
Effects of Endogenous Bioregulators from Mammalian Blood Serum and Bone Tissue on Amphibian Limb Regeneration
*Corresponding Author(s):
Mikhail S KrasnovNesmeyanov Institute Of Organoelement Compounds, Russian Academy Of Sciences, Moscow, Russian Federation
Tel:+7 9164921797,
Email:embrmsk@mail.ru
Abstract
The effects of bioregulators purified from mammalian blood serum and bone tissue on amphibian limb regeneration have been studied. The bioregulators from blood serum at a high concentration (10-1 mg/mL) has a strong morphogenetic effect on Xenopus laevis limb regeneration following amputation through the proximal part of the femur and a protective effect on the tissues of newt (Pleurodeles waltl) limb regenerates cultured in a roller system. The bioregulators purified from the bone tissue at an ultralow concentration (10-12 mg/mL) also has a strong protective effect on the cultured newt limb regenerates, especially with respect to the viability and structure of the cartilaginous tissue. The results show that hind limb regeneration in metamorphosing Xenopus laevis depends on the level of amputation along the proximodistal axis. Stimulation of limb regeneration by either of the bioregulators has been found to decrease with an increase in the regeneration capacity of amphibians.
Keywords
Amphibians; Bioregulators; Regeneration
INTRODUCTION
Analysis of regeneration processes in vertebrates is an important research field in modern developmental biology. A great number of experiments on regeneration have been performed on tailed amphibians (Urodela), since their capacity for tissue recovery and regeneration is much higher than in tailless amphibians (Anura), not to mention higher vertebrates. Thus, adult tailed amphibians can completely regenerate functional limbs and tissues of damaged organs, whereas adult tailless amphibians are incapable of epimorphic regeneration [1,2]. Despite the abundance of experimental data on regeneration processes, relatively little is known about its molecular mechanisms. In this respect, of interest is the new group of Membranotropic Homeostatic Tissue-specific Bioregulators (MHTBs), which are active at ultralow doses (10-8-10-15 mg/ml) and have an effect on basic biological processes such as cell adhesion, migration, proliferation, differentiation, and apoptosis [3]. It has been shown that these agents have a morphogenetic potential and are involved in processes of tissue recovery and repair in vertebrates [4,5].
This study deals with MHTBs purified from bovine blood serum and rat bone tissue. As shown previously, the MHTB from blood serum at a concentration corresponding to 10-4 mg protein/mL proved to have a morphogenetic effect on the tissues of newt tail regenerate in vitro. In particular, the state of the cultured regenerate was similar to that of the in situ regenerate: the cartilage completely retained its structure and showed segmentation typical of the given regeneration stage, with the pathological death of chondrocytes being absent; segments of the spinal cord and muscle fibers were well defined; mature skin glands produced abundant secretion; the dermis contained numerous poorly differentiated cells and small aggregates of pigment cells [4]. This MHTB at an ultralow dose of 10-11 mg/mL also had a protective effect on all tissues of the tail regenerate, but this effect was less apparent than that of the high dose. The blood serum MHTB at a high concentration (10-1 mg/ml) facilitated the formation of regenerates with fingers after forelimb amputation in Xenopus laevis [6]. The MHTB from bone tissue showed a tissue-specific protective effect at an ultralow dose of 10-14 mg/mL, promoting cell survival in the cartilage tissue and maintaining its histological structure, but no such effect was observed at a higher concentration of this agent (10-8 mg/mL) [4].
In this study, we analyzed the effect of the above two MHTBs on the state of newt (Pleurodeles waltl) forelimb regenerates at the tree-finger stage in roller culture and on hind limb regeneration in Xenopus laevis at stage 64 (the end of metamorphosis) after amputation at two levels: through the distal and proximal parts of the femur. It should be noted that tailless amphibians are capable of complete limb regeneration only at early stages of development (until metamorphosis); at later stages, the regenerate (if any) is represented by a morphologically and functionally undeveloped spike-like structure [2].
MATERIALS AND METHODS
Frogs (Xenopus laevis) and newts (Pleurodeles waltl) were from the aquarium centers of Department of Embryology, Biological Faculty of Moscow State University and Koltsov Institute of Developmental Biology, Russian Academy of Sciences. All manipulations with them were performed in accordance with the Directive 86/609/EEC on the protection of animals used for experimental and other scientific purposes.
MHTB PREPARATION
The MHTBs were extracted and purified as described [5,7,8]. Fibroblast growth factor (FGF-β) (Sigma, United States) was used as a reference substance. All test solutions and media were sterilized by ultra filtration through 0.22 μm Millipore filters. Testing for the effect of MHTBs on X. laevis limb regeneration in vivo experiments were performed with juvenile frogs at the end of metamorphosis (Nieuwkoop and Faber stage 64 [9]). In the first series, the right hind limb was amputated through the proximal one-third of the femur, perpendicular to its axis; in the second series, through the distal one-third of the femur (Figure 1). Amputation was performed under narcosis with 0.3% tricaine methanesulfonate (MS-222) solution, with a regeneration blastema developing 2 days later. Thereafter, MHTB solutions in normal saline or saline alone were injected in the blastema three times per week for the specified period (see below), and images of the regenerating limb were taken once a week. In control experiments, limb regenerates were cultured in the medium without additives (negative control) and in the presence of FGF-β (positive control). The total experimental period was 6 months.
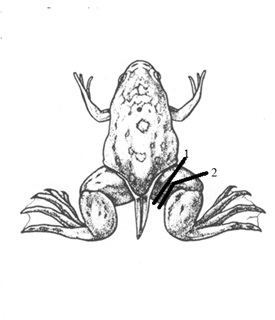 Figure 1: Scheme of hind limb amputation in Xenopus laevis (Nieuwkoop and Faber stage 64) through (1) proximal and (2) distal parts of the femur.
Figure 1: Scheme of hind limb amputation in Xenopus laevis (Nieuwkoop and Faber stage 64) through (1) proximal and (2) distal parts of the femur.
Four groups of animals were included in each series:
Series 1 (proximal amputation, injections were made for 4 months):
- group 1 (n = 8), injected with 0.5 μL of MHTB-S at a concentration of 10-1 mg/mL
- group 2 (n = 10), injected with 0.5 μL of MHTB-B at 10-12 mg/mL
- group 3 (n = 7), injected with 0.5 μL of normal saline and
- group 4 (n = 6), no injection
Series 2 (distal amputation, injections were made for 4 months):
- group 1 (n = 7), injected with 0.5 μL of MHTB-S at 10-1 mg/mL
- group 2 (n =11), injected with 0.5 μL of MHTB-B at 10-12 mg/mL
- group 3 (n = 7), injected with 0.5 μL of normal saline and
- group 4 (n = 6), no injection
At the end of experiments, the frogs were narcotized with MS-222, and the regenerates were cut off proximal to the amputation plane, fixed in Bouin's fluid, and embedded in paraffin. Serial sections (7 μm) were stained with Mallory’s trichrome or hematoxylin-eosin.
Testing for the effect of MHTBs on newt limb regeneration in vitro
Newts were narcotized with MS-222 to perform transtibial amputation of the left hind limb. The right hind limb was amputated through the distal one-third of the shin, perpendicular to its axis. After 40 days, when the regenerates reached the stage of three to four digits, they were cut off (under MS-222 narcosis) proximal to the amputation plane, disinfected in 70% ethanol, and placed in 10 mL dark glass vials containing 4 mL of culture medium with 40 μL of the test reagent or without it (control). The composition of culture medium was as follows: medium 199, 350 mL; distilled water, 150 mL; fetal calf serum, 50 mL; 1 M HEPES, 150 μL; 4% gentamicin sulfate, 1 mL; antibiotic-antimycotic solution (Sigma, United States), 5 mL.
The vials were placed in an RM5 Assistant roller mixer (Karl Hecht, Germany), and the regenerates were cultured at 35 rpm, 22°C for 12 days, replacing the medium every 3 days.
The experiment included the following variants of treatment:
1) Medium without additives (control) (n = 10)
2) MHTB-S, 10-12 mg/mL (n = 10)
3) MHTB-S, 10-1 mg/mL (n = 10)
4) MHTB-B, 10-12 mg/mL (n = 10) and
5) FGF-β, 10-10 mg/mL (n = 10)
RESULTS
Experiments with X. laevis showed that the ability of late-metamorphosing frogs (stage 64) to regenerate the lost limb was dependent on the amputation level. In agreement with previous experimental data [10], periodic trauma to the postamputation wound area (normal saline injections) promoted regeneration in both experimental series (Figure 2). However, the treatment with high-dose MHTB-S (10-1 mg/mL) proved to stimulate regeneration only in frogs with proximal amputation (Figures 3-5). It is noteworthy that the limb regenerated in one frog injected with MHTB-S had a morphologically defined toe with a claw, as could be clearly seen in histological sections (Figure 5). This is evidence that MHTB-S has a morphogenetic potential and promotes limb regeneration in tailless amphibians, which confirms previous data on the effect of this bioregulator [7]. In contrast, no stimulation of regeneration by the MHTBs was observed after distal amputation (Figures 6,7). Natural limb regeneration in vivo in case of amputation in the distal area better, than in case of amputation in the proximal area. Thus, it appears that the stimulating action of MHTB-S manifested itself under conditions where the natural regeneration capacity was at the lowest level.
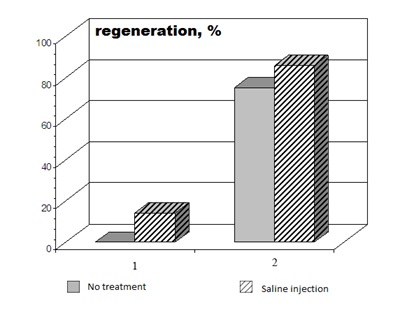 Figure 2: Comparison of regeneration capacity between groups of X. laevis frogs with hind limb amputation through (1) proximal and (2) distal parts of the femur.
Figure 2: Comparison of regeneration capacity between groups of X. laevis frogs with hind limb amputation through (1) proximal and (2) distal parts of the femur.
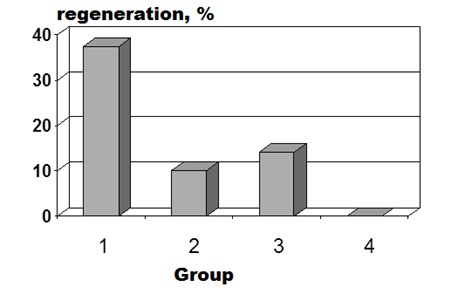
Figure 3: Percentages of hind limb regeneration at 4 months after proximal amputation in different experimental groups of X. laevis frogs: (1) injection of MHTB-S at 10-1 mg/mL, (2) injection of MHTB-B at 10-12 mg/mL, (3) injection of normal saline, (4) no treatment (here and in figures 4,6,7).
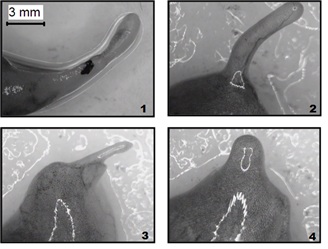
Figure 4: Hind limb regenerates formed 4 months after proximal amputation in different experimental groups of X. laevis frogs: (1) Injection of MHTB-S at 10-1 mg/mL - formed spike regenerates together with toe and claw, (2) Injection of MHTB-B at 10-12 mg/mL - formed only spike regenerates, (3) Injection of normal saline - formed only spike regenerates, (4) No treatment - no formed spike regenerates.
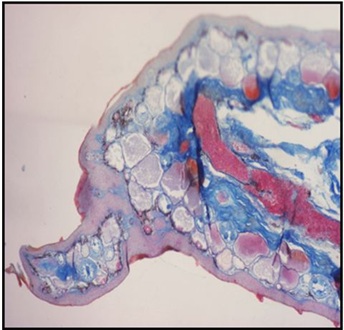
Figure 5: Histological section through a hind limb regenerate formed 4 months after proximal amputation in X. laevis treated with MHTB-S (10-1 mg/mL), Mallory’s staining. A morphologically defined toe with a claw.
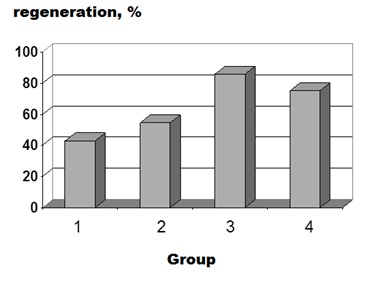
Figure 6: Percentages of hind limb regeneration at 4 months after distal amputation in different experimental groups of X. laevis frogs. For designations, see figure 3.
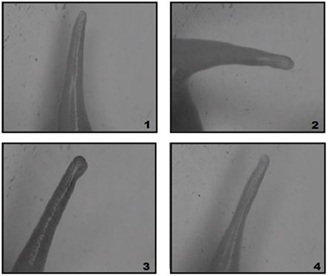 Figure 7: Hind limb regenerates formed 4 months after distal amputation in distal area in different experimental groups of X. laevis frogs: (1) Injection of MHTB-S at 10-1 mg/mL - formed only spike regenerates, (2) Injection of MHTB-B at 10-12 mg/mL - formed only spike regenerates, (3) Injection of normal saline - formed only spike regenerates, (4) No treatment - formed only spike regenerates.
Figure 7: Hind limb regenerates formed 4 months after distal amputation in distal area in different experimental groups of X. laevis frogs: (1) Injection of MHTB-S at 10-1 mg/mL - formed only spike regenerates, (2) Injection of MHTB-B at 10-12 mg/mL - formed only spike regenerates, (3) Injection of normal saline - formed only spike regenerates, (4) No treatment - formed only spike regenerates.
Histological sections shown in figure 8 illustrate the morphology of newt hind limb regenerates cultured in a roller system under different variants of treatment. A regenerate at the three-digit stage prior to culturing (Figure 8a) was covered with a stratified epithelium, and the cartilaginous structures of the regenerating digits were well defined. Spaces between them were filled with mesenchymal-like cells, collagen fibers, and blood vessels, with pigment cells forming small clusters under the epithelium and in the mesenchyme. After 12-day culturing in the medium without additives (negative control), chondrocyte death and structural disturbances in the cartilage of regenerating digits were revealed (Figure 8b-8f). The interdigital spaces contained numerous vessels filled with red blood cells, while mesenchymal cells and collagen fibers were almost absent. Pigment cell clusters were found in the mesenchyme but not under the epithelium. In general, the histological picture was indicative of inflammation and tissue degeneration.
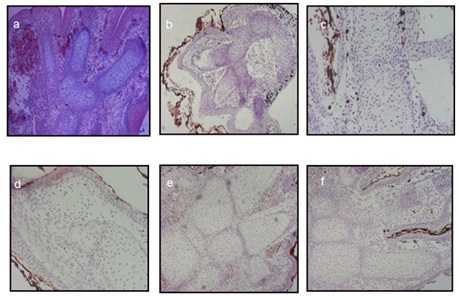
Figure 8: Histological sections of Pleurodeles waltl hind limb regenerates cultured in a roller system for 12 days: (a) A regenerate prior to culturing was covered with a stratified epithelium, and the cartilaginous structures of the regenerating digits were well defined.; (b) Culturing in the medium without additives (negative control) chondrocyte death and structural disturbances in the cartilage of regenerating digits were revealed; (c) Treatment with MHTB-S at 10-12 mg/mL provided chondrocyte death and deterioration of cartilage structure were observed; (d) Treatment with FGF-β at 10-10 mg/mL (positive control) provided for the maintenance of cartilage structure, with chondrocytes in small lacunae being sparsely distributed within a fibrous matrix; (e) Treatment with MHTB-B at 10-12 mg/mL provided cartilage tissue integrity was maintained more efficiently, with cartilaginous structures being surrounded by large vessels filled with blood cells; (f) Treatment with MHTB-S at 10-1 mg/mL provided the regenerated cartilage remained viable, had a typical structure (with numerous chondrocytes and well-developed matrix), and was surrounded with blood vessels . Magnification: (a,b,e,f) ocular 10×, objective lens 4×; (c, d) ocular 10×, objective lens 10×.
Treatment with FGF-β (Figure 8d) provided for the maintenance of cartilage structure, with chondrocytes in small lacunae being sparsely distributed within a fibrous matrix. In the epithelium, however, cell desquamation and death were observed. The connective tissue contained numerous fibroblast-like cells, muscle cells, and collagen fibers but showed almost no vascularization. Pigment cells were arranged in small clusters under the epithelium in the posterior part of the regenerate.
The effect of MHTB-S proved to differ depending on its concentration. In the regenerates treated with the ultralow concentration (10-12 mg/mL), chondrocyte death and deterioration of cartilage structure were observed. The epithelium was desquamating, and small clusters of pigment cells were found under it. The mesenchyme contained small amounts of fibroblast-like cells and vessels, but collagen fibers were missing (Figure 8c). On the whole, the state of the regenerates was better than in the control but inferior to that in the case of FGF-β treatment.
When MHTB-S was added at the high concentration (10-1 mg/mL), the regenerated cartilage remained viable, had a typical structure (with numerous chondrocytes and well-developed matrix), and was surrounded with blood vessels. The mesenchyme contained fibroblast-like cells. The epithelium showed almost no desquamation, and small clusters of pigment cells were distributed under it (Figure 8f). Thus, MHTB-S in this case proved to have a distinct homeostatic effect, in agreement with previous data on the influence of different concentrations of this bioregulator on newt tail regeneration [5].
The most evident protective effect on the tissues of cultured regenerates was observed under treatment with MHTB-B (10-12 mg/mL). Compared to the variant with FGF-β, cartilage tissue integrity was maintained more efficiently, with cartilaginous structures being surrounded by large vessels filled with blood cells. The mesenchyme contained numerous fibroblast-like cells and, in places, muscle fibers, but collagen fibers were indistinct. Aggregations of pigment cells were distributed under the epithelium, where subcutaneous glands were also visible (Figure 8e).
DISCUSSION
Limb amputation is followed by the formation of blastema, a mound of morphologically undifferentiated, proliferating cells that gives rise to regeneration of all the structures distal to the amputation plane [11].
All Urodela species have a lifelong ability to completely regenerate limbs, tail, jaws, and eye lens. A major role in limb regeneration is played by the Prod 1 protein, which is expressed at the surface of blastemal cells and regulates cell adhesion, migration and cell division. The expression of Prod 1 is graded in both normal and regenerating limbs, with its amount being greater in the proximal part and decreasing distally. This proximodistal gradient specifies which part of the growing regenerate will become the upper arm, forearm, or hand [12]. Stimulation of Prod 1 expression in a distal blastema (at the forearm or wrist level) leads to regeneration of an upper arm-like structure; i.e., blastemal cells behave as if they were located closer to the limb girdle [13]. The secreted protein named nAG (newt anterior gradient protein) has been identified as a ligand for Prod 1, and it’s over expression has been shown to rescue the denervated blastema and promote regeneration of the entire missing part of the limb [14]. It belongs to the family of anterior gradient proteins originally defined by the XAG2 protein, which is expressed in the cement gland of Xenopus tadpoles [14].
Anura species are capable of complete limb and tail regeneration at premetamorphic stages. However, the ability to regenerate forelimbs, then hind limbs, and finally the tail is gradually lost in the course of development. If the hind limb of a X. laevis tadpole is amputated before Nieuwkoop and Faber stage 53, at which toes begin to form [10], it is regenerated completely. The period of development between stages 53 and 57 is refractory in terms that the limb does not regenerate at all, and only simple wound healing and skin repair occur on the amputation stump. After stage 57, a morphologically undeveloped cartilaginous spike covered with skin is formed in the amputation site; i.e., hypomorphic regeneration takes place [15].
It has been shown that regeneration of both a complete limb in anuran larvae (before stage 52) and of a spike at later developmental stages involves cell dedifferentiation, blastema formation, and nerve-dependent proliferation of blastemal cells; i.e., this process is typically epimorphic, as in Urodela [16,17].
According to Polezhaev [18]: the loss of limb regeneration capacity in X. laevis during ontogeny is apparently due to more advanced limb tissue differentiation. The genes responsible for cell dedifferentiation and proliferation in X. laevis tadpoles are expressed at a high level. Therefore, the block of limb regeneration in mature frogs may be explained by change in the pattern of their expression [17,18].
However, the molecular mechanisms controlling anteroposterior limb patterning during regeneration remain partially active in mature Anura. For example, the spikes regenerating after forelimb amputation in male Xenopus laevis sometimes contain nuptial pads, and the expression of Hox 13 gene (an autopod marker) and some other genes responsible for anteroposterior axis formation is observed in the blastema [17-19].
The cartilage of spike regenerates in X. laevis remains unsegmented, without articulations and developed perichondrium. This appears to be due to the lack of gdf5 and bmp4 gene expression, which is characteristic of the perichondrium of the developing limb bud [18].
Fibroblast growth factor (FGF-β) was used as a reference substance. There is evidence that the positional identity of limb blastemal cells is controlled by the FGF8 protein (together with other FGF-family proteins). [20,21]. This factor has been found in limb regenerates, and it has been shown that cytokines of the FGF family are involved in the initiation of mitotic activity in the regeneration blastema and in anteroposterior patterning of the regenerating limb. FGF2 treated cells generally produces higher amounts of osteogenic and chondrogenic differentiation than untreated stem cells [22].
In our previous study [5], MHTB-S and MHTB-B from mammalian tissues proved to have a strong protective effect on the tissue structure of Pl. waltl tail regenerates cultured in a roller system. Therefore, we used the same technique to study the influence of these factors on newt hind limb regenerates. MHTB-S was tested at two concentrations (10-1 and 10-12 mg/mL) to evaluate the dose dependence of its effect. MHTB was tested only at the ultralow concentration (10-12 mg/mL), since its high concentrations in previous experiments showed no significant effect on the state of cartilage tissue in regenerates cultured in vitro.
The results of this study allow us to conclude that the ability to regenerate hind limb structures in metamorphosing X. laevis depends on the level of amputation along the proximodistal axis. Stimulation of limb regeneration by either of the bioregulators has been found to decrease with an increase in the regeneration capacity of amphibians.
MHTB-S at a concentration of 10-1 mg/mL has a distinct morphogenetic effect on X. laevis limb regeneration in vivo in case of amputation in the proximal area and a protective effect on the tissues of Pl. waltl limb regenerates cultured in a roller system. MHTB-B at a concentration of 10-12 mg/mL also has a strong protective effect on the cultured newt limb regenerates, especially with respect to the viability and structural integrity of the cartilaginous tissue.
REFERENCES
- Carlson B? (2011) Principles of Regenerative Biology. Elsevier, London, Pg no: 400.
- Stocum DL (1996) A conceptual framework for analyzing axial patterning in regenerating urodele limbs. Int J Dev Biol 40: 773-783.
- Yamskova VP, Krasnov MS, Yamskov IA (2012) New experimental and theoretical aspects of bioregulation. Action Mechanism of Membranotropic Homeostatic Tissue-specific Bioregulators. Saarbrucken: Lambert Academic Publ, Pg no: 136.
- Krasnov ?S, Bogdanov VV, Kulikova ?G, Ilina ?P, Berezin BB, et al. (2014) Studies on the wound-healing effect of a new group of bioregulators extracted from mollusk tissues (Margaritifera margaritifera) and plants. Fundament Issled 5: 63-70.
- Yamskova VP, Krasnov MS, Yamskov IA (2010) On the mechanisms underlying the processes of tissue recovery and repair. Klet Tekhnol Biol Med 2010: 32-35.
- Tuchkova SY, Yamskova VP, Reznikova MM (1992) Effect of 6D polypeptide from mammalian blood serum on forelimb regeneration in Xenopus. Ontogenez 23: 319-320.
- Yamskova VP, Reznikova MM (1991) [A low-molecular polypeptide of the blood serum in warm-blooded animals: its effect on cell adhesion and proliferation]. Zh Obshch Biol 52: 181-191.
- Rybakova EY, Krasnov MS, Yamskova VP, Margasuk DV, Bitko SA, et al. (2009) A new bioregulator extracted from rat bone tissue: Physicochemical properties and the effect on cartilage tissue in vitro. Dokl Ross Akad Nauk 427: 136-138.
- Nieuwkoop PD, Faber J (1994) Normal Table of Xenopus Laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis. Garland Pub, Spokane, USA, Pg no: 252.
- Polezhaev LV (1973) Loss and Restoration of Regenerative Capacity in Tissues and Organs of Animals. The Quarterly Review of Biology 48: 33-34.
- Echeverri K, Tanaka EM (2005) Proximodistal patterning during limb regeneration. Dev Biol 279: 391-401.
- da Silva SM, Gates PB, Brockes JP (2002) The newt ortholog of CD59 is implicated in proximodistal identity during amphibian limb regeneration. Dev Cell 3: 547-555.
- Kumar A, Godwin JW, Gates PB, Garza-Garcia AA, Brockes JP (2007) Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science 318: 772-777.
- Sive HL, Hattori K, Weintraub H (1989) Progressive determination during formation of the anteroposterior axis in Xenopus laevis. Cell 58: 171-180.
- Slack JM, Beck CW, Gargioli C, Christen B (2004) Cellular and molecular mechanisms of regeneration in Xenopus. Philos Trans R Soc Lond B Biol Sci 359: 745-751.
- Endo T, Tamura K, Ide H (2000) Analysis of gene expressions during Xenopus forelimb regeneration. Dev Biol 220: 296-306.
- Suzuki M, Yakushiji N, Nakada Y, Satoh A, Ide H, et al. (2006) Limb regeneration in Xenopus laevis froglet. Sci World J 6: 26-37.
- Polezhaev LV (1977) Regeneration. Moscow: Znanie, Saint Petersburg, Russia.
- Gardiner DM, Blumberg B, Komine Y, Bryant SV (1995) Regulation of HoxA expression in developing and regenerating axolotl limbs. Development 121: 1731-1741.
- Nurtazin S?, Vsevolodov EB (2005) Biology of Individual Development. Almaty Kazak Univ, Kazakhstan, Russia Pg no: 231.
- Tsonis PA (2000) Regeneration in vertebrates. Dev Biol 221: 273-284.
- Lee TJ, Jang J, Kang S, Jin M, Shin H, et al. (2013) Enhancement of osteogenic and chondrogenic differentiation of human embryonic stem cells by mesodermal lineage induction with BMP-4 and FGF2 treatment. Biochem Biophys Res Commun 430: 793-797.
Citation: Aguillon-Gutierrez DR, Krasnov MS, Burlakova OV, Rybakova EY, Yamskova VP, et al. (2020) Effects of Endogenous Bioregulators from Mammalian Blood Serum and Bone Tissue on Amphibian Limb Regeneration. J Cytol Tissue Biol 7: 025.
Copyright: © 2020 David R Aguillon-Gutierrez, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

