
Journal of Clinical Studies & Medical Case Reports Category: Medical
Type: Case Report
Eltrombopag Induced Myelofibrosis in an Immune Thrombocytopenic Patient: Case Report
*Corresponding Author(s):
Iman MoustafaKing Abdulaziz Medical City, AlHasa, Saudi Arabia
Tel:+966 11 801 1111,
Email:emooo74@yahoo.com
Received Date: Jun 13, 2019
Accepted Date: Jun 25, 2019
Published Date: Jun 29, 2019
Abstract
Immune Thrombocytopenia (ITP) is a disease caused by autoimmune destruction. It impairs the production of platelets with the absence of other causes of thrombocytopenia. It causes a characteristic purpuric rash and increases the tendency for bleeding. A defect in Thrombopoietin-Receptor (TPO) / Myeloproliferative Leukemia Virus (MPL) / Janus Kinase 2 (JAK2) (TPO/MPL/JAK2) genes leads to some hematological disorders such as thrombocytopenia or pancytopenia through the inhibition of the megakaryopoiesis process. Thrombopoietin-Receptor (TPO) agonists as eltrombopag increase platelet counts by stimulating the TPO receptor. We reported this case to discuss the founding of eltrombopag related to myelofibrosis in an ITP patient.
In this article we presented; A 38- year- old male with chronic ITP treated with eltrombopag for three years with very good response, that ended by marked reticulin fibrosis of the Bone Marrow (BM ) grade 2/4, and atypical megakaryocytes, which did not reverse following discontinue the medication.
In conclusion
Eltrombopag may induce myelofibrosis after a long duration of treatment. We recommended that any patient administered TPO antagonist as eltrombopag, need to give him careful observation for any signs of bone marrow changes or any abnormal results in blood tests.BM biopsy will be needed for early discover for any type of BM fibrosis that may start firstly as an abnormal blood test. We have to discontinue the medication if myelofibrosis is established.
In this article we presented; A 38- year- old male with chronic ITP treated with eltrombopag for three years with very good response, that ended by marked reticulin fibrosis of the Bone Marrow (BM ) grade 2/4, and atypical megakaryocytes, which did not reverse following discontinue the medication.
In conclusion
Eltrombopag may induce myelofibrosis after a long duration of treatment. We recommended that any patient administered TPO antagonist as eltrombopag, need to give him careful observation for any signs of bone marrow changes or any abnormal results in blood tests.BM biopsy will be needed for early discover for any type of BM fibrosis that may start firstly as an abnormal blood test. We have to discontinue the medication if myelofibrosis is established.
Keywords
Eltrombopag; Immune thrombocytopenia; Myelofibrosis
INTRODUCTION
Immune Thrombocytopenia (ITP) is an autoimmune bleeding disorder that diminishes the production of platelets. It is a clinical syndrome that exhibits a tendency of bleeding , easy bruising (purpura), or blood extravasation from capillaries into the skin and mucous membranes (petechiae). Intracranial hemorrhage may occur when the platelet count drops below 10 × 109/L (< 10 × 103/µL) [1].
Therapy has to be initiated when the platelet counts fall to ? 20 to 30 x 109/L in a patient with no symptoms to reduce bleeding risk. The initial management of ITP is corticosteroid and Immunoglobulins (IVIG). The Food and Drug Administration (FDA) approved the orphan drug anti-D for the treatment of ITP for Rh-positive patient and who have not undergone a splenectomy. Rituximab reduces Immunoglobulin G (IgG) antibody used after corticosteroids are tapered and withdrawn. Splenectomy used in cases affected individuals fail to respond to steroids [2].
Unfortunately, 85% of patients lose response with corticosteroids within 6 to 12 months 33% of patient lack response to rituximab within 12 months and the third of patients relapse after splenectomy [3-5].
Other second-line treatments include azathioprine, cyclophosphamide, cyclosporine, danazol, mycophenolate mofetil, and Vincristine. Food and Drug Administration (FDA) in 2008 appeoved eltrombopag and romiplostim as a treatment for refractory chronic ITP. Eltrombopag is a Thrombopoietin (TPO) receptor, agonist. However; the development of bone marrow fibrosis is an immense concern [2].
Therapy has to be initiated when the platelet counts fall to ? 20 to 30 x 109/L in a patient with no symptoms to reduce bleeding risk. The initial management of ITP is corticosteroid and Immunoglobulins (IVIG). The Food and Drug Administration (FDA) approved the orphan drug anti-D for the treatment of ITP for Rh-positive patient and who have not undergone a splenectomy. Rituximab reduces Immunoglobulin G (IgG) antibody used after corticosteroids are tapered and withdrawn. Splenectomy used in cases affected individuals fail to respond to steroids [2].
Unfortunately, 85% of patients lose response with corticosteroids within 6 to 12 months 33% of patient lack response to rituximab within 12 months and the third of patients relapse after splenectomy [3-5].
Other second-line treatments include azathioprine, cyclophosphamide, cyclosporine, danazol, mycophenolate mofetil, and Vincristine. Food and Drug Administration (FDA) in 2008 appeoved eltrombopag and romiplostim as a treatment for refractory chronic ITP. Eltrombopag is a Thrombopoietin (TPO) receptor, agonist. However; the development of bone marrow fibrosis is an immense concern [2].
CASE REPORT
A 38 years male is known with DM type 2 and psychiatric illness. The patient was admitted in May 2015, under internal medicine with generalized ecchymosis and epistaxis. The lab tests values of White Blood Cells (WBC), platelets and Hemoglobin (hgb) are very low. The patient was diagnosed as acute ITP. He received immunoglobulin 1 gm/kg for 3 days in addition to steroids. Patient had good initial improvement. Corticosteroid increased the blood glucose level and the blood sugar cannot be controlled. The response of the patient was decreased under this line of treatment. We offered two management options to the patient. the first option was splenectomy, the second option medications like eltrombopag or romiplostim. He refused splenectomy so, we initiated eltrombopag. Eltrombopag was started as 50 mg orally daily and gave a good response. The case was stable from 2016 to 2019. In March 2019, after three years of good response with eltrombopag, the platelet count dropped and platelets did not increase by eltrombopag and petechiae appeared on the skin of the patient. The patient underwent a bone marrow biopsy that showed grade 2 of 3 myelofibrosis. Eltrombopag was discontinued, after few months of discontinuing; the biopsy was taken showing no improvement of bone marrow status.
The report from hematology included the following; With bone marrow trephine: the specimen gives good length fragment 34 mm in length examined at three levels intact architecture. Variable cellularity; most of the section has normal to increased cellularity (approximately 70%) alternating with a small area to reduced cellularity (nearly 30%). one small region shows in a fibroblastic activity. Normal erythroid and myeloid cells present. Megakaryocytes are increased in numbers with few loose clusters also evident. Nearly 10% of megakaryocytes exhibit dysplasia (hyperlobated megakaryocytes, mononuclearity, and wide separation of nuclei (Figure 1a).
The report from hematology included the following; With bone marrow trephine: the specimen gives good length fragment 34 mm in length examined at three levels intact architecture. Variable cellularity; most of the section has normal to increased cellularity (approximately 70%) alternating with a small area to reduced cellularity (nearly 30%). one small region shows in a fibroblastic activity. Normal erythroid and myeloid cells present. Megakaryocytes are increased in numbers with few loose clusters also evident. Nearly 10% of megakaryocytes exhibit dysplasia (hyperlobated megakaryocytes, mononuclearity, and wide separation of nuclei (Figure 1a).
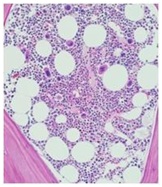
Figure 1a: HE staining scale bar 200: focus of dysplastic megacaryocytes.
With reticulin stain in most of the section, occasional fine individual fibers and foci of fine fiber network (grade 1/4) observed. In a few focal areas there is an increase in reticulin fibers in the form of diffuse fiber network with scattered thick coarse fibers (grade 2/4) (Figure 1b).
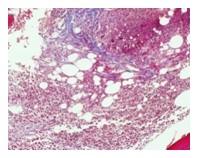
Figure 1b: Trichrome stain. Focal areas of peri-trabecular reticulin fibers accentuation and collagen formation.
Result of the pathology report: This is atypical morphology of megakaryocytes and increased fibrosis in ITP patients on treatment with thrombopoietin agonist (eltrombopag) this can lead to diagnostic confusion with a myeloproliferative neoplasm and less likely myelodysplasia.
By applied Naranjo Algorithm for Adverse Drug Reaction Probability Scaleto assess whether there is a causal relationship between myelofibrosis as an adverse effect and long administration of eltrombopag. The Naranjo Algorithm questionnaire result was scoring9 to give a definite adverse event. So there is a link between myelofibrosis and eltrombopag [6].
DISCUSSION
Immune Thrombocytopenia (ITP) is a disease caused by autoimmune destruction and impaired the production of platelets. ITP is distinguished by low platelet count with normal bone marrow and absence of any other causes of thrombocytopenia.
Defects in this TPO/MPL/JAK2 axis leads to hematological diseases such as thrombocytopenia or pancytopenia through the inhibition of the megakaryopoiesis process [7]. It causes a characteristic purpuric rash and increase the tendency of hemorrhagic. A platelet count less than 10 x 109/L was established as the threshold for diagnosis [2].
Recent advances in the understanding of ITP pathogenesis have highlighted the role of unfunctional platelet reproduction; this led to a new generation of Thrombopoietin (TPO)-receptor agonist therapies, including eltrombopag and romiplostim [2].
On August 24, 2015, the FDA approved eltrombopag for the treatment of thrombocytopenia in pediatric patients 1 year and older with ITP who have had an insufficient response to corticosteroids, immunoglobulin’s, or splenectomy [8]. Clinically eltrombopag demonstrates clear evidence that it produces a rapid and maintainable increase in platelet counts that reduces bleeding and well tolerated in ITP patients [7]. The mechanism of action of eltrombopag remains incompletely understood since it could not be studied in preclinical mouse models [9].
Eltrombopag bind to TPO receptor(c-MPL) on stem cell by activating Janus protein tyrosine Kinases (JAK) and subsequent downstream signaling leading to differentiation toward the megakaryocytic lineage, which mean eltrombopag induces human megakaryopoiesis. Megakaryopoiesis is the process leading to platelet production in the blood from the differentiation of bone marrow progenitors to platelet precursors called Megakaryocytes (MKs). The major cytokine regulating megakaryopoiesis is the Thrombopoietin (TPO) [7].
Myelofibrosis may be induced by a mutation in the Janus Kinase 2 (JAK2) gene. This gene controls certain enzymes that are involved in cell growth and the immune response or mutation in the gene called Calreticulin (CALR), which is involved in making proteins that needed for proper cell function, or in the thrombopoietin receptor gene (MPL), which is involved in cell growth [10].
Eltrombopag may increase the risk for development or progression of reticulin fiber deposition within the bone marrow [10]. Long-term use of Eltrombopag (in our case was three years) bone marrow changed by showing increased reticulin and bone marrow fibrosis (grade 2/4). Myelofibrosis considers life-threatening blood problems. So according to this abnormal biopsy of bone marrow, we discontinued Eltrombopag.
Defects in this TPO/MPL/JAK2 axis leads to hematological diseases such as thrombocytopenia or pancytopenia through the inhibition of the megakaryopoiesis process [7]. It causes a characteristic purpuric rash and increase the tendency of hemorrhagic. A platelet count less than 10 x 109/L was established as the threshold for diagnosis [2].
Recent advances in the understanding of ITP pathogenesis have highlighted the role of unfunctional platelet reproduction; this led to a new generation of Thrombopoietin (TPO)-receptor agonist therapies, including eltrombopag and romiplostim [2].
On August 24, 2015, the FDA approved eltrombopag for the treatment of thrombocytopenia in pediatric patients 1 year and older with ITP who have had an insufficient response to corticosteroids, immunoglobulin’s, or splenectomy [8]. Clinically eltrombopag demonstrates clear evidence that it produces a rapid and maintainable increase in platelet counts that reduces bleeding and well tolerated in ITP patients [7]. The mechanism of action of eltrombopag remains incompletely understood since it could not be studied in preclinical mouse models [9].
Eltrombopag bind to TPO receptor(c-MPL) on stem cell by activating Janus protein tyrosine Kinases (JAK) and subsequent downstream signaling leading to differentiation toward the megakaryocytic lineage, which mean eltrombopag induces human megakaryopoiesis. Megakaryopoiesis is the process leading to platelet production in the blood from the differentiation of bone marrow progenitors to platelet precursors called Megakaryocytes (MKs). The major cytokine regulating megakaryopoiesis is the Thrombopoietin (TPO) [7].
Myelofibrosis may be induced by a mutation in the Janus Kinase 2 (JAK2) gene. This gene controls certain enzymes that are involved in cell growth and the immune response or mutation in the gene called Calreticulin (CALR), which is involved in making proteins that needed for proper cell function, or in the thrombopoietin receptor gene (MPL), which is involved in cell growth [10].
Eltrombopag may increase the risk for development or progression of reticulin fiber deposition within the bone marrow [10]. Long-term use of Eltrombopag (in our case was three years) bone marrow changed by showing increased reticulin and bone marrow fibrosis (grade 2/4). Myelofibrosis considers life-threatening blood problems. So according to this abnormal biopsy of bone marrow, we discontinued Eltrombopag.
WHAT ARE NEW AND CONCLUSION
This case report will add evidence signifying that myelofibrosis is a serious and chronic, adverse effect due to treatment with TPO agonist. This major issue should be taken in our consideration. We recommended that any patient administered TPO antagonist need to give him required careful observation and the signs of bone marrow changes will show up as abnormal results in blood tests. BM biopsy may be needed and we have to discontinue the medication if this side effect is established.
ACKNOWLEDGMENT
We are grateful to Dr. M Taher Yacoubi and Dr. Safoorah Segheer for their kind participation in a pathology report.
REFERENCES
- Wenche JY, Horstman LL, Arce M, Ahn YS (1992) Clinical significance of platelet microparticles in autoimmune thrombocytopenias. J Lab Clin Med 119: 334-345.
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, et al. (2009) Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 113: 2386-2393.
- McCrae K (2011) Immune thrombocytopenia: No longer “idiopathic”. Cleve Clin J Med 78: 358-373.
- Patel VL, Mahévas M, Lee SY, Stasi R, et al. (2012) Cunningham-Rundles S,, et al. Outcomes 5 years after response to rituximab therapy in children and adults with immune thrombocytopenia. Blood 119: 5989-5995.
- Provan D, Stasi R, Newland AC, et al. (2000) The Four Branches of the Emotional Intelligence Ability Model Emotion Perception-Identification. 115: 1999
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, et al. (1981) A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 30: 239-245.
- Kühne T, Imbach P (2010) Eltrombopag: An update on the novel, non-peptide thrombopoietin receptor agonist for the treatment of immune thrombocytopenia. Ann Hematol 89: 67-74.
- FDA (2016) Eltrombopag approved in ITP. FDA, Maryland, USA
- Raslova H, Vainchenker W, Plo I (2016) Eltrombopag, a potent stimulator of megakaryopoiesis. Haematologica 101: 1443-1445.
- Highlights, Information P, Approval IUS, et al. PROMACTA (eltrombopag) tablets 2008.
Citation: Moustafa I, Fawzy ME, Badawy ME, Dossary I (2019) Eltrombopag Induced Myelofibrosis in an Immune Thrombocytopenic Patient: Case report. J Clin Stud Med Case Rep 6: 067.
Copyright: © 2019 Iman Moustafa, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

Journal Highlights
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!
