
Effect of Vitamin C and Exercise on Alveoli and Cytokines: Analysis of a Chronic Obstructive Pulmonary Disease (COPD) and Skeletal Muscle Atrophy Model in SMP30/GNL Knockout Mice
*Corresponding Author(s):
Hiroshi MaruokaSchool Of Health And Social Services, Saitama Prefectural University, Saitama, Japan
Tel:+81 0489710500,
Email:maruoka-hiroshi@spu.ac.jp
Abstract
Introduction: This study examined the effects of the presence and absence of Vitamin C (VC) intake and exercise on alveoli and cytokines in mice.
Materials and Methods: Twenty VC synthesis-deficient mice were used for the study and were randomly divided into four groups based on the presence or absence of VC intake and exercise. All groups were administered the Chronic Obstructive Pulmonary Disease (COPD) model treatment from 8 to 10 weeks of age, and then the disuse muscle atrophy (amyotrophia) model was administered from 11 to 12 weeks of age to create the COPD muscle atrophy model. Subsequently, exercise was performed from 13 to 20 weeks (7 weeks). The analysis included mRNA expression using real-time polymerase chain reaction (PCR), morphological analysis of histological images using general staining, macrophage expression through immunostaining, and spirometry examination.
Results: VC intake and exercise resulted in an increase in interleukin-4 (IL-4) and a decrease in interleukin-6 (IL-6) in the lungs and drops in dynamin-related protein-1 (Drp-1), IL-6, and nuclear respiratory factor-2 (NRF-2) levels in skeletal muscles. Moreover, a decrease in mean linear intercept was observed in the absence of VC intake and the presence of exercise.
Conclusion: These results indicate that VC intake and exercise affect cytokines and cause morphological changes in the lungs and affect cytokines in the skeletal muscles.
Keywords
Chronic obstructive pulmonary disease; Exercise; Skeletal muscle atrophy; SMP30/GNL knockout mice; Vitamin C
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a systemic spill-over disease of pulmonary inflammation that results in alveolar destruction and increased levels of cytokines and Reactive Oxygen Species (ROS). Systemic comorbidities, such as skeletal muscle dysfunction, are also observed. The management of systemic comorbidities is exceptionally critical to improving the prognosis, yet spill-over is still unknown and effective treatment n strategies to control the condition have not been established [1,2]. Comprehensive treatments for systemic comorbidities include smoking cessation guidance; pharmacotherapy, and respiratory rehabilitation, exercise has not yet been shown to have anti-inflammatory effects or effects on spill-over. We produced COPD muscle atrophy model mice using cigarette smoke to examine the effects of exercise. The results showed that exercise decreased inflammatory cytokines and macrophages in the lungs, increased myokines involved in skeletal muscle metabolism, and improved fibrotic alveolar walls [3]. However, the molecular mechanisms underlying the effects of exercise on pro-inflammatory cytokines and alveolar walls are yet to be elucidated.
In patients with COPD, skeletal muscle dysfunction due to muscle atrophy is observed at a frequency of 30 %- 40 %, and the involvement of spill-over and other factors has been suggested [1,2]. Typically, skeletal muscle dysfunction can be considered an imbalance in protein synthesis, degradation, or a disorder of mitochondrial dynamics. In particular, mitochondria-derived ROS damage Mitochondrial DNA (mtDNA), and the accumulation of damaged mtDNA leads to a decrease in mitochondrial function [4]. Quality control is performed by fusing mitochondria in response to mtDNA damage, aggregating the accumulated mutant mtDNA, and dividing the aggregated parts. Exercise increases mitochondrial mass, in contrast some reports show no change in fission, and few studies have used COPD disease models [5-8].
Generally, various antioxidants, including vitamin C (VC), protect the human body from ROS [9]. VC is a water-soluble antioxidant that efficiently eliminates ROS and enhances antioxidant capacity [10]. However, whether VC intake affects the mitochondria through exercise is not well understood [11-13]. Recently, it was reported that VC intake may restore alveolar cells in COPD and is associated with muscle atrophy and physical performance [14,15]. However, few studies have examined its effects in COPD disease models [16]. Since wild-type mice can synthesize VC in their bodies, unlike humans [10,16], we assumed that using VC synthesis-deficient mice might be useful for studying the effects of exercise on the COPD model with and without VC intake.
In this study, we examined the effects of VC intake and exercise on the suppression of alveolar destruction and improved inflammation and skeletal muscle atrophy in a mouse model of COPD generated from VC synthesis-deficient mice by combining the disuse muscle atrophy (amyotrophia) model with hind limb suspension.
Materials And Methods
Research design
This study was conducted under the approval of the Ethical Review Board of the institution (Approval Number 2019-6). We used twenty VC synthesis-deficient mice (SMP30/GNL Knockout, male, 7-weeks old) and randomly divided into four groups (n=5 per group) according to the presence or absence of VC intake and exercise. Group A, with VC intake + exercise; Group B, without VC intake + exercise; Group C, with VC intake + no exercise; and Group D, without VC intake + no exercise.
At the start of the study, all mice were 7 weeks old. We made the disease model based on the previous studies in 8-10 weeks old (21 days) mice, and then created the amyotrophia model in 11-12 weeks old (14 days) mice [3]. We made them exercise on the treadmills for small animals from 13-20 weeks old onward. Frequency, duration, and intensity of exercise were equivalent to those of previous studies, which is a moderate exercise load (frequency: 5 times/week; duration: 15 min/day at the beginning and gradually increased to 3 min/day, up to 60 min/day; intensity: 15 m/min; slope: 5%) [3]. We slaughtered them at 20 weeks old and collected tissues (right upper lobe and middle lobe of the lung and lateral head of gastrocnemius muscle). The tissues were collected at five hours after exercising, according to previous studies [6].
All mice were raised at a room temperature of 20 ± 1°C, a relative humidity of approximately 50%, and under 12-h (7-19:00) light-dark cycles. They were allowed to freely eat VC-free solid food (CL-2, Nippon Clare), and no restrictions were imposed on their behaviors. For VC intake, based on the previous study [17], the mice with VC intake received 100% of the daily VC requirement (1.5 g/L VC) in drinking water, and those without VC intake received 2.5% of the minimum dose of VC (0.0375 g/L VC) in drinking water to prevent scurvy. All groups were given drinking water containing 1.5 g/L VC until the age of 7 weeks, and from the age of 8 weeks, they were divided into groups with and without VC intake. The mice used in this study are model mice of accelerated aging that are very similar to humans and unable to produce VC [17].
Creation of the COPD amyotrophia model
In this study, we combined the CDPD disease model and the COPD amyotrophia model mice. For the creation of the COPD disease model, we intratracheally administered a tobacco solution using a syringe under inhalation anesthetics (isoflurane; flow rate 0.5 L/min; concentration 2%). The frequency of administration was five times/week, and each dosage 121 volume was 50 μL. Administration of the mixture of tobacco solution (four-fold dilution) + Lip Polysaccharide (LPS; 10 μg) was performed when mice were 12 and 13 weeks old, followed by the administration of an only-tobacco solution (four-fold dilution) at 14 weeks old [3,18]. The tobacco solution was created by connecting the filter mouthpiece to the introduction tube of a suction pump and the connection tube was bubbled in saline (50 mL). The tobacco used to make tobacco solution was Peace 40 piece package (Nicotine 2.3 mg/piece), and the measurement value of absorbance was 0.902 (500-fold dilution). For the creation of the COPD amyotrophia model, we performed hind limb suspension, according to the previous studies [6].
Muscle strength, breathing function, and body weight
The muscle strength of mice was measured three times and the mean values were calculated using a grip strength meter for small animals GPM-100B (Melquest Ltd., Japan) [3]. As the breathing function, we measured the respiratory minute volume, the tidal volume, and respiratory rate at rest using a flow head for mice spiratory Floheads (AD Instruments, USA) [3]. The body weight was measured using a balance for animals K.N. type (Natsume Seisakusho Co., Ltd., Japan). Muscle strength and breathing function were measured at 12 weeks old (pre) and at the time of slaughter (post), and the body weight was measured once a week.
Histological Analysis
After washing the collected tissues (the right middle lobe) with saline, they were fixed with formalin to create paraffin blocks, and then horizontal slice sections (6 μm thick) were created. For morphological analysis of the alveolar spaces, we performed Victoria Blue staining, which is commonly used. We took images of the stained slice sections using the fluorescence microscope BZ-X700 (KEYENCE Co., Japan). We used the Hybrid cell count BZ-H3C (KEYENCE Co., Japan) for analysis to measure the mean alveolar diameter in the randomized tissue images (350 × 280 μm). The paraffin sections were deparaffinized using xylene and ethanol and then the antigens were retrieved using Proteinase K (OMEGA bio-tek, USA). For the primary antibody, the F4/80 antibody, tissue sections were boiled in 1 mM EDTA, pH 8.0 for 10 min, followed by cooling at room temperature for 20 min.
Next, for suppression of endogenous peroxidase activation, we soaked them in a PBS solution including 3% hydrogen peroxide solution (FUJIFILM, Japan) for ten minutes, performed avidin blocking (VEC Labo, USA) for 15 minutes for non-specific staining blocking, and made them react in the PBS solution for 30 minutes by adding goat serum for non-specific reaction blocking. As the primary antibody, we used the CD68 antibody (ab125212, Abcam plc., Japan; diluted concentration 1/2500), CD206 antibody (ab64693, Abcam plc., Japan; diluted concentration 1/1000) and F4/80 antibody (ab100790, Abcam plc., Japan; diluted concentration 1/150).
After dropping the antibody, we made it react in a refrigerator controlled at 4°C for eight hours. For the secondary antibody, after dropping the IgG antibody derived from goat (VEC Labo., USA), we made it react at room temperature for 30 minutes. For the amplification reaction, we performed the ABC process on the VECTAIN ABC Rabbit IgG Kit (VEC Labo., USA) to emit colors. For contrast staining, we dipped it for one minute and enclosed the section. We used goat serum added PBS without the primary antibody during the staining of the primary antibody to create negative controls. We took the images of the stained sections using fluoroscope BZ-X700 (KEYENCE Co., Japan). During the image analysis, we removed the nucleus, defined a threshold by using Photoshop (Adobe Inc., USA), and measured the positive cell count semi quantitatively. Two independent co-investigators measured the randomly picked images and calculated their mean values. The primary antibodies used at this point were CD68 and CD206, as the alveolar 179 macrophage makers, and F4/80 as the interstitial lung marker.
mRNA Analysis
The RNAs of the collected tissues (right upper lobe and right lateral head of gastrocnemius muscle) were stabilized by the RNA later Stabilization Solution (Thermo Fisher Scientific K.K., Japan) and stored at -20°C. We extracted the total RNA for the RNA analysis of the tissues, according to the protocol of the RNeasy Fibrous Tissue Mini Kit (QIAGEN K.K., Japan). After extraction of the total RNA, we measured the total RNA concentration using Nano Drop Lite (Thermo Fisher Scientific K.K., Japan). Each sample was diluted until the total RNA concentration became even and cDNA was synthesized according to the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific K.K., Japan) protocol. For real-time PCR, we performed the Taqman probe method using the PCR analysis system (Chromo4; BIO-RAD, USA) for 40 cycles. To do a comprehensive analysis based on the previous studies [4-7,11-13], we determined the target genes as factors associated with inflammation (TNF-α, Interleukin-4, among others), nine factors of mitochondria (peroxisome proliferator-activated receptor gamma coactivator 1α: PGC-1α, among others), two factors associated with amyotrophia (MuRF1 and MAFbx), six factors of protease associated with emphysema (Cathepsins-L, etc.), and GAPDH as the endogenous standard gene (Table 1). The relative value was calculated from Ct values obtained by the comparative Ct method (ΔΔCt method), and they were compared setting the B group as the reference value (1.0). Furthermore, for the difference of exercise durations, we calculated the mean value from each Ct value from the B and the D groups to obtain the ratio of the A and the C groups (A/B and C/D). From the ratios obtained, we calculated the relative values and made comparisons by setting A/B as the reference value (1.0).
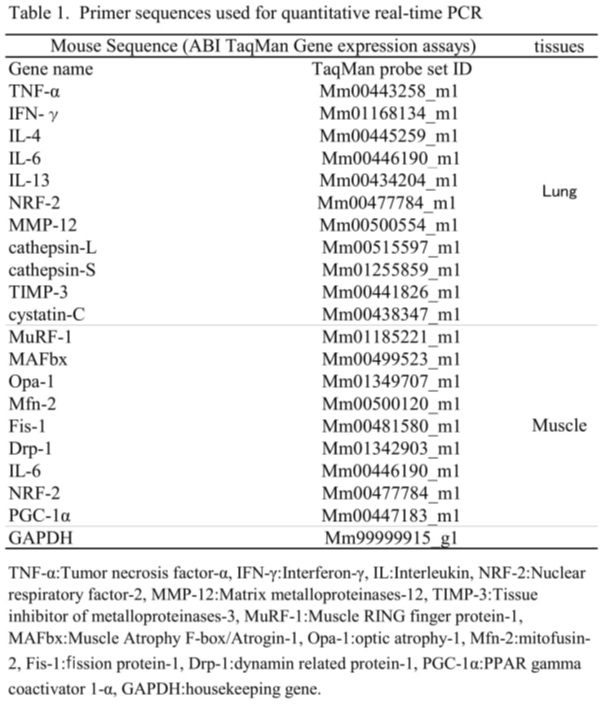 Table 1: Primer sequences used for quantitative real-time PCR.
Table 1: Primer sequences used for quantitative real-time PCR.
Analysis of VC concentration
VC concentration was measured by High Performance Liquid Chromatography (HPLC) for the reduced (ascorbic acid: AA) and oxidized (dehydroascorbic acid: DHA) forms in the liver, and the total VC (AA + DHA: Total) concentration (µmol/g) was calculated.
Statistical Analysis
We used the statistical software SPSS (Ver26 for Windows) to validate the normality by using the Shapiro-Wilk test. We examined significant differences in muscle strength and mean linear intercept, and compared macrophages using variance analysis, Tukey’s test, and T-test. All significance levels were set at < 5%. Muscle strength, respiratory function, and body weight were examined using post-pre difference (Post-Pre) values. We compared the difference between groups A and C (groups A and C) for the effects of exercise with VC intake and the difference between groups B and D (groups B and D) for exercise without VC intake.
Results
Comparison of body weight, muscle strength, and respiratory functions
In comparison between pre and post, there was a significant increase in body weight in groups A and C in post and a substantial decrease in muscle strength in all post groups (all p < 0.05, Table 2). Additionally, a significant decrease in muscle strength (mean 63%-72%) was observed in groups B and D, indicating a reduction in muscle strength regardless of whether or not exercise was performed in the absence of VC intake. Furthermore, no differences in body weight, muscle strength, or respiratory function were observed between groups A and C and B-D, indicating that the presence or absence of VC intake did not affect exercise (Table 3). In post, there was a significant decrease in VC concentration in group B compared to group A and group D compared to group C (Table 2), and a substantial reduction in groups A-C compared to groups B-D (all p < 0.05, Table 3). Implying that VC concentration declined in the presence of VC intake and the presence of exercise. In this study, we observed four dropout cases (group B and group D: two cases each), all of which died due to unknown causes.
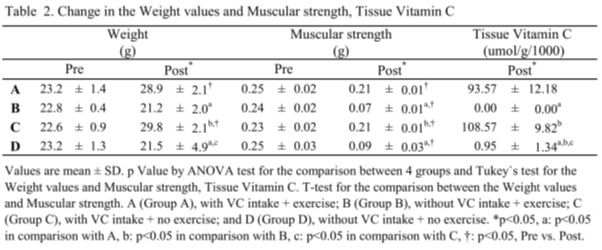 Table 2: Change in the Weight values and Muscular strength, Tissue Vitamin C.
Table 2: Change in the Weight values and Muscular strength, Tissue Vitamin C.
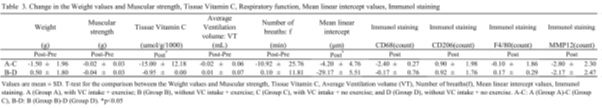 Table 3: Change in the Weight values and Muscular strength, Tissue Vitamin C, Respiratory function, Mean linear intercept values, Immunol staining.
Table 3: Change in the Weight values and Muscular strength, Tissue Vitamin C, Respiratory function, Mean linear intercept values, Immunol staining.
Comparison of mean linear intercept, macrophages, and other factors
The mean linear intercept decreased significantly in groups B-D compared to groups A-C, indicating a decrease in the absence of VC intake and the presence of exercise (p < 0.05, Table 3). The mean linear intercept decreased significantly in Group D (Figure 1). Additionally, there was no difference in CD206 or F4/80 between groups A and C and B-D, indicating that the presence or absence of VC intake did not affect exercise (Table 3). 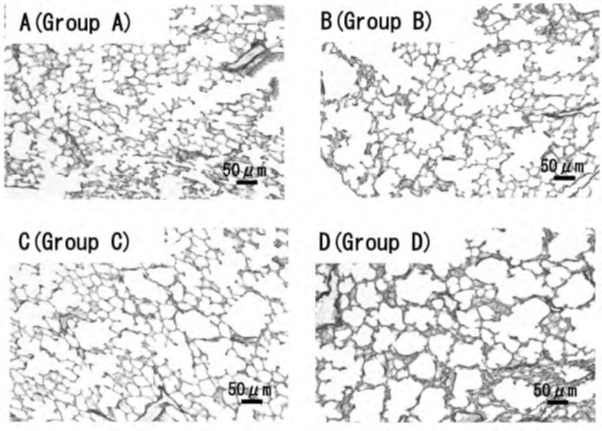
Figure 1: Representative photomicrographs of victoria blue staining pulmonary parenchyma.
A-D Representative photomicrographs of victoria blue staining pulmonary parenchyma of A (Group A), with VC intake + exercise; B (Group B), without VC intake + exercise; C (Group C), with VC intake + no exercise; and D (Group D), without VC intake + no exercise. Scale bars: 50 μm. The mean linear intercept decreased significantly in Group D.
Comparison of mRNA
In the lungs, a significant increase in interleukin-4 (IL-4) was observed in groups A-C compared to groups B-D, and a significant increase in IL-6 was observed in groups B-D compared to groups A-C (all p < 0.05, Figure 2). This means that IL-4 increased and IL-6 levels decreased in the lungs in the presence of VC intake and exercise. Drp-1, IL-6, and nuclear respiratory factor-2 (NRF-2) showed a significant increase in skeletal muscles in groups B-D compared to groups A-C (all p < 0.05, Figure 3). Specifically, Drp-1, IL-6, and NRF-2 decreased in the skeletal muscles in the presence of VC intake and exercise.
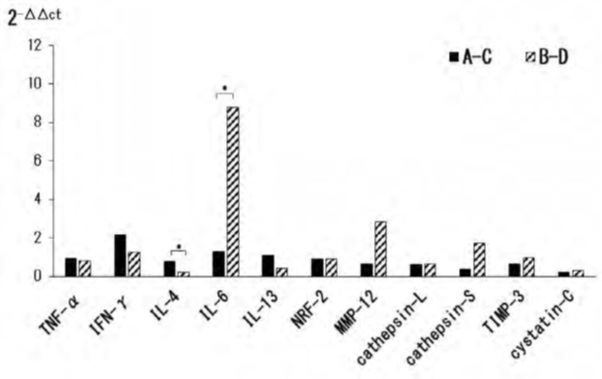 Figure 2: mRNA expression levels of cytokines factors enzymes (lung tissues).
Figure 2: mRNA expression levels of cytokines factors enzymes (lung tissues).
Effects of chronic obstructive pulmonary disease (COPD) and skeletal muscle atrophy model mice on mRNA expression levels of cytokines factors enzymes. mRNA was prepared from lung tissues and relative gene expression was determined by real-time PCR. T-test for the comparison between the mRNA. A (Group A), with VC intake + exercise; B (Group B), without VC intake + exercise; C (Group C), with VC intake + no exercise; and D (Group D), without VC intake + no exercise. A-C: A (Group A)-C (Group C), B-D: B (Group B)-D (Group D). *p < 0.05.
This means that IL-4 increased and IL-6 levels decreased in the lungs in the presence of VC intake and exercise.
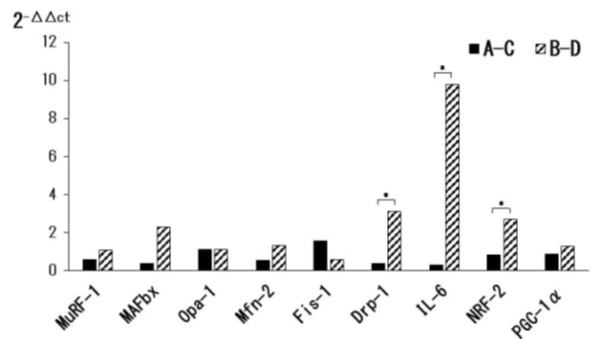 Figure 3: mRNA expression levels of cytokines factors enzymes (muscle tissues).
Figure 3: mRNA expression levels of cytokines factors enzymes (muscle tissues).
Effects of chronic obstructive pulmonary disease (COPD) and skeletal muscle atrophy model mice on mRNA expression levels of cytokines factors enzymes. mRNA was prepared from muscle tissues and relative gene expression was determined by real-time PCR. T-test for the comparison between the mRNA. A (Group A), with VC intake + exercise; B (Group B), without VC intake + exercise; C (Group C), with VC intake + no exercise; and D (Group D), without VC intake + no exercise. A-C: A (Group A)-C (Group C), B-D: B (Group B)-D (Group D). *p < 0.05.
This means that Drp-1, IL-6, and NRF-2 decreased in the skeletal muscles in the presence of VC intake and exercise.
Discussion
The effects of VC intake in humans prevent upper respiratory tract infection and lymphocyte depletion after exercise [19]. Takisawa, et al. reported that VC deficiency (using VC synthesis-deficient mice raised without VC for 16 weeks) causes muscle atrophy, muscle mass loss, and physical performance decline. Moreover, Scientists report that a reduction in VC concentration increases ROS, while VC replenishment decreases ROS and improves exercise capacity [15,20]. In this study, a significant decrease in muscle strength was observed without VC intake, regardless of the presence or absence of exercise. Therefore, VC deficiency resulted in lower muscle strength due to muscle atrophy, showing results similar to those of previous studies [15,20]. In the previous study, VC concentrations (VC synthesis-deficient mice with no control) had an average of 96.2 ± 14.7 µmol/g/1000 with VC intake and 2.3 ± 0.7 µmol/g/1000 without VC intake (mice raised for 4 weeks without VC: 12 weeks old, Maruoka, unpublished). Since VC deficiency leads to high ROS, such as in skeletal muscles and the lungs, we considered the effect of ROS on the organism [16]. Typically, aerobic exercise increases ROS levels in tissues, including the skeletal muscles, lungs, and liver [21]. In contrast, continuous exercise, along with the intake of exogenous antioxidants such as VC, enhances the actions of oxidative enzymes, including Superoxide Dismutase (SOD), and improves the protective functions against ROS [10,22]. Moreover, vitamins efficiently protect the body against ROS, which causes a tissue-specific decrease in vitamins, resulting in an increase in blood vitamin levels (redistribution) [23]. In this study VC concentration in the tissues decreased in the presence of VC intake and exercise and showed a decrease compared to previous studies in the presence of VC. It is possible that exercise caused VC redistribution in the liver and other tissues in response to increased ROS levels. ROS affects muscle atrophy; therefore, it is possible that the protective function against ROS was working in the mice with VC intake, resulting in no significant decrease in muscle strength or dropout cases [15].
VC is a powerful antioxidant that plays an essential role in health. Several epidemiological studies have reported that VC has a protective effect on lung function and reduces the risk of lung damage caused by cigarette smoke [14,24]. Furthermore, scientists report that exposure to cigarette smoke causes enlargement of airspaces, enhancement of ROS, and induction of apoptosis by alveolar destruction in VC synthesis-deficient mice [16,17]. In contrast, exercise induces antioxidant and anti-inflammatory activities and reduces ROS [25]. Furthermore, exercise in a mice model of COPD has been reported to affect lung tissues, including a decrease in mean alveolar diameter, and the mechanism involves cytokines and ROS [3,26-28]. In this study, a decrease in the mean linear intercept was observed in the absence of VC intake and exercise. Scientists report that in the absence of exogenous antioxidants such as VC, exercise can enhance the action of oxidative enzymes such as SOD, causing a protective function against ROS [27,28]. Accordingly, the decrease in the mean linear intercept might be due to reduced ROS production in the lungs and an increase in antioxidant enzymes in the inflammatory cells of the alveolar walls. In general, IL-6 is a cytokines that affects various processes, ranging from immunity to tissue repair, and metabolism. In a previous study, it was reported that VC intake decreased ROS and IL-6 produced by exercise [29]. This study observed decreased IL-6 levels in the lungs and skeletal muscles of mice with VC intake and exercise. These results were similar to those obtained in previous studies in COPD disease model mice. Scientists report that the production of IL-6 is dependent on ROS and activates the nuclear factor-kappa B (NF-KB) pathway. Therefore, VC intake may inhibit the activation of the NF-dB pathway by eliminating ROS [30]. In addition, since exercise-induced the activation of antioxidant enzymes and anti-inflammatory activities and reduced ROS, VC intake and exercise may have synergistic effects on anti-inflammatory activities [25].
Shibata et al. reported on the processes of emphysema formation in COPD model mice produced using elastase. According to this report, emphysema is caused by monocytes infiltrating the lungs, which are transformed into pulmonary interstitial macrophages by IL-4 secreted by basophils, and proteases produced by pulmonary interstitial macrophages [31]. In this study, we observed an increase in IL-4 levels in the lungs with VC intake and exercise. In our previous study, it was reported that IL-4 increase is associated with an increase in macrophages and proteases [31]. However, based on our findings, exercise with or without VC intake did not show any difference in macrophages or proteases in response to the increase in IL-4.
Particularly, since there was no increase in both IL-4 and macrophage factors observed in this study, a different molecular mechanism from that of the previous study may have occurred. Typically, the effects of exercise on cytokines have been reported to increase both pro-inflammatory (e.g., tumor necrosis factor-α: TNF-α, IL-6) and anti-inflammatory (e.g., IL-4) cytokines, and an increase in IL-4 has been reported in contrast to a decrease in TNF-α [32,33]. Moreover, previous studies found that IL-4 functions affect macrophage inhibition and anti-inflammatory activity [34]. However, since many cytokines may be involved in the morphological changes of the alveoli caused by exercise, it is necessary to study the effects of cytokines and proteins by a comprehensive expression.
COPD is caused by a disruption of the equilibrium between oxidants and antioxidants found in large amounts in cigarette smoke [1,2]. NRF-2, a transcription factor for antioxidant genes, regulates defense genes against ROS. Accordingly, NRF-2 is activated when oxidants are taken into the cells, escaping the regulation from Ketch-like ECH-associated protein 1 (Keap1), and a defense response is induced [35]. In contrast, exercise induces NRF-2 expression in skeletal muscles and promotes gene expression for ant oxidative and anti-inflammatory effects [36]. In this study, we observed a reduction in NRF-2 in skeletal muscle in the presence of VC intake and exercise (or an increase in the absence of VC intake and exercise). This suggests that NRF-2 increased to eliminate ROS in response to the increase in ROS induced by exercise. Since exercise affects NRF-2/ Keap1, it is therefore necessary to clarify the molecular mechanism of spill-over [37].
Typically, mitochondria are dynamic organelles that are bound together by fusion processes and are divided by fission processes. A balance between fusion and fission maintains the mitochondrial structure, as long networks are formed when fusion is activated [5-8]. In contrast, fission increases the number of mitochondria in the cell when it is activated. ROS affects the morphology of the mitochondria and other factors in skeletal muscle cells [38]. It has also been reported that the increase in ROS coincides with the incidence rate of mitochondrial fragmentation and is induced by Drp-1 [38,39]. In this study, Drp-1 decreased in skeletal muscles in the presence of VC intake and exercise (or increased in the absence of VC intake and exercise). Regarding the effect of exercise on mtDNA, scientists report that Drp-1increases at high-intensity load rather than low-intensity load [40]. Accordingly, since the exercise intensity remained the same in this study, the increase in Drp-1 could be influenced by the presence or absence of VC intake. It is possible that exercise without VC intake increased Drp-1, because the ROS deletion mechanism was not induced in response to the increase in ROS by exercise. These results indicate that VC intake and exercise affect cytokines in the lungs and the skeletal muscles.
In this study, we used mice as an experimental animal model to show that exercise interventions may improve various COPD symptoms induced by cigarettes. Nevertheless, the limitation of this study is the difficult to directly link the results obtained by experimental animals to the development of human exercise programs. There are still some issues to be resolved in further studies; for example, the mice were only 7-12 weeks old, and the cytokines interacted with each other.
Conclusion
In the COPD model mice with muscle atrophy, the presence of VC intake and exercise affected the balance of inflammatory cytokines (IL-6) and non-inflammatory cytokines (IL-4) in the lungs, as well as inflammatory cytokines (IL-6), cytokines acting to eliminate ROS (NRF-2), and mtDNA (Drp-1) in skeletal muscles. This indicates that VC and exercise have anti-inflammatory effects.
Conflicts of Interest
The authors declare no conflicts of interest associated with this manuscript.
Acknowledgement
This work was supported by JSPS KAKENHI Grant Number 18K10751.
References
- Jap Respir Soci COPD guidelines Creation Committee (2019) The JRS guidelines for the management of chronic obstructive pulmonary disease. 5th Ed, Jap Respir Soci, Tokyo, Pg No: 8-132.
- Betsuyaku T (2014) Recent progress in research of pathogenesis of chronic obstructive pulmonary disease (COPD). Pulm Med 3: 323-328.
- Maruoka H, Tanaka K, Takayanagi M, Zenda M, Ogawa A, et al. (2021) The influence of the different exercise length on alveolar and cytokine-The analysis of chronic obstruct-tive pulmonary disease (COPD) and skeletal muscle atrophy model mice. J Clin Physiol 51: 23-34.
- Chen H, Vermulst M, Wang YE, Chomyn A, Prolla TA, et al. (2010) Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell 141: 280-289.
- Nassan SA, Fujita N, Kondo H, Murakami S, Fujino H, et al. (2012) Chronic exercise training down-regulates TNF-α and Atrogin-1/MAFbx in mouse gastrocnemius muscle atrophy induced by hindlimb unloading. Acta Histochem Cytochem 45: 343-349.
- Maruoka H, Tanaka K, Zenda M, Ogawa A, Kido S, et al. (2019) Effect of exercise on muscle protein and mitochondrial function in mice model of skeletal muscle atrophy. Int J Anal Bio-Sci 7: 19-25.
- Jamart C, Naslain D, Gilson H, Francaux M (2013) Higher activation of autophagy in skeletal muscle of mice during endurance exercise in the fasted state. Am J Phyasiol Endocrinol Metab 305: E964-E974.
- Barreiro E, Peinado VI, Galdiz JB, Ferrer E, Marin-Corral J, et al. (2010) Cigarette smoke–induced oxidative stress. Am J Respir Crit Care Med 182: 477-488.
- Yamamoto Y (2005) Oxidative stress and its marker. Anti-aging medicine 1: 102-106.
- Kondo Y, Sasaki T, Sato Y, Amano A, Aizawa S, et al. (2008) Vitamin C depletion increases superoxide generation in brains of SMP30/GNL knockout mice. Biochem Biophys Res Commun 377: 291-296.
- Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, et al. (2008) Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87: 142-149.
- Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, et al. (2009) Antioxidants prevent health promoting effects of physical exercise in humans. Proc Natl Acad Sci USA 106: 8665-8670.
- Yada K, Matoba H (2014) Vitamin C supplementatien does not alter high-intensity endurance training-induced mitochondrial biogenesis in rat epitrochlearis muscle. J Physiol Sci 64: 113-118.
- Park HJ, Byun MK, Kim HJ, Kim JY, Kim YI, et al. (2016) Dietary vitamin C intake protects against COPD: the Korea National Health and Nutrition Examination Survey in 2012. Int J Chron Obstruct Pulmon Dis 31: 2721-2728.
- Takisawa S, Funakoshi T, Yatsu T, Nagata K (2019) Vitamin C deficiency causes muscle atrophy and a deterioration in physical performance. Scientific Reports 9: 01.
- Sato T, Seyama K, Sato Y, Mori H (2006) Senescence marker protein 30-protect mice lungs from oxidative stress, Aging and smoking. Am J Respir Crit Care Med 174: 530-537.
- Ishigami A (2012) Aging regulation of vitamin C. Hormone Frontier in Gynecology 19: 231-236.
- Amano H, Murata K, Matsunaga H, Tanaka K (2014) p38 Mitogen-activated protein kinase accelerates emphysema in mouse model of chronic obstructive pulmonary disease. J Recept Signal Transduct Res 34: 299-306.
- Moreira1 A, Kekkonen RA, Delgado L, Fonseca J (2007) Nutritional modulation of exercise-induced immunodepression in athletes: a systematic review and meta-analysis. Eur J Clin Nutr 61: 443-460.
- Paschalis V, Theodorou AA, Kyparos A, Dipla K (2016) Low vitamin C values are linked with decreased physical performance and increased oxidative stress: reversal by vitamin C supplementation. Eur J Nutr 01: 45-53.
- Dalla Corte CL, Carvalho NR, Amaral GP, Puntel GO (2013) Antioxidant effect of organic purple grape juice on exhaustive exercise. ApplPhysiol Nutr Metab 05: 558-565.
- Hatao H, Ohishi S, Itoh M, Leeuwenburgh C (2006) Effects of acute exercise on lung antioxidant enzymes in young and old rats. Mech Ageing Dev 127: 384-390.
- Takanami Y, Iwane H, Kawai Y, Shimomitsu T (2000) Vitamin E supplementation and endurance exercise are there benefits?. Sports Medicine 29: 73-83.
- Kaluza J, Larsson SC, Orsini N, Linden A (2017) Fruit and vegetable consumption and risk of COPD: a prospective cohort study of men. Thorax 72: 500-509.
- Ji LL (2008) Modulation of skeletal muscle antioxidant defense by exercise: role of redox signaling. Free RadicBiol Med 44: 142-152.
- Toledo AC, Magalhaes RM, Hizume DC, Vieria RP, Biselli PJC, et al. (2012) Aerobic exercise attenuates pulmonary injury induced by exposure to cigarette smoke. Eur Res J 39: 254-264.
- Koike K, Ishigami A, Sato Y, Hirai T, Yuan Y, et al. (2014) Vitamin C prevents cigarette smoke-induced pulmonary emphysema in mice and provides pulmonary restoration. Am J Respir Cell Mol Biol 50: 347-357.
- Koike K, Berdyshev EV, Mikosz AM, Bronova IA, Bronoff AS, et al. (2019) Role of glucosylceramide in lung endothelial cell fate and emphysema. Am J RespirCrit Care Med 200: 1113-1125.
- Righi NC, Schuch FB, Nardi ATD, Pippi CM, Righi GA, et al. (2020) Effects of vitamin C on oxidative stress, inflammation, muscle soreness, and strength following acute exercise: meta-analyses of randomized clinical trials. European Journal of Nutrition 59: 2827-2839.
- MacDonald J, Galley HF, Webster NR (2003) Oxidative stress and gene expression in sepsis. Br J Anaesth 90: 221-232.
- Shibata S, Miyake K, Tateishi T, Yoshikawa S, Yamanishi Y, et al. (2018) Basophils trigger emphysema development in a murine model of COPD through IL-4 mediated generation of MMP12 producing macrophages. Pro Nat Aca Sci (PNAS) 115: 13057-13062.
- Pedersen BK, Hoffman-Goetz L (2000) Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev 80: 1055-1081.
- Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S, et al. (1999) Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA 281: 1722-1727.
- Hamuro J (2000) IL-4. Biotherapy 14: 819-834.
- Kobayashi M, Yamamoto M (2005) Molecular mechanisms activating the NRF2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal 7: 385-394.
- Li T, He S, Liu S, Kong Z, Wang J, et al. (2015) Effects of different exercise durations on Keap1-NRF2-ARE pathway activation in mouse skeletal muscle. Free Radical Research 49: 1269-1274.
- Gao L, Kumar K, Vellichirammal NN, Park SY, Rudebush TL, et al. (2020) Functional, proteomic and bioinformatic analyses of NRF2 and Keap1 null skeletal muscle. J Physiol 598: 5427-5451.
- Iqbal S, Hood DA (2014) Oxidative stress-induced mitochondrial fragmentation and movement in skeletal muscle myoblasts. Am J Physiol Cell Physiol 306: C1176-1183.
- Iqbal S, Ostojic O, Singh K, Joseph AM, Hood DA, et al. (2013) Expression of mitochondrial fission and fusion regulatory proteins in skeletal muscle during chronic use and disuse. Muscle Nerve 48: 963-970.
- Tareda K, Kitaoka Y, Takemasa T (2018) High-intensity intermittent swimming training increases mitochondrial dynamics proteins in mouse skeletal muscle. AdvExerc Sports Physiol 24: 13-16.
Citation: Maruoka H, Tanaka K, Takayanagi M, Zenta M, Ishigami A (2021) Effect of Vitamin C and Exercise on Alveoli and Cytokines: Analysis of a Chronic Obstructive Pulmonary Disease (COPD) and Skeletal Muscle Atrophy Model in SMP30/GNL Knockout Mice. J Phys Med Rehabil Disabil 7: 62.
Copyright: © 2021 Hiroshi Maruoka, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

