
Effectiveness of an 8 Week Home Balance Training Program in CMT1A: A Pre and Post Intervention Study
*Corresponding Author(s):
Steven K. Baker2,Division Of Physical Medicine And Rehabilitation, Department Of Medicine, McMaster University, Hamilton, Ontario, Canada
Email:bakersk@mcmaster.ca
Abstract
Background: Charcot-Marie-Tooth (CMT) disease is a hereditary genetic disorder characterized by peripheral, sensory and motor polyneuropathy. Given the lack of curative treatments, management of CMT usually involves utilization of rehabilitative therapies that help alleviate disease symptoms. Balance impairment has been implicated as one of the most significant deterrents of functionality and overall quality of life for CMT patients. Hence, it is of significant importance to explore effective rehabilitative interventions within this context.
Objective: The primary objective of this study was to evaluate the effectiveness of an 8 week home balance training program in improving gait and balance amongst CMT1A patients between the ages of 18 and 65.
Design & Setting: An 8 week prospective, single arm, pre and post intervention study was conducted at an outpatient neuromuscular clinic.
Participants: Eight participants were enrolled into the study. Patients at the outpatient neuromuscular clinic made up the sampling frame of the study and participants were recruited using a consecutive sampling strategy via email and phone call advertising.
Intervention: An 8-week home balance training program consisting of a combination of six balance exercises which were to be carried out five days per week for the total study duration of 8 weeks.
Main Outcome Measures: Balance and gait were assessed at study entry (baseline), mid-point (week 4), and study exit (week 8) through a combination of single leg balance assessments, a 10-metre heel-to-toe walk test, and static and dynamic balance assessments using the Biodex Balance System.
Results: An improvement in overall median scores was observed at study exit compared to baseline across all clinical evaluations. The improvements observed at both week-4 and at week-8 were statistically significant, when compared to baseline, for the single leg balance and heel-to-toe walk assessments. The changes in the sway index scores noted across all Biodex Balance System assessments were relatively modest and not statistically significant.
Conclusion: Our investigation supports the role of at-home, balance training in improving gait and balance among CMT1A patients. The results and methodological considerations from our study may inform future studies in this area. Larger, comparative studies should be performed to elucidate the precise short-term and long-term effects of regular, at-home balance training in CMT disease.
Keywords
Balance; CMT1A; Home exercise training
ABBREVIATIONS
CMT: Charcot-Marie-Tooth
BBS: Biodex Balance System SD
mCTSIB: Modified Clinical Test of Sensory Integration of Balance
INTRODUCTION
Charcot-Marie-Tooth (CMT) disease is a hereditary genetic disorder characterized by peripheral, sensory and motor polyneuropathy [1,2]. While it is estimated that the disorder affects approximately 1 in 1,214 - 2,500 people, the prevalence of the disease has been shown to be varied across different population groups in recent literature [3-6]. The neuropathy often leads to progressive, symmetrical atrophy of the muscles in the limbs. As such, common clinical manifestations include foot deformities such as pes cavus, reduced deep tendon reflexes, weakening of distal muscles, increased fatigue, postural instability, dysfunctional gait, sensory impairment and chronic pain [7]. Altogether; these symptoms significantly reduce the overall quality of life of CMT patients [8-12].
As a hereditary condition with a complex genetic basis involving more than 80 genes, CMT represents a set of genetically heterogeneous disorders [13]. The disease is clinically classified into different types such as CMT1, CMT2, and CMT4 based on factors like inheritance patters (autosomal dominant, autosomal recessive, or X-linked), neuropathy type (demyelinating, axonal), and nerve conduction velocity [1,14-18]. Each of these types are further classified into various subtypes based on the genes involved [1,14]. The most common subtype is CMT1A which involves duplication of the PMP22 gene and accounts for approximately 70% of all CMT1 cases and approximately 50% of all CMT cases [18-21].
Currently, there is no known cure for CMT disease [22]. Hence, at this stage, optimizing the management of CMT involves exploration of rehabilitative techniques and therapies that could mitigate the disease symptoms. Balance impairment has been implicated as one of the most significant deterrents of functionality and overall quality of life in CMT patients given its association with increased risk of falls and fall related injuries, decreased daily physical activity, decreased physical performance, and reduced balance confidence [23-29]. Therefore, it is of significant importance to evaluate the effectiveness of therapeutic interventions that could improve gait and balance in CMT patients and consequently improve their overall quality of life. While a few different disease management strategies have been previously explored, there is lack of consensus regarding the most optimal therapeutic option [22]. Previous studies have supported the utility of exercise, including self-directed at-home resistance training, as rehabilitative interventions for strength and functional improvements in CMT patients [30-32]. However, few studies have investigated the role of exercise programs for balance improvement in this patient population [33]. Interestingly, while clinicians often recommend the regular practice of simple balance exercises at home, the literature within this context is limited. Hence, the aim of the our study was to evaluate the effectiveness of an 8 week, at-home, static and dynamic balance training program in improving gait and balance amongst CMT1A at our centre.
METHODS
A prospective single arm, pre and post intervention cohort study was conducted to evaluate the effectiveness of an eight-week home exercise intervention program in improving balance in CMT-1A patients. Participant balance was assessed at study entry (baseline), mid-point (week 4), and study exit (week 8) at the neuromuscular clinic. The study was approved by the local Research Ethics Board and informed written consent was obtained from each participant before enrollment into the study. Patients at an outpatient neuromuscular clinic made up the sampling frame of the study and participants were primarily recruited using a consecutive sampling strategy via email and phone call advertising focused towards the CMT-1A patients at the clinic. To be eligible, patients had to be between 18 and 65 years of age and have a genetically confirmed diagnosis of CMT-1A. The exclusion criteria included being pregnant, and having permanent physical disability or long-term immobilization from injury. All interested subjects were screened before enrollment for their physical ability to carry out the home exercises safely, and without assistance or supervision.
INTERVENTION
The home exercise routine consisted of the following balance training exercises-one leg balance with eyes open and eyes closed, one leg balance with anterior-posterior and medial-lateral leg swings, one leg balance with rotating arms (clock like motions), heel-to-toe walk, and double leg balance on a BOSU ball. Participants were required complete these balance exercises five days a week for 8 weeks and record their best performance scores on each day in the performance tracker that was provided to them to account. The amount of time spent on each exercise and the frequency of repetitions were specified. A detailed description of the intervention routine is provided in Supplementary Table 1. Given the risk of falling with these exercises, multiple safety measures were put in place. Firstly, our preliminary screening process ensured that those enrolled were physically able to carry out the exercises and take part in this study. Secondly, all participants were trained by the research team on how to carry out the exercises safely and effectively and a detailed instructions manual with schematics was provided to each participant for reference.
|
N=16 |
Median |
IQR |
Minimum |
Maximum |
|
Baseline |
4.875 |
3.38 |
2.25 |
17.00 |
|
4 weeks |
2.750 |
3.06 |
1.50 |
11.75 |
|
8 weeks |
2.250 |
1.63 |
1.00 |
10.00 |
Table 1: Median balance error scores for the single leg balance assessment program.
OUTCOME ASSESSMENT
Clinical assessment of balance was performed at three time points: at baseline (week 0), after 4 weeks of the intervention, and after 8 weeks of the intervention. All assessments were carried out bare foot. The following outcome measures were used to assess the effect of the home exercise training routine on balance outcomes.
Single leg balance assessment
Single leg balance was evaluated using four standing positions of varying difficulties. The four tested standing positions, which were also a part of the home-exercise intervention routine, included the one leg balance with eyes open (20 seconds), one leg balance with eyes closed (20 seconds), single leg balance with anterior-posterior leg swings (30 seconds), and single leg balance with lateral leg swings (30 seconds). Each test was scored by counting the total number of errors in balance accumulated by the participant. Errors included stepping off for support or stumbling, and/or using the upper limbs for external support. Balance for each leg was assessed individually.
Heel to toe walk test
A 10 meter heel-to-toe walk test was conducted to evaluate gait. Participants were required to walk in a straight line, across a 10-meter stretch, while placing the heel of one foot near the toe of the other foot with each step. The total number of incorrect foot placement errors (i.e. number of missteps deviating from the reference line) and the deviation distance for each misstep from the reference line were recorded. Additionally, the total time taken to complete the 10-meter distance was also recorded.
Biodex balance system assessment
The Biodex Balance System SD (Biodex Medical Systems, Inc., New York, USA) is an electronic balance training and assessment platform that has been shown to quantitatively measure both static and dynamic balance with high reliability [34]. The Biodex Balance System (BBS) particularly measures a participant’s ability to control their centre of mass and offers a variety of testing options, involving a range of stationary, foamy, and dynamic surface settings, which ultimately allows for a comprehensive assessment of static and dynamic balance. For each of its inbuilt tests, the BBS software provides a score of sway index along with a breakdown of scores for each of the involved components in the assessment. We included the following two BBS tests as part of our clinical balance assessment for all participants:
Postural stability test (Static and Dynamic)
The BBS static and dynamic postural stability tests involved the assessment of postural sway on stable and unstable surfaces respectively. To assess dynamic balance, we used a platform setting of ‘8’ for all participants (i.e. a moderately dynamic setting on 6 point scale where in 12 represents the minimal platform sway setting and 6 represents the maximum platform sway setting). For both tests, participants were required to stand as still as possible while maintaining their centre of mass at the centre of the circular platform, as visually indicated by a marker on the screen that depicted the patient’s sway. Each condition was tested thrice with each trial lasting 20 seconds followed by a 10 second break. For each test, the overall, averaged sway index recorded by the BBS was used for analysis.
Modified Clinical Test of Sensory Integration of Balance (mCTSIB)
The BBS mCTSIB test assessed postural sway across four different conditions: (1) with Eyes Open on Firm Surface, (2) with Eyes Closed on Firm Surface, (3) with Eyes Open on Foam Surface, and (4) with Eyes Closed, Foam Surface. Each condition was assessed for 30 seconds followed by a break where participants transitioned into the next condition. During conditions 3 and 4, the patients were instructed to reposition their feet on a foam mat that was provided by the Biodex system. This mat was placed on the circular platform with the same markings as those present on the firm surface. For each condition, the participant was required to stand still as possible for the 30 second test duration, and the overall, averaged sway index recorded by the BBS was used for analysis.
STATISTICAL METHODS
All statistical analyses were performed using IBM SPSS Version 25 statistical package. Descriptive analysis was carried out to assess the preliminary distribution of traits within the study sample. All data was assessed graphically and statistically for normality of distribution prior to analysis. Given the small sample size and non-normal distribution of data, the non-parametric, related samples Friedman test was used to compare within group changes in balance scores across the three time points (baseline, week 4, and w eek 8) with post-hoc analysis conducted using the Wilcoxon sign rank test for pair wise comparisons. The level of statistical significance was set at p<0.05 for the omnibus test and at p<0.0167 for the post hoc test after the Bonferroni correction was made for three independent comparisons. Missing data was imputed using the last observation carried forward method.
RESULTS
Eight patients with clinically dialogised CMT1a enrolled into the study. The average participant age in the study was 46.38 ± 12.95 years and female participants represented 62.5% of the sample. After enrollment, seven participants completed the study with 8 weeks of follow-up, while one female participant was lost to follow up after 4-weeks due to an unrelated leg injury (i.e. not caused by the exercise training in the study). Data for this participant at week 8 was imputed using the last observation carried forward method.
Single leg balance assessment
Single leg balance was assessed though four exercises. Balance for both the right and left legs were assessed independently and performance for each leg was considered separately for analysis (i.e. 16 data points for 8 patients). The measure used to assess balance was a count of the total number of errors (i.e. use of corrective steps, stumbles, use of external support) with a lower score indicating lesser number or errors and hence better single leg balance. The performance scores for each leg were averaged across all four exercises to determine the overall average performance scores per leg for the entire single leg balance-testing program, at each time point. Table 1 and figure 1 summarize the aggregated, overall single leg balance scores at each time point.
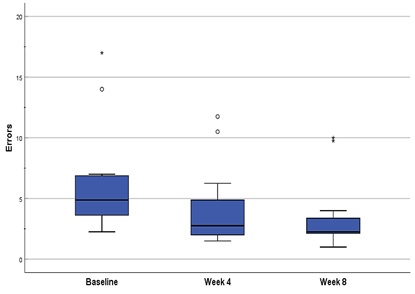 Figure 1: Single Leg Balance Assessment - Performance was assessed across four single balance positions and scored by counting the total number of errors in balance accumulated by participants.
Figure 1: Single Leg Balance Assessment - Performance was assessed across four single balance positions and scored by counting the total number of errors in balance accumulated by participants.
An improvement in balance was observed at follow up visits, with an overall decline in median number of corrective steps noted at both week 4 and week 8 (omnibus p=1.334x10-8). Particularly, post-hoc analysis indicated significant improvement at week 4 vs. baseline (0.000031), and at week 8 vs. baseline (0.000061). However, the change noted at week 8 was not significant when compared to week 4 (p=0.0183).
Heel to toe walk test
The parameters measured for the test included the total number of missteps away from the reference line, the deviation distance from the reference line for each misstep, and the total time taken to walk the 10m stretch. Based on these data parameters, three customized performance metrics were developed and utilized for a comprehensive evaluation of gait. The three metrics included: 1) total step error (i.e. the cumulative deviation distance from the reference line for all missteps), 2) gait efficiency (i.e. total time x the cumulative deviation distance from the reference line for all missteps) and 3) step error product (i.e. total number of missteps x the cumulative deviation distance from the reference line for all missteps). In all cases, a lower or reduced score indicates improved balance. Table 2 and figures 2-4 summarize the aggregated scores at each time point.
|
N=8 |
Median |
IQR |
Minimum |
Maximum |
|
|
Total Step Error (cm) |
Baseline |
48.500 |
99.725 |
5.5 |
148.7 |
|
4 weeks |
5.000 |
26.8 |
0 |
58.8 |
|
|
8 weeks |
7.550 |
23.5 |
0 |
82.0 |
|
|
Gait Efficiency (cm*s) |
Baseline |
2667.000 |
4372.325 |
539.0 |
13680.4 |
|
4 weeks |
216.450 |
1744.3 |
0 |
2528.4 |
|
|
8 weeks |
348.450 |
968 |
0 |
8036.0 |
|
|
Step Error Product (cm*missteps) |
Baseline |
145.500 |
1437.975 |
5.5 |
1933.1 |
|
4 weeks |
8.000 |
202.525 |
0 |
470.4 |
|
|
8 weeks |
7.50 |
47 |
0 |
328.0 |
|
Table 2: Median scores for the 10 metre Heel-to-Toe walk test.
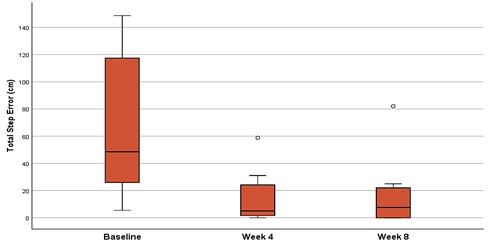 Figure 2: Heel-to-Toe Walk Test: Total Step Error - Performance was assessed by measuring the cumulative distance deviation distance from the reference line for all missteps.
Figure 2: Heel-to-Toe Walk Test: Total Step Error - Performance was assessed by measuring the cumulative distance deviation distance from the reference line for all missteps.
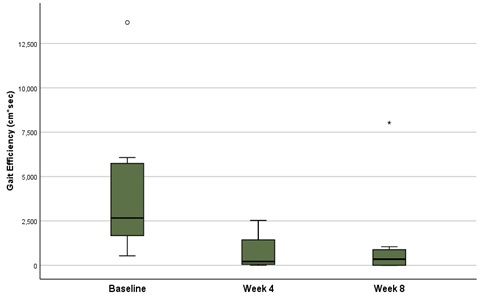 Figure 3: Heel-to-Toe Walk Test: Gait Efficiency - Performance was assessed by factoring in the total time taken to walk the 10-meter distance and the cumulative deviation distance from the reference line for all missteps.
Figure 3: Heel-to-Toe Walk Test: Gait Efficiency - Performance was assessed by factoring in the total time taken to walk the 10-meter distance and the cumulative deviation distance from the reference line for all missteps.
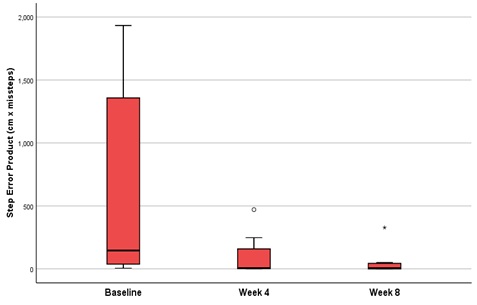 Figure 4: Heel-to-Toe Walk Test: Step Error Product - Performance was assessed by factoring in the total number of missteps and the cumulative deviation distance from the reference line for all missteps.
Figure 4: Heel-to-Toe Walk Test: Step Error Product - Performance was assessed by factoring in the total number of missteps and the cumulative deviation distance from the reference line for all missteps.
A significant reduction in median scores was observed at follow up relative to baseline in all cases (Table 2). The omnibus test noted a significant change with respect to the three performance metrics, including the total step error, gait efficiency, and step-error product, with significance values of 4.57 x 10-4, 4.57 x 10-4, and 2.86 x 10-4 respectively. Post-hoc analysis indicated a significant reduction in step error rate (p=0.008), gait efficiency (p=0.008), and step error product (p=0.008), at both subsequent follow up visits when compared to baseline.
In comparison, only a slight improvement was observed in the step error product at week 8 relative to week 4, while a slight deterioration was noted in total step error and gait efficiency.
However, the changes observed between week 4 and week 8 were non-significant in all cases (p=0.813, 1.000 and 0.625 for total step error, gait efficiency, and step error product respectively).
Biodex balance system assessment
The static postural stability, dynamic postural stability, and the sensory integration of balance tests, offered by the Biodex Balance Assessment system, were conducted at each clinic visit to assess balance on stable and unstable platforms. The BBS reported aggregate sway scores where a lower score indicates higher stability while a larger score represents greater amount of postural sway and instability. Table 3 and figure 5 summarize the aggregated scores at each time point.
|
N=8 |
Median |
IQR |
Minimum |
Maximum |
|
|
Overall Sway (Static Postural Stability) |
Baseline |
0.4 |
0.325 |
0.2 |
4.6 |
|
4 weeks |
0.3 |
0.175 |
0.2 |
0.5 |
|
|
8 weeks |
0.3 |
0.1 |
0.1 |
0.5 |
|
|
Overall Sway (Dynamic Postural Stability) |
Baseline |
1.100 |
0.6 |
0.5 |
1.2 |
|
4 weeks |
0.700 |
0.175 |
0.4 |
1.7 |
|
|
8 weeks |
0.600 |
0.35 |
0.3 |
0.9 |
|
|
Composite Score (mCTSIB) |
Baseline |
1.5050 |
0.48 |
1.14 |
2.14 |
|
4 weeks |
1.5950 |
0.28 |
1.18 |
1.69 |
|
|
8 weeks |
1.4350 |
0.4 |
1.13 |
1.68 |
|
Table 3: Median error scores for the Biodex balance assessments.
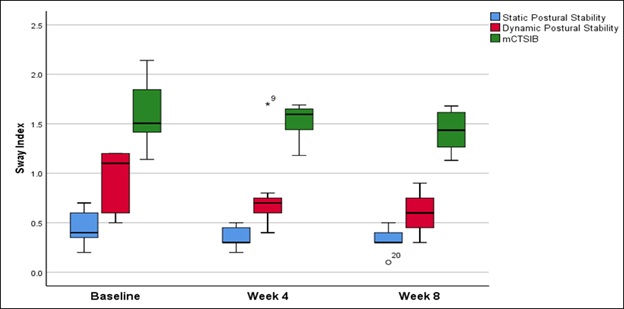 Figure 5: Biodex Balance System Assessments - Performance was assessed across three different testing conditions including tests of static postural stability, dynamic postural stability, and the Modified Clinical Test of Sensory Integration of Balance (mCTSIB).
Figure 5: Biodex Balance System Assessments - Performance was assessed across three different testing conditions including tests of static postural stability, dynamic postural stability, and the Modified Clinical Test of Sensory Integration of Balance (mCTSIB).
A reduction in median sway scores was noted for the static postural stability test (stable platform condition) at follow-up compared to baseline, as assessed by the overall static sway score (Table 3). However, there was no change in the median noted between follow-up visit 2 (week 4) and visit 3 (8 week). The omnibus tested indicated a significant change (p=0.039) with the post-hoc analysis revealing a statistically significant improvement at week 8 compared to baseline (p=0.0156).
Similarly, a reduction in median sway scores was observed in dynamic postural stability performance (unstable platform condition) at follow-up visits compared to baseline, as assessed by the overall dynamic sway score. Nonetheless, the treatment effect did not reach the statistical significance for the omnibus test (p=0.0503).
In the mCTSIB test, deterioration in postural sway was noted after 4 weeks compared to baseline. This was assessed by the composite score, which provided aggregated scores from four-test conditions - eyes open firm surface, eyes closed firm surface, eyes open foam surface, eyes closed foam surface. After 8 weeks, however, an improvement was observed relative to both baseline and week 4. However, statistical significance was not achieved for the omnibus test (p=0.103).
DISCUSSION
In this study, we investigated the role of an extended, at-home balance training intervention in improving gait and balance amongst eight patients with genetically confirmed CMT1A disease. An improvement in overall gait and balance was observed at study exit compared to baseline across all clinical evaluations. Particularly, for most assessments, a remarkable portion of the of the improvement was noted within the first four weeks of carrying out the home exercises, as opposed to between week 4 and week 8 wherein the amount of change was relatively modest. The improvements observed after both week-4 and week-8 were statistically significant, when compared to baseline, for the single leg balance and heel-to-toe walk assessments.
Interestingly, the BBS mCTSIB assessment and the heel-to-toe assessments of gait efficiency and total step error showed slightly different trends. The mCTSIB scores indicated a slight deterioration between baseline and week 4 while the gait efficiency and total step error scores indicated deterioration between week 4 and week 8. Nevertheless, despite minor deteriorations, significant improvements in scores were noted at study exit, when compared to baseline, in all of these cases. In comparison, however, none of the changes observed between week 4 and week 8 met the Bonferroni threshold for statistical significance. Similarly, none of the changes in the sway index scores observed across all the BBS assessments met the threshold for statistical significance for the omnibus test. However, this may be attributed to the lack of sufficient power. In any case, given that notable improvements in median scores was noted across these tests, a viable intervention effect may still be considered and further explored in future investigations.
Given its negative impact on overall quality of life, balance impairment has been deemed a critical patient important outcome of CMT disease [23]. Recent research has suggested that 86% of individuals with CMT have reported falling or having near fall experiences, with 29.9% reporting falling once a month while 14% reporting falling as frequently as once a week [27]. Indeed, distal muscle weakness has been implicated as a critical contributing factor [35-37]. But while some studies have investigated the effect of exercise interventions on muscle strength in CMT1A patients, the literature on the role of exercise training within the context of balance improvement is limited [30-33]. In a previous study, Matjacic et al. evaluated the impact of a 12-day dynamic balance training and muscle strengthening intervention in patients that had hereditary sensory motor neuropathy, and elucidated the potential role of dynamic balance training in balance improvement [33]. Similarly, Kobesova et al. evaluated the effect of a 3-week intensive inpatient rehabilitation program in a CMT patient and reported significant improvements in gait and balance [38]. In this context, our study investigated the effect of an extended (8-week) at-home, static and dynamic balance training intervention. Based on the results of our study, extended, at-home, balance training appears as a promising candidate in improving gait and balance and it may offer benefits similar to onsite, supervised rehabilitative exercise programs.
Our study has several strengths. Firstly, we were able to perform a comprehensive assessment of balance and gait through the use of various quantitative clinical measures including static and dynamic balance tests, a walk test, and assessments using the Biodex Balance System. Secondly, the intervention plan we used was comprehensive, including a range of exercises for unilateral and bilateral, static and dynamic balance components. Our intervention was also appropriately tailored for our target population group, allowing the participants to carry out the exercises at home without professional support or supervision. Apart from that, we were able to test the effect of a relatively long-term physical intervention (8-weeks) with continuous follow-up and a high degree of compliance. Limitations of our study include a small sample size (N=8) which is insufficiently powered to detect subtle interventional effects and impacts the generalizability of the results. Our study also did not include a control group or account for potential confounding variables, which consequently limited out ability to assess the effect of the intervention independent of other modifiable factors. Furthermore, participant performance in this case may have been susceptible to potential practice effect given the repeated measures study design. Lastly, since the balance assessments in the clinic required a significant amount of focus for completion with minimal error, concentration level on the day of the clinic appointment may have also had an impact.
CONCLUSION
In conclusion, our investigation supports the role of an at-home, static and dynamic, balance training program in improving gait and balance amongst CMT1A patients. To the best of our knowledge, this is one of the few studies that has addressed the effect of an extended, at-home balance training intervention amongst CMT1A patients. Given the limited amount of research evidence on the role of home exercise in CMT1A, the results of our investigation highlight a potential therapeutic avenue that should be further explored within this clinical context. Our study further presents a uniquely tailored intervention strategy and a comprehensive outcome assessment approach which may help inform future studies in this area. Further studies, including large scale prospective cohort studies and RCTs with extended follow-up, should be conducted to elucidate the precise short-term and long-term effects of regular, at-home balance training in CMT disease.
ACKNOWLEDGEMENTS
We are indebted to all participants of this study. We would also like to thank Anneke Nijenhuis for administrative assistance.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
AUTHOR’S CONTRIBUTION
S.B. designed the study; T.S, M.K and S.B conducted the study; T.S and S.B analyzed the data; T.S, M.K and S.B wrote and reviewed the manuscript for important intellectual content; all authors read and approved the final manuscript.
PRODUCTS/EQUIPMENT USED IN THE STUDY
- BOSU® Balance Trainer (BOSU Ball)
- Biodex Balance System SD (Biodex Medical Systems, Inc., New York, USA)
REFERENCES
- Bird TD (2020) Charcot-Marie-Tooth (CMT) hereditary neuropathy overview. In Gene Reviews. University of Seattle, Washington.
- Patzkó Á, Shy ME (2011) Update on Charcot-Marie-tooth disease. Curr Neurol Neurosci Rep 11: 78-88.
- Braathen GJ, Sand JC, Lobato A, Hoyer H, Russell MB (2011) Genetic epidemiology of Charcot-Marie-Tooth in the general population. Eur J Neurol 18: 39-48.
- Skre H (1974) Genetic and clinical aspects of Charcot-Marie-Tooth’s disease. Clin Genet 6: 98-118.
- Barreto LC, Oliveira FS, Nunes PS, de França Costa IM, Garcez CA, et al. (2016) Epidemiologic study of Charcot-Marie-Tooth disease: a systematic review. Neuroepidemiology 46: 157-165.
- Theadom A, Roxburgh R, MacAulay E, O’Grady G, Burns J, et al. (2019) Prevalence of Charcot-Marie-Tooth disease across the lifespan: a population-based epidemiological study. BMJ Open 9: 029240.
- Pareyson D, Marchesi C (2009) Diagnosis, natural history, and management of Charcot-Marie-Tooth disease. Lancet Neurol 8: 654-667.
- Padua L, Aprile I, Cavallaro T, Commodari I, La Torre G, et al. (2006) Variables influencing quality of life and disability in Charcot Marie Tooth (CMT) patients: Italian multicentre study. Neurol Sci 27: 417-423.
- Padua L, Aprile I, Cavallaro T, Commodari I, Pareyson D, et al. (2008) Relationship between clinical examination, quality of life, disability and depression in CMT patients: Italian multicenter study. Neurol Sci 29: 157-162.
- Cordeiro JL, Marques W, Hallak JE, Osorio FL (2014) Charcot-Marie-Tooth disease, psychiatric indicators and quality of life: a systematic review. ASN Neuro 6: 00145.
- Boentert M, Dziewas R, Heidbreder A, Happe S, Kleffner I, et al. (2010) Fatigue, reduced sleep quality and restless legs syndrome in Charcot-Marie-Tooth disease: a web-based survey. J Neurol 257: 646-652.
- Redmond AC, Burns J, Ouvrier RA (2008) Factors that influence health-related quality of life in Australian adults with Charcot-Marie-Tooth disease. Neuromuscul Disord 18: 619-625.
- Rossor AM, Polke JM, Houlden H, Reilly MM (2013) Clinical implications of genetic advances in Charcot-Marie-Tooth disease. Nat Rev Neurol 9: 562-571.
- Schenone A, Nobbio L, Monti Bragadin M, Ursino G, Grandis M (2011) Inherited neuropathies. Curr Treat Options Neurol 13: 160-179.
- Dyck PJ, Lambert EH (1968) Lower motor and primary sensory neuron diseases with peroneal muscular atrophy. I. Neurologic, genetic, and electrophysiologic findings in hereditary polyneuropathies. Arch Neurol 18: 603-618.
- Harding AE, Thomas PK (1980) The clinical features of hereditary motor and sensory neuropathy types I and II. Brain 103: 259-280.
- Davis CJF, Bradley W, Madrid R (1978) The peroneal muscular atrophy syndrome. Clinical, genetic, electrophysiological and nerve biopsy studies. I. Clinical, genetic and electrophysiological findings and classification. J Genet Hum 26: 311-349.
- Szigeti K, Lupski JR (2009) Charcot-Marie-Tooth disease. Eur J Hum Genet 17: 703-710.
- Lupski JR, de Oca-Luna RM, Slaugenhaupt S, Pentao L, Guzzetta V, et al. (1991) DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell 66: 219-232.
- Timmerman V, Nelis E, Van Hul W, Nieuwenhuijsen BW, Chen KL, et al. (1992) The peripheral myelin protein gene PMP-22 is contained within the Charcot-Marie-Tooth disease type 1A duplication. Nat Genet 1: 171-175.
- Pareyson D, Saveri P, Pisciotta C (2017) New developments in Charcot–Marie–Tooth neuropathy and related diseases. Curr Opin Neurol 30: 471-480.
- Young P, De Jonghe P, Stogbauer F, Butterfass-Bahloul T (2008) Treatment for Charcot?Marie?Tooth disease. Cochrane Database Syst Rev 1: CD006052.
- Johnson NE, Heatwole CR, Dilek N, Sowden J, Kirk CA, et al. (2014) Quality-of-life in Charcot–Marie–Tooth disease: The patient’s perspective. Neuromuscul Disord 24: 1018-1023.
- Anens E, Emtner M, Hellström K (2015) Exploratory study of physical activity in persons with Charcot-Marie-Tooth disease. Arch Phys Med Rehabil 96: 260-268.
- Eichinger K, Odrzywolski K, Sowden J, Herrmann DN (2016) Patient Reported Falls and Balance Confidence in Individuals with Charcot-Marie-Tooth Disease. J Neuromuscul Dis 3: 289-292.
- Roberts-Clarke D, Fornusek C, Saigal N, Halaki M, Burns J, et al. (2016) Relationship between physical performance and quality of life in Charcot-Marie-Tooth disease: a pilot study. J Peripher Nerv Syst 21: 357-364.
- Ramdharry GM, Reilly-O'Donnell L, Grant R, Reilly MM (2018) Frequency and circumstances of falls for people with Charcot-Marie-Tooth disease: A cross sectional survey. Physiother Res Int 23: e1702.
- Tozza S, Aceto MG, Pisciotta C, Bruzzese D, Iodice R, et al. (2016) Postural instability in Charcot-Marie-Tooth 1A disease. Gait Posture 49: 353-357.
- Lencioni T, Rabuffetti M, Piscosquito G, Pareyson D, Aiello A, et al. (2014) Postural stabilization and balance assessment in Charcot-Marie-Tooth 1A subjects Gait Posture 40: 481-486.
- Kilmer DD (2002) Response to resistive strengthening exercise training inhumans with neuromuscular disease. Am J Phys Med Rehabil 81: 121-126.
- Sman AD, Hackett D, Fiatarone Singh M, Fornusek C, Menezes MP, et al. (2015) Systematic review of exercise for Charcot-Marie-Tooth disease. J Peripher Nerv Syst 20: 347-362.
- Djordjevic D, Fell S, Baker S (2017) Effects of Self-Selected Exercise on Strength in Charcot-Marie-Tooth Disease Subtypes. Can J Neurol Sci 44: 572-576.
- Matjaci? Z, Zupan A (2006) Effects of dynamic balance training during standing and stepping in patients with hereditary sensory motor neuropathy. Disabil Rehabil 28: 1455-1459.
- Cachupe WJ, Shifflett B, Kahanov L, Wughalter EH (2001) Reliability of biodex balance system measures. Measurement in physical education and exercise science 5: 97-108.
- Lencioni T, Piscosquito G, Rabuffetti M, Bovi G, Calabrese D, et al. (2015) The influence of somatosensory and muscular deficits on postural stabilization: insights from an instrumented analysis of subjects affected by different types of Charcot-Marie-Tooth disease. Neuromuscul Disord 25: 640-645.
- Guillebastre B, Calmels P, Rougier P (2013) Effects of muscular deficiency on postural and gait capacities in patients with Charcot-Marie-Tooth disease. J Rehabil Med 45: 314-317.
- Nardone A, Tarantola J, Miscio G, Pisano F, Schenone A, et al. (2000) Loss of large-diameter spindle afferent fibres is not detrimental to the control of body sway during upright stance: evidence from neuropathy. Exp Brain Res 135: 155-162.
- Kobesova A, Kolar P, Mlckova J, Svehlik M, Morris CE, et al. (2012) Effect of functional stabilization training on balance and motor patterns in a patient with Charcot-Marie-Tooth disease. Neuro Endocrinol Lett 33: 3-10.
Citation: Sharma T, Khudadad M, Baker S (2020) Effectiveness of an 8 Week Home Balance Training Program in CMT1A: A Pre and Post Intervention Study. J Phys Med Rehabil Disabil 6: 045.
Copyright: © 2020 Tanmay Sharma2,, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

