agonist non donor fresh IVF/ICSI cycles. Taiwan J Obstet Gynecol 55: 503-506.
- Melo MA, Meseguer M, Garrido N, Bosch E, Pellicer A, et al. (2006) The significance of premature luteinization in an oocyte-donation programme. Hum Reprod 21: 1503-1507.
- Vanni VS, Somigliana E, Reschini M, Pagliardini L, Marotta E, et al. (2017) Top quality blastocyst formation rates in relation to progesterone levels on the day of oocyte maturation in GnRH antagonist IVF/ICSI cycles. PLoS One 12: 0176482.
- Huang B, Ren X, Wu L, Zhu L, Xu B, et al. (2016) Elevated progesterone levels on the day of oocyte maturation may affect top quality embryo IVF cycles. PLoS One 11: 0145895.
- Bieszczad RR, McClintock JS, Pepe GJ, Dimino MJ (1982) Progesterone secretion by granulosa cells from different sized follicles of human ovaries after short term incubation. J Clin Endocrinol Metab 55: 181-184.
- Hugues JN, Massé-Laroche E, Reboul-Marty J, Boîko O, Meynant C, et al. (2011) Impact of endogenous luteinizing hormone serum levels on progesterone elevation on the day of human chorionic gonadotropin administration. Fertil Steril 96: 600-604.
- Younis JS, Matilsky M, Radin O, Ben-Ami M (2001) Increased progesterone/estradiol ratio in the late follicular phase could be related to low ovarian reserve in in vitro fertilization-embryo transfer cycles with a long gonadotropin-releasing hormone agonist. Fertil Steril 76: 294-299.
- Aparicio IM, Garcia-Herreros M, O'Shea LC, Hensey C, Lonergan P, et al. (2011) Expression, regulation, and function of progesterone receptors in bovine cumulus oocyte complexes during in vitro Biol Reprod 84: 910-921.
- O'Shea LC, Mehta J, Lonergan P, Hensey C, Fair T (2012) Developmental competence in oocytes and cumulus cells: Candidate genes and networks. Syst Biol Reprod Med 58: 88-101.
- Stanger JD, Yovich JL (1985) Reduced in-vitro fertilization of human oocytes from patients with raised basal luteinizing hormone levels during the follicular phase. Br J Obstet Gynaecol 92: 385-393.
- Nagy ZP, Liu J, Joris H, Devroey P, Van Steirteghem A (1994) Fertilization and early embryology: Time-course of oocyte activation, pronucleus formation and cleavage in human oocytes fertilized by intracytoplasmic sperm injection. Human Reproduction 9: 1743-1748.
- Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R (2004) Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online 8: 45-58.
- Vangelov I, Dineva J, Todorova K, Stefanova Ts, Nikolov G, et al. (2010) Relationship of follicular fluid nitric oxide concentrations with the serum steroid (progesterone, estradiol,) levels, apoptosis of granulosa luteinized cells and with the outcomes after COH/ IVF. Journal of Reproductive Immunology 86: 107.
- Maiese K, Chong ZZ, Hou J, Shang YC (2010) Oxidative stress: Biomarkers and novel therapeutic pathways. Exp Gerontol 45: 217-234.
- Long X, Peng C, Lu G (2009) Isolation and identification of genes differentially expressed in premature luteinization granulosa cell during controlled ovarian hyperstimulation. Fertil Steril 92: 1767-1771.
- Hill MJ, Royster GD 4th, Healy MW, Richter KS, Levy G, et al. (2015) Are good patient and embryo characteristics protective against the negative effect of elevated progesterone level on the day of oocyte maturation? Fertil Steril 103: 1477-1484.
- Çiftlik DG, Ergüven M, Irez T (2021) P–236 The evaluation of apoptosis and luteinization process in cumulus cell culture of IVF patients in terms of embryo development and pregnancy.
Citation: Alnasser R, Alghazawi M (2023) Effect of Progesterone Levels on the Day of hCG Trigger on Quality of Embryos in ICSI Cycles. J Reprod Med Gynecol Obstet 8: 119.
Copyright: © 2023 Rami Alnasser, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

