
Endemic Plants from Mauritius Islands as Potential Resources for Sugar Substitutes
*Corresponding Author(s):
Bhajan CheetraUniversity Of Mauritius, Department Of Food And Agricultural Science, Mauritius
Email:cheetrabhajan@gmail.com
Abstract
The high prevalence of type 2 diabetes in Mauritius during the last decade has been an alarming situation that needs scrutiny for a healthier future Mauritian population. The widespread consumption of sugar combined with sedentary lifestyle is often associated with this problem and have led to the search for alternative sources of sweetness. To date, a countless number of sugar substitutes, both of natural and synthetic origins, have been introduced on the market. Many of these have the advantages of being sweeter than sugar, containing a low number of calories, as well as acting as prebiotics. One of the most common natural sweeteners consumed by Mauritians is stevia, while the most used artificial sweetener is aspartame. Lately, the safety of existing sweeteners has been a controversial subject and highly debated because various studies have suggested that some of them may also be the causative agents of disease like cancer. Hence, the search for new sugar substitutes that are devoid of health hazards is ongoing worldwide. The island of Mauritius is home to a plethora of endemic plants which has had little attention when it comes to its sweetening potential. Coincidently, some of these plants are from families that have been documented to possess sweeteners. These include economically important plant families such as the Asteraceae, Fabaceae, and Sapotaceae, among others. The review highlights the need for sugar alternatives in Mauritius while listing some Mauritian endemic plants that represent an array of potential for naturally occurring sweet biomolecules.
Keywords
Diabetes; Mauritian endemic plants sugar; Natural sweeteners; Substitutes
Introduction
Diabetes mellitus is the collective name of metabolic abnormalities primarily caused by a defect in the secretion of insulin hormone by the pancreatic islets. A decrease in the effectiveness of insulin on target tissues consequently leads to a series of irregularities that affect both the biochemistry and physiology of the human body. The disease is chiefly manifested in the form of elevated levels of blood glucose. Poorly controlled diabetes can lead to the damage of multiple organs, including the eyes, kidneys, nerves, and cardiovascular system [1] Among the two most common types of diabetes, Type 1 occurs when the insulin producing cells in the pancreas are destroyed by the immune system and there is usually no cure, while in Type 2 diabetes there is insulin resistance and relative insulin deficiency [2]. Genetic susceptibility and environmental influences seem to be the most common factors responsible for the development of the condition. Diet, on the other hand, is considered a universal factor linked to type 2 diabetes [3].
The multi-ethnic island Mauritius which is around 1.2 million according to World Bank, 2017, has an estimated 257,442 people between the ages of 25 and 79 years living with type 2 diabetes. The island was ranked fourth in the IDF's 2019 list of countries having the greatest prevalence of type 2 diabetes, with a rate of 22%. In 2020, the percentage climbed to 25.3%, and since the outbreak of COVID-19, these values are projected to change much further. Type 2 diabetes is the second leading cause of death on the island, with a death percentage of 24.1% in 2016. The disease kills one out of every four Mauritians. During World Diabetes Day in November 2017, it was stated by the Minister of Health that 450 amputations are due to diabetes, while around 1500 people with diabetes undergo dialysis annually. Furthermore, it is estimated that 30-40% of people with type 2 diabetes will develop chronic renal disease. There is also a high occurrence of pre-type 2 diabetes in the country, a condition associated with an increased risk of subsequent diabetes. These figures are alarming and constitute a significant threat in terms of the future social and economic burden of type 2 diabetes complications for Mauritius. This relates to both the direct medical costs and national productivity due to the impact of the disease on the workforce.
Sucrose, commonly known as table sugar, is the most widely used additive to provide sweet taste to edibles across the world. Unfortunately, its use has been linked to various disease states, including obesity, diabetes mellitus and tooth decay among others. Adramatic rise in the prevalence of type 2 diabetes mellitus worldwide has been paralleled by the increasing dietary consumption of sugar [4]. Due to ongoing climate change, Mauritius experiences unseasonably hot weather from November to April, with the highest temperature hovering around 38°C. Individuals with a preference for sweetened beverages are more likely to give in to their cravings during heat waves and drinking fizzy sugary drinks can end up contributing to most of a person’s daily sugar intake and the result is a higher risk of type 2 diabetes. The main source of sugar in Mauritius is cane sugar, although there are other sources. Being a tropical island with ideal conditions for sugarcane growth, the crop is presently cultivated on 72, 000 hectares, representing 85% of the arable land. An average of 600 000 tonnes of sugar is produced each year, of which about 40 000 tonnes are for domestic consumption and the rest are for export to the European Union. When refined, the sugar is made of pure carbohydrates, which has devastating effects on the body and health in general. It is considered "empty calories" as it offers no nutritional substance and only elevates blood sugar levels. Furthermore, during processing, there are harmful ingredients that are added to the sugar, such as phosphoric acid, sulfur dioxide, and formic acid [5].
Growing health awareness, increasing public interest in fitness and figures, and the rising imbalance between calorie consumption and expansion are factors that led to the demand for ood products that support better health. Alternative sources of sweetness that lack the disastrous effects of sugar help people satisfy their sweet tooth without suffering the aftermath. The use of sugar substitutes is one way to overcome the dangers of sugar consumption [6]. These are natural or chemical food additives that are used to mimic the sweet taste of sugar and enhance the flavor of foods and drinks. Sweeteners usually do not provide calories, do not influence blood glucose, and can be metabolized without insulin. Unlike sugar, they are thought to be non-contributive to tooth decay and obesity. However, the safety of these sweeteners has been dubious for a long time due to studies proving that they may lead to a number of diseases. Even the alleged health benefits of sweeteners regarding type 2 diabetes control have been lately subjected to clinical controversy. Despite the fact that sweeteners were originally devised to aid diabetics in controlling blood sugar, epidemiological data suggests the contrary. Though both forward and reverse causalities have been proposed, it is apparent that the rise in the percentage of the population that has diabetes correlates with the increase in the widespread consumption of food products sweetened with sugar substitutes.
Currently, about 677 plant species have been spotted in Mauritius, with 277 being endemic to the island and 147 exclusively found in the Mascarene Islands [7]. This represents a hub of Mauritian plants to be explored in the quest for biomolecules with sweetening capacity. Moreover, several of these endemic and indigenous plants belong to the same plant families from which natural sweeteners have been extracted in other countries. For example, the common natural sweetener Stevia is obtained from the plant Stevia rebaudiana, which belongs to the Asteraceae family. Psiadia arguta, Psiadia lithospermifolia, Psiadia viscosa, Distephanus populifolius, and Psiadia terebinthina are all Mauritian endemic plants that equally belong to the Asteraceae family but have not been thoroughly explored. Other plant families include the Fabaceae and Sapotaceae. After living an almost furtive life, hidden in unreachable regions, the Mauritian endemic species have a chance of getting recognised on the international level thanks to the work of dedicated specialists. The high rate of type 2 diabetes, coupled with the increasing interest in Mauritian endemic plants, creates opportunities for the discovery of novel sweeteners.
Sucrose Metabolism And Diabetes
Sugar has been used for sweetening worldwhile since ancient times and is highly sought for its taste and led to humans having a penchant for it [8]. These sugary foods can however take a toll on our health. Every gram of sugar provides the human body with 4 calories [9]. Over the years, its evolution in taste, availability, and popularity has made it an essential food ingredient and is now considered a necessity. Sucrose is composed of one molecule of glucose and one molecule of fructose linked by a glyosidic bond (Figure 1). While glucose is an important energy source that is needed by all the cells and organs of the human body, especially the muscles and brain, sucrose and fructose are not essential components of men’s feeding. Yet sucrose represents 10–25% of total energy intake in most parts of the world, and sweetened beverages are the major contributors [10]. There is strong evidence that the fructose component of sucrose is essentially responsible for the adverse metabolic effects of a high sucrose diet. 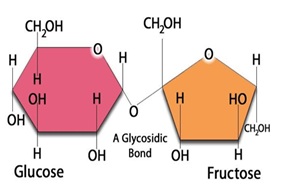
Figure 1: Structural formula of sucrose.
Upon ingestion, as sucrose passes through the gut, it is split into glucose and fructose for absorption into the portal blood (Figure 2). Glucose is the main catabolic and anabolic substrate for most organs in the body. It undergoes intestinal absorption, hepatic production, and extrahepatic usage, mainly by the brain, the skeletal muscle, and the adipose tissue. Once inside the intestinal lumen, glucose is taken up by enterocytes that express two glucose transporters: sodium-glucose co-transporter 1 (SGLT1 on the apical membrane) and GLUT2 (on the basal membrane). SGLT1 helps the transport of one glucose molecule and two sodium ions, which provides the energy to drive glucose accumulation in the enterocyte against is concentration gradient. Sodium is then transported into the blood vessels by the Na+/K+-ATPase. Glucose is phosphorylated and accumulates within the cell. Dephosphorylated glucose is then passively transported out of the cell through the basolateral membrane by GLUT2. As a result, accumulated glucose in the enterocyte diffuses out of the cell through GLUT2 into the blood stream. GLUT2 transports the glucose to the liver cells where it is converted into glucose-6-phosphate by glucokinase in the presence of insulin. Glucose-6-phosphate then enters the glycolysis pathway where it is converted into pyruvate. Fructose also undergoes intestinal absorption. It is then mainly mediated by the liver and, to a lesser extent, by the kidneys. In the intestinal lumen, fructose is taken up by enterocytes in the presence of GLUT5, where it is phosphorylated by fructokinase to fructose-1-phosphate using ATP as a phosphate donor and enters into the circulation through facilitated passive transport across enterocyte membranes by GLUT5 itself. Once absorbed in the intestine, fructose reaches the liver through the hepatic portal vein, where it is metabolized by hepatocytes. Glyceraldehyde and dihydroxyacetone phosphate are produced by the splitting of fructose-1- phosphate by the enzyme aldolase B. Triokinase catalyzes the phosphorylation oglyceraldehyde by ATP to form glyceraldehyde-3-phosphate. Thi process does not require the presence of the hormone insulin and does not depend on the energy status of liver cells. Therefore, all of the fructose that goes into the liver is metabolized into triose phosphates, which are secondarily converted into glucose, glycogen, lactate, and lipids. Due to the hepatic synthesis of this large amount of triose phosphates, which are lipogenic precursors, fructose is probably the most potent lipogenic nutrient in a diet [11]. A high amount of fructose consumption rapidly produces a slight but significant increase in fasting plasma glucose and in hepatic glucose production, leading to some degree of hepatic insulin resistance [12]. 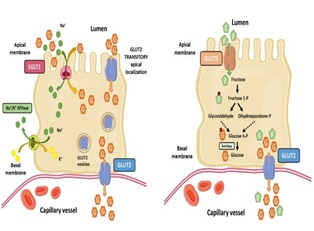
Figure 2: Uptake of glucose versus uptake of fructose by enterocytes [13].
There are several other mechanisms by which fructose may, in the long term, induce insulin resistance (Figure 3). Experiments showed that short-term fructose administration significantly increases intrahepatic and intramuscular lipid concentrations. Because ectopic lipid deposition seems to cause insulin resistance, the observation is a warning that the long-term effects of fructose consumption may be associated with significant lipotoxicity and eventually lead to more generalized insulin resistance. Another alarming fact about fructose is that its intake may contribute to the development of non-alcoholic fatty liver disease. Furthermore, high consumption of sucrose or fructose, when associated with a hyperenergetic diet, is expected to increase body weight and body fat mass, which may secondarily cause insulin resistance. Fructose administration produces oxidative stress through the generation of reactive oxygen species and triggers an endoplasmic reticulum stress response, which may be associated with impaired insulin signaling. Fructose also increases the production of uric acid because it is rapidly and completely phosphorylated to fructose-1-phopshate, which produces massive hepatic degradation of ATP to ADP and AMP. The ensuing hyperuricemia may cause endothelial cell dysfunction, resulting in impaire postprandial muscle vasodilation, and this phenomenon may also contribute to insulin resistance. 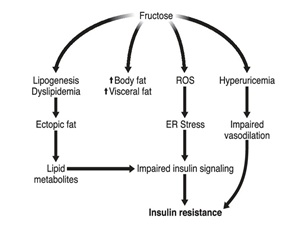
Figure 3: Potential mechanisms for fructose-induced insulin resistance.
ROS: Reactive oxygen species ER: Endoplasmic Reticulum
Sugar Substitutes
Sugar substitutes are usually made up of molecules other than sucrose, glucose, or fructose. Hence, they do not trigger a demand for insulin and lack the diabetes-causing effect with the additional benefit of being sweeter than sugar. They are required in smaller amounts to provide the same level of sweetness as sugar and have a low number of calories or may even have no calories. Existing sugar alternatives are either of natural or synthetic origin. These are intensively used in the food industry to sweeten sugar-free products such as baked goods, carbonated beverages, powdered drink mixtures, jams, jellies, and dairy products. Such products are usually consumed for weight loss, dental care, diabetes mellitus, and reactive hypoglycemia due to the small number of calories they contain. More than half of all human consumption is accounted for by the use of artificial sweeteners in beverages [14]. Artificial sweeteners are also available in tablets, powders, and liquids for use as table-top sweeteners. Other uses of artificial sweeteners are to mask the unpleasant tastes of pharmaceuticals, especially those used by diabetics. They are used in cosmetics, mainly in oral hygiene products like toothpaste and mouthwash. Artificial sweeteners provide health benefits but can be toxic at high concentrations in the long run.
According to studies, their consumption causes mild to severe side effects ranging from headaches to severe brain damage. Experiments showed that the artificial sweeteners saccharin, acesulfame-K and aspartame induced DNA damage in human peripheral lymphocytes, while sucralose has been found to deplete beneficial microorganisms [15].
The most popular artificial sweetener, aspartame, a non-nutritive artificial sweetener, was discovered in 1965. Even though aspartame contains the same 4 kcal/g as sucrose, it is 200 times sweeter. Therefore, the amount needed to attain the same degree of sweetness is so small that its caloric contribution is negligible [16]. Aspartame is made up of the amino acids L-aspartic acid and L-phenylalanine (Figure 4). When consumed, aspartame is hydrolyzed in the intestinal lumen and converted into the hydrolytic products phenylalanine (50%), aspartic acid (40%), and methanol (10%) [17]. These are further broken down to form formaldehyde, formic acid, and diketopiperazine [18]. Unlike sucrose, none of these processes are known to cause insulin resistance. However, studies suggest that the methanol produced contributes to toxicities to some extent. The hydrolysis of aspartame in the gut has been related to gastrointestinal problems. Moreover, the production of the amino acid phenylalanine can be hazardous to people suffering from the genetic disease phenylketonuria [19]. Phenylalanine has also been thought to cause brain damage as it plays an important role in neurotransmitter regulation. Hence, the possible harmful properties of synthetic sweeteners increase the demand for natural sweetening agents, especially those that are non-sacchariferous, since they are highly potent, useful, safe, and low-calorie sugar alternatives with fewer fatal to no side effects. 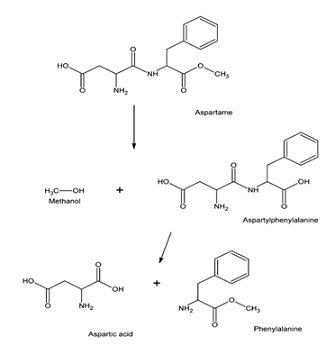
Figure 4: Structural formula of Aspartame and its hydrolytic products [20].
By the year 2002, over 100 plant-derived sweet compounds, of which 20 were major structural types had been isolated from around 25 different plant families. Several of these products are marketed as pure compounds, compound mixtures, or refined extracts, in different countries [21]. Plant extracts have also been used to screen compounds with inhibitory effects on intestinal GLUT2 and GLUT5 transporters for the prevention of fructose-induced diseases. In vitro studies showed that natural phenol phloretin of plants inhibits GLUT2 in mammalian cells. Chamomile tea containing epigallocatechin gallate effectively inhibits fructose transport through GLUT2. Green tea catechins on the other hand inhibit D-fructose. Chinese blackberry tea extracts prevented the transport of GLUT5-mediated fructose in liposomes reconstituted with human GLUT5. Likewise, astragalin-6-glucoside inhibited GLUT5-mediated fructose transport in proteoliposomes [22,23] found that demethoxycurcumin and curcumin from turmeric extracts inhibits fructose transport by GLUT2- and GLUT5-mediated fructose uptake, respectively. Catechin from guava leaf inhibits GLUT5-mediated fructose uptake, whereas quercetin inhibits both GLUT5- and GLUT2-mediated fructose transport. The effects of hesperidin, a flavonoid present in oranges, on fructose uptake in Caco-2 cell monolayers was studied by [24]. The compound inhibited fructose uptake in cells when fructose was the only source of sugar.
The most popular natural sugar substitute is stevia. Discovered in 1899, the zero-calorie natural sweetener has been used for centuries. It is obtained from the plant Stevia rebaudiana, the leaves of which are extracted and purified to obtain a high-purity stevia leaf extract containing steviol glycosides. Steviol glycosides pass through the gut intact. Upon reaching the colon, gut bacteria hydrolyze them into steviol, which is then absorbed via the portal vein and primarily metabolized by the liver, forming steviol glucuronide, which is excreted in the urine (Figure 5). Research shows that there is no accumulation of stevia or any component or by-product of tevia in the body after excretion [26]. Stevia seems to be gaining more popularity in many countries due to its unique characteristics of zero glycemic indexes and zero calories. 
Figure 5: Graphical representation of stevia pathway in the human body upon ingestion [26].
Steviol glycoside biosynthesis in Stevia appears to be a deviation from the biosynthesis of the plant growt hormone gibberellin [27].Following bifurcation, the steps catalyzed by ent-kaurenoic acid-13 hydroxylase and three UDP-glycosyltransferases encoded by genes SrKA13H, SrUGT85C2, SrUGT74G1, and SrUGT76G1 are involved in the synthesis of steviol glycosides. UGTs are multisubstrate acting enzymes that are both regio-specific and regio-selective. Previously, SrUGTs of steviol glycoside biosynthesis were investigated for their reactivity against various known substrates of the pathway [28]. SrUGT74G1 is predicted to catalyze the conversion 284 of steviol to 19-O-b-glucopyranosyl steviol. This compound is the source of rubusoside and stevioside. Furthermore, SrUGT74G1 is predicted to act on steviolmonoside to synthesize rubusoside. Rubusoside is metabolized again to stevioside. HPLC assays revealed that SrUGT74G1 has an equal affinity for steviolmonoside and steviolbioside. SrUGT74G1 reacts with steviolmonoside to produce rubusoside. Steviol glycosides and 289 gibberellins share a common origin. Following the common metabolite ent-kaurenoic acid, these biosynthetic routes split into two distinct pathways. (Figure 6). As a result, there is a direct metabolic flux of SrKA13H and SrUGT85C2 between the steviol glycoside and gibberellin biosynthetic pathways. This could be because these genes encode enzymes that catalyze the downstream steps of steviol glycoside biosynthesis. Data also suggests the presence of alternative steps in the biosynthesis of steviol glycosides 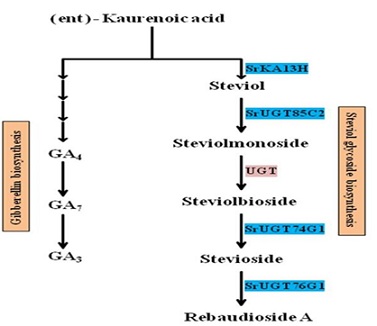
Figure 6: The steviol glycoside biosynthetic pathway is derived from the same commo substrate, ent-kaurenoic acid, which also provides the basis for the gibberellin biosynthetic route.
Apart from stevia and aspartame, many other sweeteners are now being discovered. In fact, the sugar substitute market is estimated to grow in developing countries due to the rise in health problems related to sugar consumption and increasing awareness and preference for low caloric food and beverage products. The global market for sugar substitutes has been growing exponentially in the last few years. The market was projected to be valued at USD 13.26 billion in 2015 and was expected to rise to USD 16.53 billion by 2020. The food industry has responded to people’s demand for sugar substitutes by producing sugar alcohols (polyols or polyhydric alcohols) as sweeteners. These are compounds produced by catalytic hydrogenation of carbohydrates, while some are also found in nature in fruits, vegetables, or mushrooms. These low digestible carbohydrates are obtained by the substitution of an aldehyde group with a hydroxyl one. The majority of sugar alcohols are synthesized from their corresponding aldose sugars [29]. Polyols are widely utilized in the food, beverage, confectionery, and pharmaceutical industries throughout the globe. They are actually used in bulk because they are less sweet than sugar and are known to promote dental health and exert a prebiotic effect. Their lower number of calories might help consumers reduce their energy intake and lose weight [30]. Sugar alcohols also do not induce a rise in blood glucose or insulin secretion and can be eaten by diabetics. Some sugar alcohols have even been found to increase the bioavailability of minerals in humans and rats. Due to slow and incomplete absorption in the intestine, sugar alcohols are low in nutritional value when compared to sugars and are thus metabolized through fermentative degradation by the intestinal flora. The products of fermentation include short-chain fatty acids and gases. Hence, polyols are known for their potent laxative effect and other gastrointestinal symptoms such as flatulence, bloating, and abdominal discomfort when consumed in excess. Products containing more than 10% of these polyhydric alcohols usually include the advisory statement "excessive consumption may produce laxative effects" [25]. Only seven sugar alcohols are defined as nutritive sweeteners according to EU legislation. These are sorbitol, mannitol, isomalt, maltitol, lactitol, xylitol, and erythritol.
Prebiotics are low-digestible food ingredients that are beneficial to the growth or activity of some probiotic bacteria in the intestine [31,32]. These bacteria usually include bifidobacteria and some gram-positive bacteria. Administration of sweeteners usually affects the intestinal microbiome. The roots of Stevia rebaudiana are known to contain inulin and fructans that have a positive effect on human health [20]. Fructans have fermentation capacity as they are used as substrates by gut microorganisms. Reported that fructans derived from S. rebaudiana improve the growth of bifidobacteria and lactobacilli that are important for bowel function. In the gut, the sweetener glycyrrhizin is de-glycosylated to glycyrrhetic acid by Eubacterium and Bacteroides and to 18-glycyrrhetic acid 3-O-monoglucuronide by Bacteroides and Streptococcus. The production of glycyrrhetic acid from 8-glycyrrhetic acid Omonoglucuronide is also mediated by Eubacterium spp. These glycyrrhizin metabolites are potent cytotoxic agents against tumor cells and exert potent inhibitory effects on viruses and platelet aggregation activity [33]. Some data suggest that the relationship between glycyrrhizin and the intestinal microbiota exerts positive effects on the host. Polyols, including isomaltose and maltitol as they reach the colon, increase bifidobacteria numbers.
Mauritian Endemic Plants As Target In The Search For Sugar Substitutes
The science of chemotaxonomy is employed for the classification of plants based on their chemical constituents. All plants produce secondary metabolites that are derived from primary metabolites. The chemical structure of the secondary metabolites and their biosynthetic pathways is often specific and restricted to taxonomically related organisms [34]. Many popular plant families have been studied through chemotaxonomy. It can be deduced that taxa (species, genera, and families) have characteristic compounds. Thus, it is highly expected that plants belonging to the same family produce many similar biomolecules due to analogous biosynthetic pathways. Biomolecules also include sweeteners. Figure 7 shows a schematic representation of the different molecules obtained from plants that are used as sewwteners, 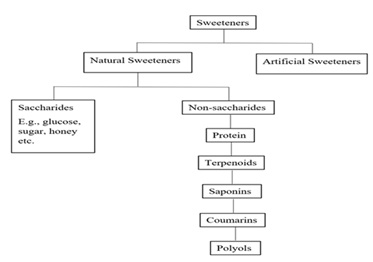
Figure 7: Schematic representation of plant derived sweeteners.
The African continent in which Mauritius is situated is diverse in plant species, and southern. Africa has even been reported to contain almost 10% of the world’s flora. The neighboring islands of Reunion, Rodrigues, and Madagascar equally harbor unique plant species, accounting for around 10,000 plants in total. Table 1 shows a list of some common natural sweeteners, their origins, and the plant families to which they belong, as well as Mauritian endemic and indigenous plants belonging to these plant families.
|
Natural Sweetener |
Type of Molecule |
Origin |
Plant family |
Endemic and indigenous plants from the same family |
Reference |
|
Glycyrrhizin |
Triterpenoid |
Roots of the South European plant Glycyrrhiza glabra (liquorice) root |
Fabaceae |
Albizia vaughanii (Apsus)
Gagnebina Pterocarpa (Acacia Indigene) |
[35] |
|
Miraculin
Glycyrrhizin |
Glycoprotein
Triterpenoid |
Triterpenoid The fruit of the west African plant Synsepalum dulcificum
Latex and fruit of Achras sapota and Lucuma glyciphylla
|
|
Sideroxylon grandiflorum (Tambalacoque)
Sideroxylon puberulum (manglier rouge)
Sideroxylon cinereum (manglier vert)
Labourdonnaisia calophylloides (Bois de Natte a Petites Feuilles) |
Theerasilp et al., 1989 |
|
Monati |
Indol derivates |
The root bark of the south African plant Sclerochiton ilicifolius |
Acanthaceae |
Barleria observatrix |
Vleggaar et al., 1992 |
|
Inulin
Stevia |
Polysaccharide
Glycosid |
The root of the European plant Cichorium intybus
The leaves of the American plant Stevia rebaudiana |
Asteraceae |
Psiadia arguta
Psiadia lithospermifolia
Psiadia viscosa
Psiadia terebinthina
Distephanus populifolius
Senecio lamarckianus (Bois Chevre)
Psathura borbonica (Bois Cassant) |
[35] |
|
Mannitol |
Sugar alcohol |
The bark of the European plant Fraxinus ornus |
Oleaceae |
Noronhia Thouars Noronhia broomeana
Noronhia obovate
Chionanthus broomeana (Bois de Cœur Bleu) |
[36] |
|
Naringin |
Flavonoid glycoside |
Citrus paradisi |
Rutaceae |
Euodia chapelieri
Euodia obtusifolia
Zanthoxylum heterophyllum
Toddalia asiatica (Patte Poule Piquant) |
Nielsen et al., 2006 |
Table 1: Some sweeteners, their origins, plant families to which they belong and Mauritian endemic and indigenous plants belonging to those plant families. 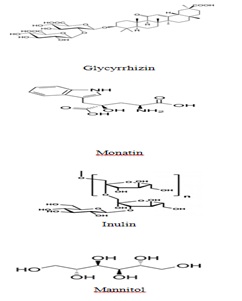
Figure 8: Molecular structure of plant-derived sweeteners.
Fabaceae
The Fabaceae, or Leguminosae, family is an important economic family of flowerin plants. It is the third-largest land plant family in terms of the number of species [37]. The family features nearly 700 genera and around 20,000 species of trees, shrubs, vines, and herbs. Several Leguminosae are staple human foods, and their use is closely associated with human evolution. Cereals, fruits, and roots have been the main products consumed from the plants in the family. The Fabaceae have high nutritional values due to their ability to fix atmospheric nitrogen for protein synthesis. This may lead to protein concentrations of up to 20% and 40% dry weight in leaves and seeds in many species. The family is also rich in therapeutic properties and hence has been proven to be effective against a wide variety of human ailments [38]. The medicinal uses of the family are usually associated with compounds such as tannins, flavonoids, alkaloids, and terpenes due to their high level of biological activity [39]. The sweetener glycyrrhizin (Figure 9), also known as liquorice, is a pentacyclic triterpenoid saponin glycoside obtained from the roots and stolons of the plant Glycyrrhiza glabra of the Fabaceae family. Glycyrrhizin has been found to be 100 times sweeter than sucrose. Along with being sweet, glycyrrhizin possesses various pharmacological properties such as anti-inflammatory, immunomodulatory, anti-ulcer, and anti-allergy activities. The most remarkable property of glycyrrhizin, however, is its anti-viral activity against various DNA and RNA viruses, including HIV and the Severe Acute Respiratory Syndrome (SARS) associated coronavirus. Therefore, a large amount of liquorice and its extracts are on the market both as sweetening and medicinal agents. Abrus precatorius, another plant of the Fabaceae family, is a climbing shrub with its leaves and roots abundant in sweet-tasting triterpene glycoside principles. The leaves taste sweeter than the roots as they contain triterpene glycosides called abrusosides, A, B, C, D, and E, while the roots ontain glycyrrhizin. Hence, the plant can also be used as a substitute for liquorice. The abrusosides A, B, C, and D sweeteners have been found to be 30, 100, 50, and 75 times sweeter nthan 2% w/v sucrose, respectively. Abrusoside E, on the other hand, is marginally sweet [40]. In Mauritius, the rare plants that exist from the Fabaceae family are Albizia vaughanii and Gagnebina Pterocarpa (Figure 8). The plants usually inhabit subtropical or tropical dry forests and are underexplored, so little is known about their constituents. Since the history of the Fabaceae family points towards the existence of sweeteners in its members, the plants are potential candidates in the search for sugar substitutes from Mauritian flora. 
Figure 9: Albizia vaughanii.
Sapotaceae
Sapotaceae is a latex-yielding plant family, consisting of around 35–75 genera and 800 species, most of which are tropical trees [41]. The family is known for the production of edible flowers, fruits, and oil seeds. It is also known for its wide range of chemical constituents, especially flavonoids and polyphenolic compounds, which have several biological activities, including antidiabetic activities. The plant Synsepalum dulcificum produces a fruit called “miracle fruit," native to tropical West Africa, where it is used locally to sweeten palm wine and other beverages. It was found that the protein miraculin, which is approximately 400,000 times sweeter than sucrose on a molar basis, was responsible for the sweet taste. The plant possesses other properties like antioxidant, antibacterial, and anticancer activities. The predominant form of the plant is shrubby, but it can grow up to 20 feet high, bearing fruits after 2-3 years of growing. It produces two types of fruits: red and yellow berries. Miraculin is found in the thin parenchyma layer surrounding the pit of the fruit. The miraculin present in the fruit has taste-modifying properties and transforms sour taste into a sweet taste that can last for up to two hours [42]. The sweetener consists of 191 amino acids linked with an N-linked oligosaccharide. These berries are considered a novel food and authorization is being sought for them to be introduced to the EU market. The Sapotaceae family also yields glycyrrhizin, especially in the latex, fruits, and barks of the plants Achras sapota and Lucuma glyciphylla [43]. The Mauritian endemic plant, Sideroxylon grandiflorum, belonging to the Sapotaceae family, is a green canopy tree that can grow up to 15 meters tall and its bark is dissociated into thin patches. The tree has multiple branches that are gnarled and covered with a fine gray duvet. Its leaves are arranged in spirals and are elliptical in shape. It produces flowers that are yellowish and grow in clusters on the stems. It also produces ovoid berries that are 5 cm long, reddish green, with a fleshy pericarp. To date, no particular part of the plant has been extracted for the search of biomolecules, which presents an opportunity to discover new compounds that have the potential to be sweeteners. Some other Mauritian endemic and indigenous plants belonging to the Sapotaceae family are Labourdonnaisia glauca, Labourdonnaisia revoluta, Mimusops petiolaris, Mimusops erythroxylum, Mimusops maxima, Sideroxylon cinereum, Sideroxylon grandiflorum, and Sideroxylon puberulum, among others. Another plant, Labourdonnaisia calophylloides (Figure 10), a tree of 20 meters that has a characteristic ornamental shape as its leaves bunch together at the very tips of each twig, is equally potent for the search for sweeteners. Much research has not yet been done on the plants and needs attention on a phytochemical basis. 
Figure 10: Labourdonnaisia calophylloides
Asteraceae
The Asteraceae family is abundant in flowering plants, accounting for almost 1600 genera and over 23,000 species. Some of these species, such as chamomile and wormwood, are highly aromatic and have previously been reported to have medicinal purposes. Many species of the Asteraceae family are good sources of natural antioxidants and are thus frequently consumed. The famous Stevia rebaudiana plant, from which the widely used sweetener stevia is obtained, belongs to the Asteraceae family. In fact, the plant is often known by the names "sweet herb," "sweet leaf," "honey leaf," or "candy leaf." The plant is usually small, herbaceous, semi-bushy, and perennial [44]. Leaves of the plant have been used since ancient times to cure many chronic and non-chronic diseases. Many studies have proved that the phytoconstituents of the plant are nontoxic. The biological molecule responsible for its sweetness is the glycoside stevioside, which is 100-350 times sweeter than sucrose. Along with its sweet taste, stevia leaves also have good solubility in water and are easily metabolized by the body. Furthermore, stevia leaves are rich in nutrients. They contain 80 to 85% water together with amino acids, proteins, fiber, lipids, essential oils, vitamins, and organic acids. The plant is even considered a good source of calcium, magnesium, potassium, iron, phosphorus, sulfur, and sodium. On a pharmacological basis, the plant is known to possess antioxidant, antimicrobial, antitumor, anti-inflammatory, antifungal, and anticarcinogenic activities [45]. Steviol glycosides are also involved in the enhancement of insulin production by directly acting on -cells without modifying the activity of K+-ATP channels and cAMP levels in the islets, thus proving its efficiency to cure diabetes [46]. Stevia and its derivatives have been shown to reduce the risk of hypertension and heart disease. Another sweetener, inulin, is also present in a number of Asteraceae plants. It was first discovered in the plant Inula Helenium of the Asteraceae. Inulin is used in processed foods to replace sugar, giving only 25–35% more energy as compared to carbohydrates. The sweetness level of inulin is about 10% that of sucrose. It has health benefits such as increasing mineral absorption, being fermentable, acting as a laxative and promoting the growth of microflora in the digestive tract. Being low in calories, it is often considered appropriate for diabetics. Some Asteraceae of Mauritius include Psiadia terebinthina, the leaf decoction of which is used against fever and asthma, Psiadia viscosa, the leaf infusion of which is used against asthma, and Psiadia arguta (Figure 11), among others [47]. Most of its plants are edible, making the Mauritian Asteraceae good candidates for sweetener identification. 
Figure 11: Psiadia arguta.
Acanthaceae
The Acanthaceae family (acanthus) is composed of aroundgenera and 2500 species, most of which are herbs and shrubs. The family generates dicotyledonous flowering plants. Plants in this family grow mainly in tropical countries, including Indonesia, Malaysia, Africa, Brazil, and Central America. Many plants belonging to this family have various medicinal uses due to the presence of important biomolecules. A highly sweet amino acid, monatin (Indol-3-yl)-2- amino-4-carboxy-4-hydroxypentanoic acid, was isolated from the roots of an African plant, Sclerochiton ilicifolius, of the family, a spiny-leafed hardwood shrub. Monatin showed a sweetness capacity of 1300 times that of sucrose. S. ilicifolius is also referred to as' Molomo monate ', from the Sepedi name meaning "mouth nice". The sweetener is compatible with other sweeteners like aspartame. Five oleanane-type triterpene glycosides named strogins 1–5 were also isolated from the leaves of Staurogyne merguensis of the Acanthaceae family. After strogins 1, 2, and 4 were held in the mouth, water elicited a sweet taste. Barleria Observatrix (Figure 12) of the Acanthaceae family is endemic to Mauritius. It has dark green, glossy leaves with pale blue-purple flowers. Once planted and exposed to the right conditions, it tends to be invasive, like most barlerias. The flowers are usually attractive to bees, butterflies, and/or birds due to their bright color and sweet scent. 
Figure 12: Barleria Observatrix.
Oleaceae
The olive family consists of about 29 genera and 600 species worldwide. The multipurpose jasmine plant comes from this family. In several species of the Oleaceae, the polyol manifold usually builds up as a result of abiotic stress. It is the only sugar alcohol that comes from natural sources. In the plants Fraxinus ornus and F. excelsior of the Oleaceae, it was found that the leaf mannitol content increases in response to summer drought conditions [36,48]. Mannitol is used both as a medication and to sweeten medicines, especially those for diabetics. Many organisms, including bacteria, yeasts, fungi, algae, lichens, and many plants, produce mannitol for use as an energy and carbon storage molecule. A mannitol cycle, which is a fructose to mannitol metabolic pathway, was discovered in a type of red algae (Caloglossa leprieurii), and it is highly expected that other microorganisms employ similar pathways. A class of lactic acid bacteria, capable of multiple fermentation pathways, has been found to be able to convert three fructose molecules into two mannitol molecules. The human body does not completely absorb the sweetener, and it has laxative effects. Endemic plants from the Oleaceae family that are present in Mauritius are Noronhia Thouars, Noronhia broomeana, Noronhia obovate (Figure 13) and Chionanthus broomeana (Bois de coeur bleu). 
Figure 13: A: Noronhia broomeana; B-C: Close-up of Noronhia macrophylla; D: Noronhia obovate [49].
Rutaceae
Rutaceae, commonly known as the rue or citrus family, is a family of flowering plants with approximately 160 genera and over 2000 species. Common fruits from this family include oranges (Citrus sinensis), lemons (Citrus limon), grapefruits (Citrus paradisi), and limes (Citrus aurantifolia). Many plants in the family have medicinal uses, and some varieties are so lacking in citric acid that they are known as sweet limes. Hesperidin, a sweetener, is the predominant polyphenol consumed from citrus fruits and juices. Additionally, high antioxidant activities of plants from the Rutaceae family were also determined by ferric reducing antioxidant power (FRAP) and 1,1-diphenyl-2-picryl hydrazyl (DPPH) radical scavenging activity [50]. The flavone glycoside naringin is a second sweetener found in grapes and citrus fruits and usually has two rhamnose units attached to an aglycon portion and naringenin at the 7-carbon position. Naringin content in various citrus species varies. Both naringin and naringenin are also strong antioxidants [51]. The sugar moiety in naringin exhibits steric hindrance of the scavenging group. Naringin is moderately soluble in water. The gut microflora breaks down naringin to its aglycon naringenin in the intestine; it is then absorbed from the gut [52-60]. The plants Euodia chapelieri and Euodia obtusifolia (Figure 14) belong to this family and are present only in Mauritius and the neighboring island of Reunion. 1 Little is known about the sweetener content in the plants; hence they remain to be explored. 
Figure 14: Euodia obtusifolia.
Other plants
Many Mauritian plants, other than the ones from the above families, have been consumed by local people. In particular, the plants are traditionally used in the form of infusions or decoctions of the various parts of the plants (e.g., leaves, roots, and flowers), either dried or fresh, to extract the active constituents into water. Following interviews done with local plant users and planters, it was determined that some other plant species bear epithets that may be indicative of a sweetness sensation. Moreover, many of these plants physically have sweet scents and have been observed to attract bees. Therefore, these plants can equally be considered as candidates in future investigation for potential sweet constituents. These include the leaves of Pittosporum senacia (Bois carrot) of the Pittosporaceae family [61-65], Eugenia bojeri (Bois de clou) of the Myrtaceae family, Cassine orientalis (Bois d'olive) of the Celastraceae family, fruits of Pandanus macrocarpa of the Pandanaceae family, Tambourissa quadrifida of Monimiaceae family, Stadmania oppositifolia of the Sapindaceae family (Figure 15), Coffea macrocarpa of the Rubiaceae family and flowers of Nesocodon mauritianus (Bloody bell flower) of the Campanulaceae family, Crinum mauritianum (Swamp-lily) of the Amaryllidaceae family and hibiscus Genevii of the Malvaceae family [66-72].
 Figure 15: Other Mauritian endemic plant species which are known to exhibit sweet taste. (Credit: All pictures of Mauritian endemic plants were taken at the Native Plant Propagation Centre of Mauritius).
Figure 15: Other Mauritian endemic plant species which are known to exhibit sweet taste. (Credit: All pictures of Mauritian endemic plants were taken at the Native Plant Propagation Centre of Mauritius).
Conclusion
Many sugar substitutes have been discovered in plants and have successfully been commercialized, especially in a variety of foods and beverages as well as in pharmaceuticals. Most of these sweeteners have been found to have numerous health benefits along with being non-sacchariferous, low-calorie, nutritive, and having a sweetness more intense than sugar. Some of these include antioxidant, prebiotic, and even antidiabetic properties. Natural sweeteners are also easily metabolized by the human body, while some are only partially metabolized and then excreted. Hence, plants will remain an essential component in the discovery and development of sugar substitutes. Most natural sweeteners have been isolated from tropical plants. Therefore, the presence of potential novel sweeteners in the rich tropical Mauritian endemic flora is highly anticipated. Molecules other than carbohydrates are to be targeted, such as proteins, which may give rise to sweeteners that lack the destructive attributes of sugar while being nutritive. The presence of such molecules in Mauritius is projected to leadto the alleviation of the rising diabetic burden of Mauritians while exposing the nutraceutical potential of underutilized endemic plants, thus valorizing the Mauritian flora.
Acknowledgements
I am grateful to the Ministry of Agro Industry, Food Production and Security for giving me the authorization to access the endemic plants habitat of Mauritius. I extend my gratitude to Miss Houshna Naujeer from the National Parks and Conservation Service who provided much support and valuable information about endemic plants. I also thank Mr Kersely Pynee and the nursery officers from the Native Plant Propagation Centre for helping in tracing out endemic plant species for photo shooting, generously donating photographs and accompanying in National Parks.
References
- Galicia GU, Benito VA, Jebari S, Larrea SA, Siddiqi H, et al. (2020) Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci 21: 62.
- Cantley J, Ashcroft FM (2015) Q&A: Insulin secretion and type 2 diabetes: Why do β-cells fail?. BMC Biol 13: 33.
- Ali O (2013) Genetics of type 2 diabetes. World J Diabetes 4: 114-123.
- Amin KA, Safwat G, Srirajaskanthan R (2012) High Sucrose Diet and Antioxidant Defense. Dietary Sugars 770-778.
- Kwong RNGK (2019) Sugar Industry. Society for Sugar Research and Promotion.
- McCain HR, Kaliappan S, Drake MA (2018) Invited review: Sugar reduction in dairy products. Journal of Dairy Science 101: 8619-8640.
- Bucktowar K, Bucktowar S, Bucktowar M, Chandy V (2016) Endemic Plants belonging to a Paradise Island-Mauritius. Journal of pharmacology and phytochemistry 6: 316-321.
- Caroline MC, Annette BMA, David M, Roder DM (2019) Who drinks sugar sweetened beverages and juice? An Australian population study of behaviour, awareness and attitudes. BMC Obesity 6.
- Cseke LJ, Kirakosyan A, Kaufman PB, Warber S, Duke JA, et al. (2016) Natural products from plants. CRC press.
- Malik VS, Popkin BM, Bray GA (2010) Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation 121: 1356-1364.
- Mayes PA (1993) Intermediary metabolism of fructose. Am J Clin Nutr 58: 754S-765S.
- Faeh D, Minehira K, Schwarz JM (2005) Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes 54: 1907-1913.
- Merino B, Fernández-díaz CM, Cózar-castellano I, Perdomo G (2020) Intestinal Fructose and Glucose Metabolism in Health and Disease. Nutrients 12: 94.
- Lipinski CA (2000) Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods 44: 235-249.
- Bandyopadhyay A, Ghoshal S, Mukherjee A (2008) Genotoxicitytesting of low-calorie sweeteners: Aspartame, acesulfame-K, and saccharin. Drug Chem Toxicol 31: 447-457.
- Cammenga, HK, Figura LO, Zielasko B (1996) Glasses of sugars and sugar substitutes. J Thermal Anal 47: 427-434.
- Humphries P, Pretorius E, Naudé H (2008) Direct and indirect cellular effects of aspartame on the brain. J Clin Nutr 62: 451-462.
- George V, Arora S, Wadhwa BK, Singh AK (2010) Analysis of multiple sweeteners and their degradation products in lassi by HPLC and HPTLC plates. J Food Sci Technol 47: 408-413.
- Heber D (2004) Vegetables, fruits and phytoestrogens in the prevention of diseases. JPostgrad Med 50: 145-149.
- Magnuson BA, Burdock GA, Doull J, Kroes RM, Marsh GM, et al. (2007) Aspartame: A safety evaluation based on current use levels, regulations, and toxicological and epidemiological studies. Toxicol 37: 629-727.
- Kim NC, Kinghorn AD (2002) Highly sweet compounds of plant origin. Arch pharm Res 25: 725-746.
- Campbell-Thompson M, Rodriguez-Calvo T, Battaglia M (2015) Abnormalities of the Exocrine Pancreas in Type 1 Diabetes. Curr Diab Rep 15: 79.
- Lee Y, Lim Y, Kwon O (2015) Selected Phytochemicals and Culinary Plant Extracts Inhibit Fructose Uptake in Caco-2 Cells. Molecules 20: 17393-17404.
- Kerimi A, Gauer JS, Crabbe S, Cheah JW, Lau J, et al. (2019) Effect of the flavonoid hesperidin on glucose and fructose transport, sucrase activity and glycaemic response to orange juice in a crossover trial on healthy volunteers. Br J Nutr 121: 782-792.
- EFSA Panel on Food Additives and Nutrient Sources (2010) Scientific Opinion on the safety of steviol glycosides for the proposed uses as a food additive. EFSA J 8: 1537.
- Edusky JS (2019) Stevia: A sweet Story. Scientificmind.
- Brandle JE, Telmer PG (2007) Steviol glycoside biosynthesis. Phytochemistry 68: 1855-1863.
- Humphrey TV, Richman AS, Menassa R, Brandle JE (2006) Spatial organization of four 752 enzymes from Stevia rebaudiana that are involved in steviol glycoside synthesis. Plant Mol Biol 61: 47-62.
- Mäkinen KK (2011) Sugar alcohol sweeteners as alternatives to sugar with special consideration of xylitol. Med Princ Pract 20: 303-320.
- Grabitske HA, Slavin JL (2008) Low-Digestible Carbohydrates in Practice Food Science and Nutrition. J Am Diet Assoc 108: 1677-1681.
- Lohner S, Toews I, Meerpohl JJ (2017) Health outcomes of non-nutritive sweeteners: Analysis of the research landscape. Nutr J 16: 55.
- Carocho M, Morales P, Ferreira ICFR (2017) Sweeteners as food additives in the XXI century: A review of what is known, and what is to Food Chem Toxicol 107: 302-317.
- Kim Dh, Hong SW, Kim BT, Bae EA, Park HY, et al. (2000) Biotransformation of glycyrrhizin by human intestinal bacteria and its relation to biological activities. Arch Pharm Res 23: 172-177.
- Ankanna S, Suhrulatha D, Savithramma N (2012) Chemotaxonomical studies of some important monocotyledons. Botany Research International 5: 90-96.
- Keerthi P, Gupta VRM, Srikanth K (2011) Natural Sweeteners: A Complete Review. Journal of Pharmacy Research 4: 2034-2039.
- Guicherd P, Peltier JP, Gout E, Bligny R, Marigo G (1997) Osmotic adjustment in Fraxinus excelsior L.: Malate and mannitol accumulation in leaves under drought conditions. Trees 11:155-161.
- Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ(2002) Plant Systematics, A Phylogenetic Approach, 2nd edition. Sinauer Associates, Sunderland, MA 576.
- Leonti M, Sticher O, Heinrich M (2003) Antiquity of medicinal plant usage in two Macro-Mayan ethnic groups (Mexico). J Ethnopharmacol 88: 119-124.
- Moerman DE, Pemberton RW, Kiefer D (1999) A comparative analysis of five medicinal Floras. Journal of Ethnobiology 19: 49-67.
- Douglas AK, Djaja DS (2002) Discovery of terpenoid and phenolic sweeteners from plants. Pure Appl Chem 74: 1169-1179.
- Evans WCT (1996) Pharmacognosy. 14 th edition. London, Philadelphia, Toronto, Sydney and Tokyo: WB Saunders Company Ltd 50.
- Jbrouwer Jn, Van DER Wel H, Francke A, Henning GJ (1968) Miraculin, the Sweetness-inducing Protein from Miracle Fruit. Nature 20: 373-374.
- Machadoa (1941) Chemical study of Brazilian licorice. Rev Soc Brasil Quim10: 101-103.
- Magnuson BA, Carakostas MC, Moore NH, Poulos SP, Renwick AG, et al. (2016) Biological fate of low-calorie sweeteners. Nutr Rev 74: 670-689.
- Lemus-Mondaca R, Vega-Gálvez A, Zura-Bravo L, Ah-Hen K (2012) Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensivereview on the biochemical, nutritional and functional aspects. Food Chem132: 1121-1132.
- Gupta E, Purwar S, Sundaram S, Rai GK (2013) Nutritional and therapeutic values of Stevia rebaudiana: A review. Journal of Medicinal Plants Research 7: 3343-3353.
- Gurib-Fakim A, Subratty H, Narod F, Govinden-Soulange J, Mahomoodally F (2005) Biological activity from indigenous medicinal plants of Mauritius. Pure Appl Chem 77: 41-51.
- Marigo G, Peltier, JP (1996) Analysis of the diurnal change in osmotic potential in leaves of Fraxinus excelsior. L. Journalof Experimental Botany 47: 763-769.
- Baider C (2014) Taxonomy and Conservation of the Genus Noronhia Thouars (Oleaceae) in Mauritius. Candollea 69: 157-163.
- Al-Ashaal HA, El Sheltawy ST (2011) Antioxidant capacity of hesperidin from Citrus peel using electron spin resonance and cytotoxic activity against human carcinoma cell lines. Pharmaceutical Biology 49: 276-282.
- Jung UJ, Kim HJ, Lee JS, Lee MK, Kim HO (2003) Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin Nutr 22: 561-568.
- Choudhury R, Chowrimootoo G, Srai K, Debnam E, Rice-Evans CA (1999) Interactions of the flavonoid naringenin in the gastrointestinal tract and the influence of glycosylation. Biochem Biophys Res Commun 265: 410-415.
- Affourtit C, Brand MD (2006) Stronger control of ATP/ADP by proton leak in pancreatic beta-cells than skeletal muscle mitochondria. The Biochemical Journal 393: 151-630.
- Ahmed SH, Guillem K, Vandaele Y (2013) Sugar addiction: pushing the drug sugar analogy to the limit. Curr Opin Clin Nutr Metab Care 16: 434-439.
- Alberti K, Davidson MB, Defronzo RA, Drash A, Genuth S, et al. (1998) Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 21: 5.
- Alves AS, Adão H, Ferreroc TJ, Marques JC, Costa MJ, et al. (2012) Benthic meiofauna as indicator of ecological changes in estuarine ecosystems: The use of nematodes in ecological quality assessment. Ecol Ind 24: 462-475.
- Andzouana M, Mombouli JB (2011) Chemical Composition and Phytochemical Screening of the Leaves of Hymenocardia ulmoides and Vitex ferruginea. Pakistan Journal of Nutrition 10: 1183-1189.
- Aronson M, Budhos M (2010) Sugar changed the world: A story of magic, spice, slavery, freedom and science. Clarion Books,Boston-New York 111-166.
- Ashwell M (2015) Stevia, Nature’s Zero-Calorie Sustainable Sweetener A New Player in the Fight Against Obesity. Nutr Today 50: 129-134.
- Benzie IFF, Strain J (1996) The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power: The FRAP Assay. Analytical Biochem 239: 70-76.
- Bizeau ME, Pagliassotti MJ (2005) Hepatic adaptations to sucrose and fructose. Metabolism 54: 1189-1201.
- Carakostas MC, Curry LL, Boileau AC, Brusick DJ (2008) Overview: The history, technical function and safety of rebaudioside A, a naturally occurring steviol glycoside, for use in food and beverages. Food Chem Toxicol 46: 1-10.
- Chattopadhyay S, Raychaudhuri U, Chakraborty R (2014) Artificial sweeteners-a review. J Food Sci Technol 51: 611-621.
- Craig ME, Hattersley A, Donaghue KC (2009) Definition, epidemiologyand classification of diabetes in children and adolescents. Pediatr Diabetes10: 1-2.
- Dirlewanger M, Schneiter P, Jequier E (2000) Effects of fructose on hepatic glucose metabolism in humans. Am J Physiol Endocrinol Metab 279: 907-911.
- Domingos AI, Sordillo A, Dietrich MO (2013) Hypothalamic melanin concentrating hormone neurons communicate the nutrient value of sugar. Elife.
- Erdal G, Esengün K, Erdal H, Gündüz O (2007) Energy use and economical analysis of sugar beet production in Tokat province of Turkey. Energy 1: 35-41.
- Gardana C, Simonetti P, Canzi E, Zanchi R, Pietta P, et al. (2003) Metabolism of stevioside and rebaudioside A from Stevia rebaudiana extracts by human microflora. J Agric Food Chem 51: 6618-6622.
- George Thompson AM, Iancu CV, Nguyen TT, Kim D, Choe JY, et al.(2015) Inhibition of human GLUT1 and GLUT5 by plant carbohydrate products; insights into transport specificity. Sci Rep 26: 12804.
- Goldstein RZ, Woicik PA, Moeller SJ (2010) Liking and wanting of drug and non-drug rewards in active cocaine users: The STRAP-R questionnaire. J Psychopharmacol 24: 257-266.
- Grenby TH (1987) Developments in sweeteners 3. Elsevier Science Publishing Co Inc New York 139-140.
- Hansotia T, Drucker DJ (2005) GIP and GLP-1 as incretin hormones: lessonsfrom single and double incretin receptor knockout mice. Regul Pept 128: 125-134.
Citation: Cheetra B, Joyce GS, Vijayanti Mala RS (2022) Endemic Plants from Mauritius Islands as Potential Resources for Sugar Substitutes. J Plant Sci Curr Res 6: 015.
Copyright: © 2022 Bhajan Cheetra, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

