
Emerging pattern of Viral Diseases in a high Andean Microregion (Bolivia) producing potato seed-tubers
*Corresponding Author(s):
Coca Morante MDepartamento De Fitotecnia, Laboratorio De Fitopatología, Universidad Mayor De San Simón, Facultad De Ciencias Agrícolas Y Pecuarias, Bolivia
Email:m.cocamorante@umss.edu.bo
Abstract
In Bolivia, the commercial production of potato seed-tubers (Solanum tuberosum L) began in the high Andean area of Cochabamba. Production grew gradually and a small number of potato varieties are now cultivated, including Désirée (Dutch variety). Other high Andean areas of the country now also raise seed-tubers, but viral diseases have become a production-limiting factor. The present work provides an update on the incidence of four viruses that affect potato seed-tuber production, and examines the symptoms associated with infection plus the effect of these pathogens on potato yields in the area of influence of Lope Mendoza (a high Andean microregion of Cochabamba). Leaflet samples were collected from Désirée plants in eight communities (Lope Mendoza, Escalante, Chullchunghani, Phuyuhuasi, Qhollu Mayu, Cuesta Punta, Vélez Rancho and Chaupi Rancho) all in the Municipality of Pocona (Province of José Carrasco, Dept. of Cochabamba), and all within the above area of influence. All were analyzed for PVX, PVY, PLRV and APLV using DAS ELISA. PVX, PVY and PLRV were detected but not APLV. Some plants infected with these viruses showed typical symptoms, while others were asymptomatic. PVX+PVY and PVX+PLRV co-infections were also identified. In general, yields were low, possibly related to the incidence of infection. A new pattern of potato viral disease would appear to be emerging in this high Andean region, perhaps attributable to the gradual change in the varieties being cultivated, and to new possibilities of transmission and dissemination modes associated with changes in cultivation practices.
Keywords
Emergent Disease; Pattern Viral; Seed-Tuber.
Introduction
In Bolivia, the potato (Solanum tuberosum L.) is mainly cultivated in the high Andean region, at altitudes between 3000 and 4500 m. The mean area under potato production for the years 2010-2020 was 182,449 ha, and the mean national yield 5 t/ha [1]. According to the latter source, the Department of Cochabamba is the second most important area (with 37,000 ha devoted to potatoes) after that of La Paz (53,000 ha). In general, cultivation in Cochabamba occurs in the Andean-Amazonian transition zone, where high environmental humidity, permanent cloud cover and cold temperatures prevail (which unfortunately is also suitable for the appearance of potato late blight, caused by Phytophthora infestans) [2] (Figure 1). The area of influence around the Lope Mendoza community (altitude 2900-3500 m), in the Municipality of Pocona (Province of José Carrasco, Dept. of Cochabamba) (Figure 1), has one of the longest histories of producing Dutch varieties of potato seed-tuber in Bolivia [3,4]. Currently, the variety Désirée (S. tuberosum) and Huaych’a (S. andigena) are predominantly grown (other native varieties are little cultivated), but the former is more important in the formal certification system associated with the supply of tubers to the mesothermal valleys (altitude 1000-2000 m), the tropical east (350-450 m) of the Dept. of Santa Cruz, other high Andean areas, and the inter-Andean valleys.
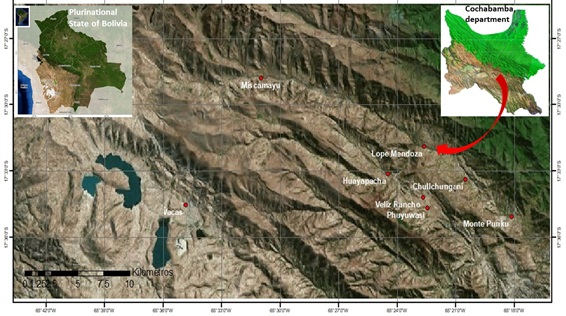 Figure 1: Location of the area of influence of Lope Mendoza (in the Municipality of Pocona, Province of José Carrasco, Dept. of Cochabamba, Bolivia.
Figure 1: Location of the area of influence of Lope Mendoza (in the Municipality of Pocona, Province of José Carrasco, Dept. of Cochabamba, Bolivia.
Like other areas in the Bolivian high Andean region, potato cultivation in the microregion of Lope Mendoza is affected by foliar pathogens and soil-borne diseases. The viral diseases affecting these crops have not been greatly studied, but Andean Potato Latent Virus (APLV), Andean Potato Mottle Virus (APMV), and Potato Leaf Roll Virus (PLRV) have been regarded as important pathogens, along with Potato Virus X (PVX) and Potato Virus Y (PVY) to a lesser extent [5] Over 40 years ago, [6] reported the most economically important virus to be PVY, with PVX, APMV and APLV also important in the highlands. [7] reported PVX, followed by Potato Virus S (PVS), to have the highest incidence in the Peruvian highlands, with PLRV, PVY, APLV and APMV showing intermediate to high values. In 1992, ELISA testing performed on material from the high Andes around Cochabamba (2900–3380 m) showed the most common viruses affecting the native Huaych’a (Solanum andigena L.) variety to be PVX, APLV, APMV and PVY, and to a lesser extent PVS and PLRV [8]. Later, [9] indicated the most common potato viruses in crops growing at altitudes >3000 m in Peru to be PVX (incidence 30–82%), PVS (20–50%), APMV (4–15%) APLV (2–6%), followed less frequently by PVY and PLRV. Similar viruses and incidences were found at higher altitudes in Ecuador. Finally, [10] reported the most common viruses in Bolivian microcentres of potato genetic diversity to be PVX, PVY and APLV, with PVY+PVX and PVY+PLRV mixed infections the most common in the Aymara region, and PVX+APLV and PVX+APMV in the Quechua region. The present work provides an update on the incidence of infection by these four viruses in the area of influence of Lope Mendoza - a potato seed-tuber-producing microregion - and examines their association with symptoms and yield.
Materials and Methods
Study area, collection of samples, and DAS ELISA analysis
In February 2018, three leaflet samples were taken at random from the mid-region of flowering Désirée plants cultivated for the production of seed-tubers in all plots in which they were detected in eight communities belonging to the area of influence of Lope Mendoza (Lope Mendoza, Escalante, Chullchunghani, Phuyuhuasi, Qhollu Mayu, Cuesta Punta, Vélez Rancho and Chaupi Rancho) (Table 1). This area of influence (17°32'58.87" S 65°22'0.08" W; altitude 2950-3170 m; (Figure 1) is characterized by permanent cloud, high humidity and cold temperatures (8-18oC), and is endemic for potato late blight (caused by P. infestans). A total of 108 samples were collected. At the sampling time, plants with viral symptoms (Mosaic, Leaf roll, Severe mosaic, Interveinal yellowing, etc.) and apparently healthy were identified. All were placed in BIOREBA Universal 12 × 15 cm extraction bags, following the manufacturer's protocol, to avoid contamination. These bags were then placed in a Tecnopor box containing ice and taken to the laboratory. The presence of PVY, PLRV, PVX and APLV in the leaf samples was sought using the Double-Antibody Sandwich-ELISA Kit from BIOREBA (these kits employ polyclonal antibodies conjugated with alkaline phosphatase for antigen capture and visualization), according to the manufacturer's instructions. Colorimetry was performed using a Labsystem Multiscan device. Frequency histograms were produced to define cut-offs and thus allow the detection of false negatives and positives. Cut-offs were determined using the formula mean+3s × 1.1, where 'mean' refers to the mean values for the negative control and 's' to its standard deviation. The incidence (percentage) of viral infection was determined as the number of positive samples/the total number of samples × 100.
|
Community / locality |
Altitude (m) |
Variety |
Origin / category |
Number of |
Total No. of samples |
|
|
|
|
|
plots sampled |
|
|
Lope Mendoza |
2950 |
Désirée |
own and non-certified seed |
6 |
18 |
|
Escalante |
3050 |
Désirée |
own and non-certified seed |
3 |
9 |
|
Chullchunghani |
2950 |
Désirée |
North of Santa Cruz and non-certified seed (*) |
9 |
27 |
|
Phuyuhuasi |
3100 |
Désirée |
North of Santa Cruz and non-certified seed (*) |
3 |
9 |
|
Qhollu Mayu |
3100 |
Désirée |
own and non-certified seed |
3 |
9 |
|
Cuesta Punta |
3050 |
Désirée |
own and non-certified seed |
6 |
18 |
|
Vélez Rancho |
3150 |
Désirée |
own and non-certified seed |
3 |
9 |
|
Chaupi Rancho |
3170 |
Désirée |
North of Santa Cruz and non-certified seed (*) |
3 |
9 |
Table 1: Communities where potato leaflet (S. tuberosum subsp. tuberosum) samples were taken within the area of influence of Lope Mendoza, Province of José Carrasco, Dept. of Cochabamba, Bolivia.
(*) North (450 msnm) Dept. of Santa Cruz, is lowland tropical region of Bolivia. Yield estimation (t.ha-1)
In each plot, yields were estimated at physiological maturity by examining a furrow length of 3 m (three repetitions per plot).
Results
Incidence of potato virus and mixed infections
PVX, PVY and PLRV were detected, but not APLV. PVX was the most widely distributed virus and showed the highest incidence in all localities (up to 100%). PVY was the second most commonly detected virus, and showed a variable incidence between localities (up to 33%). PLRV was detected in samples from only three localities (with Escalante the most affected) (Figure 2A). PVX+PVY and PVX+PLRV mixed infections were recorded, but not in all localities. The former was the more common (Figure 2B).
Symptomatology
PVX, PVY and PLRV were detected in asymptomatic plants, and in plants showing characteristic symptoms such as mild mosaicing, severe mosaicing, leaf roll, and interveinal yellowing. Severe mosaicing was the most common symptom (Figure 2C). Mild and severe mosaic symptoms are typical of PVX and PVY infections (Figure 2D).
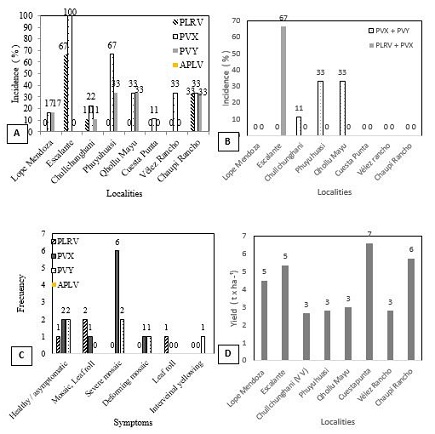 Figure 2: A: Viral diseases incidence by locality in the influence area of Lope Mendoza; B: Relationship foliar symptoms by each virus identified. Yield (txha-1) of potato Désirée (S. tuberosum) variety in different localities of Lope Mendoza high Andean influence area.
Figure 2: A: Viral diseases incidence by locality in the influence area of Lope Mendoza; B: Relationship foliar symptoms by each virus identified. Yield (txha-1) of potato Désirée (S. tuberosum) variety in different localities of Lope Mendoza high Andean influence area.
Discussion
PVX was the most commonly detected virus, followed by PVY and PLRV; APLV was not detected at all. PVX+PVY was the most common type of mixed infection. In the past, the most common infections were those caused by APLV, APMV and PLRV, with PVX and PVY only of minor importance [5]. Later [8] indicated PVX, APLV APMV and PVY to be the most common viruses, with PVS and PLRV less important (at least for the Huaych'a variety). Recently, [10] reported PVX, PVY and APLV to be the most important in the high Andean areas of Bolivia, with the most common mixed infections to be those of PVY+PVX and PVY+PLRV. The present results suggest that PVX, which showed incidence values up to 100% (Figure 2), has indeed become established as the most important of all potato viruses in the studied microregion.
PVY was also commonly detected, although to a lesser extent than PVX (incidence up to 33%). In the past, [6] reported it to be of little importance in the Andean highlands, and [5] indicated it to be the least important potato virus in Bolivia. [7] too indicated PVY to be of little importance, and [8] reported it be fourth in importance after APLV and APMV. Later, however, [9] noted the incidence of PVY to be increasing, and [10] reported it the most commonly detected virus after PVX. PVY would therefore appear to be increasing as a potato pathogen in the region.
In certain places, however, PLRV remains an important pathogen, with incidences of up to 67% (Figure 2). In the past, [5-9] all reported is to be of little importance; only suggested otherwise [10].
A high incidence (67%) of mixed PVX+PVY infection was noted in one locality, and lesser incidences (up to 33%) of PVX+PLRV infection in three others. [11] report that PVY causes more severe symptoms in mixed infections with other viruses, such as PVX. In agreement, [12] report that mixed-infections with PVX+PVYO or PVX+PVYNTN cause severe mosaic symptoms and systemic necrosis soon after inoculation.
Some of the plants in the present work were infected but remained asymptomatic (25%). [13] reported asymptomatic PVX, PVY and PLRV infections (incidence up to 17.5%) in Désirée and other varieties. PVX infection was associated with severe mosaicing, deforming mosaic, and leaf rolling. According to [14] PVX causes mosaic symptoms, including interveinal mosaicing and faint yellowing on young leaves.
PVY was seen to be associated with mosaicing and yellowing between the veins, while PLRV caused leaf roll symptoms (Figure 2B). According to [15], the N strain induces systemic vein necrosis in tobacco, but generally causes no hypersensitive reaction and only mild or zero symptoms in potato leaves. However, [16] report symptoms on the aerial part of the plant include leaf mosaicing, mottling, crinkling, and vein necrosis. In general, PVX and PVS are reported to cause mild mosaicing, while PVS and PVYNTN cause intermediate mosaicing, and PVYNTN+PVX and PVYO+PVS/PVX co-infections cause severe leaf deformation/necrosis/drop symptoms [12]. The most severe symptoms occurred in plants inoculated with PVX+PVYNTN, with dramatic synergism seen between these strains [12]. In highly susceptible varieties this co-infection can induce severe mosaicing, stunting, and yield losses of 5-20%. [16] reported that strong mosaic symptoms were most commonly seen with PVYO infection, and only seen with PVYN:O or PVYNTN in 15 and 3 varieties, respectively. [17] reported that symptoms consisted of mild to severe mottling, often associated with crinkling of the leaves, with yellowing and necrosis (vein necrosis and necrotic spots) commonly involving the lower leaves.
PVX is common worldwide, causing losses of 10–40% in single infections. It is, however, particularly damaging in combination with PVY [18] or Potato Virus A (PVA) [8]. PVY has been reported as the major viral threat to potato cultivation affecting both yield (with losses of up to 80%) and tuber quality [16]. Indeed, PVY is one of the 10 most important crop-plant viruses worldwide [19,20].
The present yields were relatively low (just 3-7 t.ha-1) the most common being around 4.4 t.ha-1 (Figure 2D). In 1954, the yields of 120 native potato varieties of the Bolivian Altiplano ranged from 1 to 7 t.ha−1, with 91 of these yielded between 1 and 5 t.ha−1 [21]. According to [21], these low yields were the consequence of “degenerative” diseases caused by viruses. In 2020, average yields were still just 5.5 t.ha−1 [22]. Currently, the Désirée variety is therefore doing even worse, a consequence of seed-tuber degeneration.
The present results suggest a new pattern of potato virus incidence to be emerging, at least, for this Andean microregion. The importance of PVX is maintained, that of PVY and PLRV virus appears to be increasing, while that APLV is decreasing (quite different to its wider distribution of the past; [5-10]. According to [8], PVY (especially) and PLRV are now the most damaging of all potato-infecting viruses. The causes favoring their emergence are complex [23,24] mentions how major agricultural changes play a pivotal role in promoting virus emergence and describes up to nine different scenarios that favor it, summarizable into four groups: (i) changes in the host plant and/or virus ecology; (ii) changes in the genetic composition of the host populations; (iii) changes in the genetic composition of the virus population; and (iv) in the case of vectored viruses, changes in the ecology and/or genetic composition of the vector [23]. In the present case, one factor could be the gradual change in the varieties grown. Traditionally, native varieties of S. andigena, such as Imilla Negra, Imilla Blanca and Qhoyllu Papas, etc., were once commonly grown in the studied microregion, but rural development projects aimed at improving growers' social and economic wellbeing, along with input from certain non-governmental organizations, began to introduce new, more productive varieties. Since end of the 1970s, Dutch potatoes varieties (S. tuberosum) such as Alpha, Radosa, Gineke and Spunta, etc., were introduced into the area of influence the Lope Mendoza [3], and in 1984 Diamant, Cardinale, Gigant, Monalisa, Baraka and Alpha were imported for planting [4]. The Alpha and Radosa varieties had a particularly strong economic impact. Later, in the 1990s, the Potato Seed Production Unit Project (UPS-SEPA) continued with the introduction of Désirée [4]. Thus, the potato production system has radically changed over the last forty years. Indeed, Désirée is now the most cultivated potato for seed-tubers within the certified seed system.
According to [25], Désirée plants vary in their resistance to virus diseases, e.g., from low to high resistance to PVX, medium to high to PVY, and very low to high to PLRV. The high incidence of PVX infection detected in the present work might be explained by the fact that this virus is transmitted mechanically and via infected tuber material, while PVY and PLRV, which were less frequent, are transmitted by grafting, sap inoculation and aphids [13]. In other species, PVY is also spread naturally, by vegetatively propagated material, and by aphids, and transmission by contact has even been reported [17]. The increasing presence of PVY and PLRV may also be due to aphids reaching higher altitudes, and by transmission through seed-tubers [9]. Certainly, different aphid species have been reported on native potato crops in the high Andean area of Cochabamba [26]. Transmission may thus be associated with the handling of seed potato tubers during harvest and post-harvest. According to [20], infected seed-tubers can be an important source of PVY inoculum; the virus then spreading within daughter and other potato crops in following seasons.
Conclusion
In conclusion, PVX, PVY and PLRV were detected, but not APLV. PVX was the most commonly found virus, with PVY and PLRV somewhat less common. PVX+PVY was the most common mixed infection. Some plants appeared to be asymptomatic when infected with PVX, PVY and PLRV, while others showed typical manifestations of disease. The low yields obtained might be a reflection of the effect of infection by these viruses (alone and in combination). The modes of transmission and dissemination of these pathogens might explain the gradual change in their incidence in the high Andean zone, at least for the microregion studied.
Acknowledgement
This research was funded by ASDI-UMSS via project PIA.ACC.06, which allows for research into the effects of climate change on Andean tuber crops. The authors thank Jaime Ortego and Marcelo Huarte, Senior Researchers at the Instituto Nacional de Tecnología Agropecuaria (INTA), Argentina, for suggestions, comments and revision of the manuscript. Thanks, also to Roger Fuentes Cadima, Director of Instituto de Investigaciones Agropecuarias, Facultad de Ciencias Agricolas y Pecuarias, Universidad Mayor de San Simon, for comments and suggestions. Thanks are also owed to Eulogio Arnéz of the Chullchunghani community, and Martin Claros Salazar, Agronomist and Director of the Oficina de Desarrollo Productivo in the Pocona Municipality for assistance in organizing the required fieldwork. Special thanks go to the Subcentral Campesina of Huayapacha in the Pocona Municipality, whose members helped in planning the field work, and to the plant pathology students of the Agriculture Faculty, San Simon University, for assistance in sample collecting and yield estimation. Finally, thanks are due to the Lourdes Zalles Cueto, haed of the immunology laboratory at The Labimed Laboratory, Faculty of Medicine-UMSS for assistance in reading virus plates.
Author’s Contribution
Conceptualization, Coca Morante M; Methodology, Coca Morante M, Lopez Salinas H, Tapia Ponce N; Formal Analysis, Coca Morante M, Lopez Salinas N; Data Curation, Coca Morante M; Writing-Original Draft Preparation, Coca Morante M; Review & Editing, Tapia Ponce N, Lopez Salinas H; Project administration and Funding Acquisition, Coca Morante M.
Conflicts of Interest
The authors declare no conflict of interest.
References
- INE (2022) Instituto Nacional de Estadísticas de Bolivia. INE, Bolivia.
- Morante MC (2016) Disease Note: First Records of Potato Late Blight Caused by Phytophthora infestans in Bolivia. J Plant Pathol Microbiol 7: 374.
- Asociación de Servicios Artesanales y Rurales (ASAR) (1983) Technical Report Carrasco project technical activities. Cochabamba ASAR, Bolivia 23.
- Acción Rural Agrícola Organizado (ARADO) (1985) Progress report production project plan seed potato seed supply emergency IBTA/ARADOM Cochabamba , Bolivia 12.
- Otazu V, Brown MW, Quiton MHD (1983) Enfermedades de las plantas en Bolivia. Ministerio de Asuntos Campesinos y Agropecuarios. Instituto Boliviano de Tecnología Agropecuaria/Consorcio Internacional para el Desarrollo. Cochabamba, Bolivia 30.
- Fribourg, C.E. 1980. Historia y Distribución de los Virus de papa en América Latina. Fitopatología. 15 (2): 13–24.
- Bertschinger L, Scheidegger UC, Luther K, Pinillos O, Hidalgo A (1990) La incidencia de virus de papa en cultivares nativos y mejorados en la sierra peruana. Revista Latinoamericana De La Papa. 3: 62–79.
- García, Gandarillas WYA (1992) Incidencia virotica en campos de tubérculo-semilla de papa en certificación y campos comerciales 21: 29–33.
- Kreuze, JF, Dias JACS, Jeevalatha A, Figueira AR, Valkonen JPT, et al. (2020) Chapter 11 Viral Diseases in Potato crop 389–429.
- Morante MC, Salazar EC, Villegas JB, Ponce NT (2021) Virus Incidence Associated with Native Potato Yield in Microcenters of Potato Genetic Diversity of Bolivian. American Journal of Potato Research 98: 384-394.
- Torrance L, Talianksy ME (2020) Review: Potato Virus Y Emergence and Evolution from the Andes of South America to Become a Major Destructive Pathogen of Potato and Other Solanaceous Crops Worldwide. Viruses 12.
- Nie X, Singh M (2013) Response of potato, tobacco and Physalis floridana plants to mixed infection with PVX, PVYNTN and PVY° strains. Canadian Journal Plant Pathol 35: 390–401.
- Deloko TDC, Achiangia NP, Chofong NG, Mbulli AI, Anoumaa M, et al. (2021) Prevalence of potato viruses on potato (Solanum tuberosum L.) grown in the Western Highlands of Cameroon. Journal of Agriculture and Food Research 5: 1-6.
- Daniela GR, Quintero OMA, Sánchez GP, Montoya MM (2016) Serological and molecular detection of Potato virus X (PVX) in seed potato tubers (Solanum tuberosum and Solanum phureja Juz. & Bukasov) from Antioquia, Colombia. Rev. Colomb. Biotecnol 18: 104-111.
- Julie Q, Vassilakos N, Moury B (2013) Potato virus Y: a major crop pathogen that has provided major insights into the evolution of viral pathogenicity. MOLECULAR PLANT PATHOLOGY 14: 439–452.
- MacKenzie TDB, Nie X, Bisht V, Singh M (2019) Proliferation of Recombinant PVY Strains in Two Potato-Producing Regions of Canada, and Symptom Expression in 30 Important Potato Varieties with Different PVY Strains. Plant Disease 103:2221-2230.
- Kerlan C, Moury B, 2008. Potato Virus Y. OA.MG 287-296.
- Lico C, Benvenuto E, Baschieri S (2015) The Two-Faced Potato Virus X: From Plant Pathogen to Smart Nanoparticle. Front Plant Sci 6.
- Scholthof KBG, Adkins S, Czosnek H, Palukaitis P, Jacquot E, et al. (2011) Review: Top 10 plant viruses in molecular plant pathology. Molecular Plant Pathology 12: 938–954.
- Lacomme Ch, Jacquot E (2017) General Characteristics of Potato virus Y (PVY) and Its Impact on Potato Production, An Overview. In: Lacomme C, Glais L, Bellstedt DU, Karasev BDAV, Jacquot E (Eds.), Potato virus Y: biodiversity, pathogenicity, epidemiology and management, 1-19.
- Rodríguez W (1954) Colección de ecotipos y especies indígenas de papa. Proyecto A-II.C-1, Subproyecto A-II-C-1a. Informe técnico Estación Experimental del Altiplano (Belén) 1953–54. Servicio Agrícola Interamericano. La Paz, Bolivia.
- Scott GJ (2010) Growth Rates for Potatoes in Latin America in Comparative Perspective: 1961–07. American Journal of Potato Research 88: 143-152.
- Elena SF, Fraile A, García-Arenal F (2014) Evolution and Emergence of Plant Viruses. Advances in Virus Research 88: 161-191.
- Jones RAC (2009) Plant virus emergence and evolution: Origins, new encounter scenarios, factors driving emergence, effects of changing world conditions, and prospects for control. Virus Research 141: 113-130.
- The European Cultivated Potato (2022) Desiree. The European Cultivated Potato, Europe.
- Evelyn CSL (2018) Incidencia de virus en papas nativas en comunidades del municipio de Colomi, Cochabamba. Facultad de Ciencias y Tecnología, Carrera de Biología. Universidad Mayor de San Simón. Tesis Licenciatura en Biología. Cochabamba, Bolivia.
Citation: Coca Morante, et al. (2023) Emerging pattern of Viral Diseases in a high Andean Microregion (Bolivia) producing potato seed-tubers. J Agron Agri Sci 6: 30.
Copyright: © 2023 Coca Morante M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

