
Efficacy of a Retinoid, Keratolytic, Anti-Inflammatory and Antibacterial Gel and Spray Formulations in Mild Common Acne
*Corresponding Author(s):
Massimo MilaniDepartment Of Medical Affair, Cantabria Labs Difa Cooper, Via Milano 160, Caronno P, Italy
Email:massimo.milani@difacooper.com
Abstract
Background and Objectives: For the treatment of mild/moderate acne, topical retinoids and antibacterial molecules are used in monotherapy or in combination. An exfoliating and anti-inflammatory action can increase the clinical efficacy of this therapeutic approach. A topical product in Gel and Spray Formulations (GF and SF) with retinoids (hydroxypinacolone retinoate and encapsulated retinol), with anti-inflammatory (niacinamide), antibacterial (biopep15) and keratolytic (glycolic and salicylic acids) activity has recently been developed. Topical retinoids have anti-inflammatory, anti-seborrheic and anticomedone-formation properties. Biopep15 is an oligopeptide with antibacterial action that can interfere with lipoteichoic acid, a component of the wall of Cutibacterium acnes. In addition, Biopep15 can perform also an antagonistic action against the Toll-Like-Receptor 2, involved in the pathogenesis of acne. Niacinamide has a well-known anti-inflammatory action. Salicylic and glycolic explain keratolytic and exfoliating activities. In this study the objective was to determine the efficacy and tolerability of GF and SF in mild/ moderate comedogenic acne.
Methods: In a 4-week, open-label, prospective trial, 32 patients between the ages of 15 and 30 have been evaluated. All participants gave their written consent. Treatment with gel (for facial lesions) and spray (for lesions located on thorax, back and shoulder) applied twice daily were used. To assess clinical efficacy, a count of comedogenic lesions (open and closed comedones; non-inflammatory lesions: NIL) and inflammatory lesions (IL; papules, and pustules) was performed and the reduction in the number of lesions after 2 and 4 weeks of treatment was evaluated. An evaluation of Total lesions count (TL; NIL+IL) was also performed.The lesion count data were analysed with a paired Student's t test.We evaluated also the exfoliating/keratolytic activity and the effect on sebum production assessed at baseline, after 2 and 4 weeks of treatment. Finally, to evaluate Cutibacterium acnes (C. acnes) skin colonization, we performed a fluorescence detection of skin porphyrin content at baseline and at day 28, by mean of Visiopor PP 34 camera.
Results: All patients completed the trial. At baseline the NIL, IL and TL count were 14.3, 8.7 and23, respectively. After 2 weeks of GF/SF treatment, NIL, IL and TL significantly decreased to 9.7 (-32%), 6.8 (-22%) and 16.5 (-29%), respectively. At the end of the treatment, a significant reduction in comparison with baseline was observed for NIL (-49%) IL (-63%) and TL (-54%). The exfoliating index evaluated in comparison with baseline value improved not significantly by 13% at day 14, and significantly (p=0.05) by 18% at day 24. The Cutibacterium acnes skin colonization area was significantly (p=0.02) reduced by 28% in comparison with baseline. Treatment was well tolerated, and local tolerability was assessed as optimal by all patients.
Conclusion: This new anti-acne combination formula based on retinoids, antibacterial oligopeptide, keratolytic and anti-inflammatory agents have shown high clinical efficacy and good tolerability in patients with mild to moderate acne. The treatment shows also a keratolytic effect and a significant reduction of C. acnes skin colonization.
Keywords
Acne; Clinical trial; Niacinamide; Topical retinoids
Introduction
Acne is a very common inflammatory skin disease affecting skin area rich in sebaceous glands, like face, thorax, shoulder and back [1]. Acne is mainly a disease of the pilosebaceous unit [2]. Hyperseborrhea, hyperkeratinisation, Cutibacterium acnes proliferation and inflammation are the four very well-known acne pathogenetic factors [3]. Specific anti-acne treatments should address ideally all these factors. For the treatment of mild/moderate acne, topical retinoids and antibacterial molecules are used in monotherapy or in combination [4]. Retinoid molecules, used orally or topically, are very effective in normalizing keratinocyte differentiation and proliferation therefore reducing the initial acne lesions (comedones) [5]. Anti-bacterial compound can reduce the presence of pathogenetic population of C. acnes [6]. An exfoliating and anti-inflammatory action can increase the clinical efficacy of this therapeutic approach. A topical product in gel and spray formulations (GF/SF) (BiretixTriactive®, Cantabria Labs, Madrid, Spain) with retinoids (hydroxy pinacolone retinoate and retinol encapsulated with glycospheres), with anti-inflammatory (niacinamide), antibacterial (biopep15) and keratolytic (glycolic and salicylic) activity has recently been developed. The gel formulation is used for acne lesions located on the face; the spray formulation is commonly used to treat large skin surfaces.The gel formulation of this product has already demonstrated to be efficacious and well tolerated in the treatment of common acne [7]. Topical retinoids have anti-inflammatory, anti-seborrheic and anticomedone formation properties [8]. Biopep15 is an oligopeptide with antibacterial action that can interfere with lipoteichoic acid, a component of the wall of Cutibacterium acnes [9]. In addition, Biopep15 can perform also an antagonistic action against the Toll-Like-Receptor 2, involved in the pathogenesis of acne and in the inflammatory process involved in acne lesion formation [10]. Niacinamide has a relevant anti-inflammatory action [11]. Salicylic and glycolic exert exfoliating activity [12]. Therefore, the composition of this innovative topical formulation could act against all the factors involved in the pathogenesis of common acne. Furthermore, the product is vehiculated as both a gel and a spray. The latter formulation could be a convenient topical composition for the treatment not only of the face but also for other area involved in the development of acne lesions such as thorax, shoulders, and the back [13].
Study Aim
To assess the clinical efficacy and tolerability of GF/SF in mild/moderate comedogenic acne.
Study Design
We conducted a 4-week, open-label, prospective trial, in subjects with mild-to-moderate acne.
Methods
The trial, conducted by Zurko Bioresearch CRO Company (Madrid Spain), was performed between January 2018 and March 2018.
Subjects
We have enrolled thirty-two subjects. All participants gave their written consent. Main inclusion criteria were, both sexes, aged between 15 and 30 years with mild to moderate common acne (< 100comedon lesions, < 50 inflammatory lesions, no more than 2 nodules).Main exclusion criteria were severe form of acne, pregnancy or breast-feeding, other clinically relevant skin diseases other than acne. Treatment with GF/SF (twice application per day on average depending on the extension of the affected area) was used in the most affected skin localization (GF for face; SF for back, chest and shoulders).
Study Outcomes
To assess clinical efficacy, a count of comedonic lesions (non-inflammatory lesions: NIL) and inflammatory lesions (IL; papules and pustules) was performed and the reduction in the number of lesions at 2 (day 14) and 4 weeks (day 28) of treatment was evaluated. Macro high-definition face pictures were performed for each enrolled subject at baseline and at day 28.An evaluation of Total lesions count (TL=NIL+IL) was also performed. We have also evaluated the exfoliating/keratolytic activity by mean of CorneofixF 20 (Courage+Khazaka GmbH) and the effect on sebum production evaluated by means of sebumeter (Sebufix F16; Courage+Khazaka GmbH) and the assessed at baseline, after 2 and 4 weeks of treatment. To evaluate P. acnes skin colonization, we performed a fluorescence detection of skin porphyrine content at baseline and at day 28.The skin porphyrin content was determined by Visiopor PP 34 camera (Courage + Khazaka, Cologne, Germany), which uses a specific UVA light (375 nm) with a measured area of 6 × 8 mm. The porphyrins are visible as fluorescent orange-red spots in the pores, which indicate the presence of P. acnes within and on the surface of the follicular impactions or comedones. The parameters analysed were the number and percentage of the area covered by orange-red spots.
Statistical Analysis
Statistical analysis was performed using GraphPad statistical software ver. 13.0 (La Jolla, CA, USA). Continuous variables were expressed asmean±SD. The primary endpoint of the trial was the evolution of acne lesions from baseline today 14 and day 28 (end of treatment). The Wilcoxon and the ANOVA tests were used for the analysis of the study outcomes (lesion count, corneometry, sebometry and porphyrin detection).
Results
All patients completed the trial. Treatment was well tolerated, and local tolerability was assessed as optimal by all patients.
Clinical efficacy on acne lesions
At baseline the number of NIL was 14.2±2.6 and the number of IL was 8.7±2.5 with a TL mean number of 23±3.The product induced a significant (p = 0.0001) reduction of NIL, IL and TL at both 14 and 28 days.NIL lesion count was reduced by32% at day 14 and by 49% at day 28. IL were reduced by 22% at day 14 and by 63% at day 28. The TL count was reduced by 29% at day 14 and by -54% at day 28. Figure 1 summarizes the evolution of acne lesions (NIL, IL and TL) from baseline and after 14 and 28 days of treatment. Treatment was well tolerated, and local tolerability was assessed as optimal by all patients. Figure 2 shows the pictures of three subjects at baseline and after 28 days of treatment.
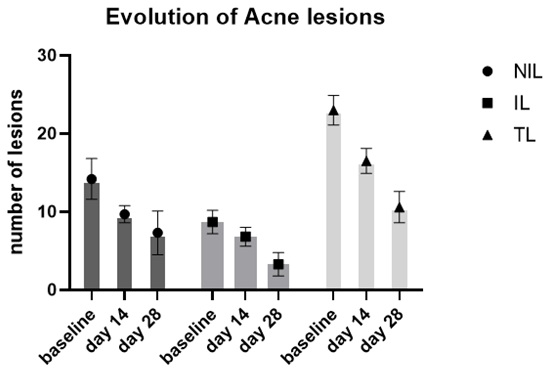 Figure 1: Figure legend: Evolution of NonInflammatory Lesions (NIL), Inflammatory Lesions (IL) and Total Lesions (TL) at baseline, after 14 and 28 days of treatment.
Figure 1: Figure legend: Evolution of NonInflammatory Lesions (NIL), Inflammatory Lesions (IL) and Total Lesions (TL) at baseline, after 14 and 28 days of treatment.
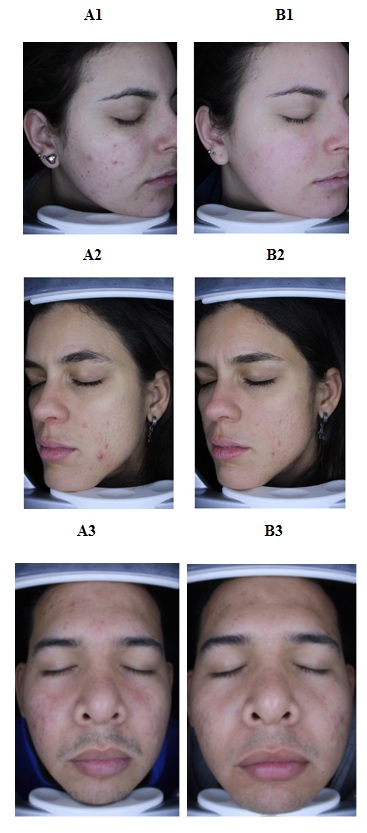 Figure 2: Figure legend: Pictures of three subjects at baseline (A1,A2, A3) and after 28 days of treatment (B1, B2, B3).
Figure 2: Figure legend: Pictures of three subjects at baseline (A1,A2, A3) and after 28 days of treatment (B1, B2, B3).
Exfoliating keratolytic effect
The exfoliating index evaluated in comparison with baseline value (mean value 5.1±1.9) improved not significantly by 13% at day 14,and significantly (p=0.05) by 18% at day 24.
Sebum production
The instrumental evaluation of seborrheadid not show significant differences between baseline value and after treatment. However, during treatment 38% (day 14) and 57% of subjects (day 28) presented an improved of seborrhea (reduction of area covered by sebum) in comparison with baseline.
acnes colonization: Porphyrin fluorescence detection
The use of the Visiopor PP34 camera at baseline and at day 28 showed that the intensity and the extension of fluorescence area were significantly (p=0.02) reduced by 28% in comparison with baseline. An improvement (i.e., reduction) in area covered by positive fluorescence was documented in 25 out of 32 subjects (76%).
Discussion
In this trial we demonstrated that a topical product in gel and spray formulation containing retinoid, keratolytic, anti-inflammatory and antibacterial compound is effective and well tolerated as monotherapy in subjects with mild to moderate common acne inducing a significant reduction in non-inflammatory and inflammatory acne lesions. In this trial we have also demonstrated that the product has also a significant keratolytic action and a direct anti C. acnes activity, supported by the data of porphyrin fluorescence. These two topical formulations contain retinoids, anti-inflammatory molecule (niacinamide) an antibacterial (biopep15) and finally two components (glycolic and salicylic) with keratolytic activity. Therefore,it could exert positive anti-acne actions toward all the relevant pathophysiology processes involved in the formation of acne lesions (comedo formation, seborrhea, inflammation, C. acnes proliferation). The clinical efficacy in term of percentage reduction of acne lesions is in line with the results of other topical drug products [14,15].
A relevant aspect to underline is the good skin tolerability of this formulation even if it contains retinoids and keratolytic agents. Topical retinoids are considered as first line therapy in mild and moderate acne [16]. However, especially during the very first days of therapy a low local skin tolerability could limit their use [17]. In this product the retinoid component (mainly hydroxypinacolone retinoate) is characterized by a tolerability profile better that other retinoid compounds [18,19]. In addition, the presence in the formulation of niacinamide, an anti-inflammatory molecule [20], could at least in part explain the good tolerability profile of the tested product. In evaluating the results of our study, we must consider some trial’s limitations. This was uncontrolled, not randomized trial. However, to improve the internal validity of the present study we performed instrumental evaluations such as corneometry, sebum production and porphyrine fluorescence evaluation. All these instrumental evaluations were in line with the clinical improvement regarding the evolution of acne lesions observed in the study.
Conclusion
This new anti-acne combination formula based on retinoids, antibacterial oligopeptide, keratolytic and anti-inflammatory agents have shown high clinical efficacy and good tolerability in patients with mild to moderate acne. The product reduces also significantly the colonization of the skin by C. acnes.
Statement of Ethics
The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. The subjects have given their written informed consent to publish their case including the images used in this article).
Study approval Statement
Ethics approval was obtained.
Consent to Publish Statement
Written informed consent was obtained from participants and in one case by legal guardians for publication of the details of their medical case and used images.
Conflict of Interest Statement
M.M., LC and MV are employees of Cantabria Labs group.
Funding Sources
This trial was funded by Cantabria Labs Difa Cooper.
Author Contributions
MM, LC and MV participate in the writing of the final manuscript and data analysis.
Data Availability Statement
Source data are available upon specific request.
References
- Mahto A (2017) Acne vulgaris. Medicine 45: 386-389.
- Williams HC, Dellavalle RP, Garner S (2012) Acne vulgaris. The Lancet 379: 361-372.
- Dagnelie MA, Corvec S, Khammari A, Dréno B (2021) Update on Cutibacterium acnes. In Acne, Springer, Cham, Page no: 1-15.
- Thielitz A, Gollnick H (2008) Topical retinoids in acne vulgaris. Am J Clin Dermatol 9: 369-381.
- Krautheim A, Gollnick HP (2004) Acne: topical treatment. Clinics in Dermatology 22: 398-407.
- Graber EM (2021) Treating acne with the tetracycline class of antibiotics: A review. Dermatological Reviews.
- Villani A, Annunziata MC, Cinelli E, Donnarumma M, Milani M, et al. (2020) Efficacy and safety of a new topical gel formulation containing retinol encapsulated in glycospheres and hydroxypinacolone retinoate, an antimicrobial peptide, salicylic acid, glycolic acid and niacinamide for the treatment of mild acne: preliminary results of a 2-month prospective study. G Ital Dermatol Venereol 155: 676-679.
- dos Santos Marubayashi BA, Simoni SE, de Fariña LO (2021) Topical treatments for acne: a bibliographic review. Brazilian Journal of Health Review 4: 3910-3922.
- Gabriel MTG, Oblepias MSM, Vitale M (2020) Safety and Efficacy of Retinsphere® Technology and Biopep-15 in the treatment of Acne in Asian Population. J Clin Cosmet Dermatol 4.
- Lotz S, Aga E, Wilde I, Van Zandbergen G, Hartung T, et al. (2004) Highly purified lipoteichoic acid activates neutrophil granulocytes and delays their spontaneous apoptosis via CD14 and TLR2. J Leukoc Biol 75: 467-477.
- Gehring W (2004). Nicotinic acid/niacinamide and the skin. J Cosmet Dermatol 3: 88-93.
- Merinville E, Laloeuf A, Moran G, Jalby O, Rawlings AV (2009) Exfoliation for sensitive skin with neutralized salicylic acid? International Journal of Cosmetic Science 31: 243-244.
- Sheth AH, Sen DJ, Doshi NB (2011) New Researches on Acne Vulgaris. Research Journal of Pharmacology and Pharmacodynamics 3: 48-57.
- Mcginley KJ, Webster GF, Leyden JJ (1980) Facial follicular porphyrin fluorescence: correlation with age and density of Propionibacterium acnes. Br J Dermatol 102: 437-441.
- Gollnick H, Schramm M (1998) Topical therapy in acne. J Eur Acad Dermatol Venereol 11: 8-12.
- Gollnick HP, Krautheim A (2003) Topical treatment in acne: current status and future aspects. Dermatology 206: 29-36.
- Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, et al. (2006) Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging 1: 327-328.
- Veraldi S, Barbareschi M, Guanziroli E, Bettoli V, Minghetti S, et al. (2015). Treatment of mild to moderate acne with a fixed combination of hydroxypinacolone retinoate, retinol glycospheres and papain glycospheres. G Ital Dermatol Venereol 150: 143-147.
- Bettoli V, Zauli S, Borghi A, Toni G, Ricci M, et al. (2016) Efficacy and safety of a 12-month treatment with a combination of hydroxypinacolone retinoate and retinol glycospheres as maintenance therapy in acne patients after oral isotretinoin. Giornale italiano di dermatologia e venereologia: organo ufficiale. G Ital Dermatol Venereol 152: 13-17.
- Wohlrab J, Kreft D (2014) Niacinamide - mechanisms of action and its topical use in dermatology. Skin Pharmacol Physiol 27: 311-315.
Citation: Milani M, Gonzales LC, Villalba M (2021) Efficacy of a Retinoid, Keratolytic, Anti-Inflammatory and Antibacterial Gel and spray Formulations in Mild Common Acne. J Clin Dermatol Ther 7: 089.
Copyright: © 2021 Massimo Milani, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

