
Enzymatic Hydrolysis of Various Pretreated Dried Distillers’ Grains with Solubles
*Corresponding Author(s):
Bishnu KarkiDepartment Of Biology And Microbiology, 1224 Medary Avenue # 228, P.O. Box 2104A, South Dakota State University, Brookings, SD, 57007, United States
Tel:+1 6056954744,
Email:bishnu.karki@sdstate.edu
Abstract
Keywords
INTRODUCTION
The environmentally-friendly process of using commercial enzymes to solubilize fibers can achieve high sugar yields in lignocellulose biomass (lignin, hemicellulose, and cellulose). However, enzymes cost is of a significant barrier in producing an economical product [3]. One way to overcome the high cost of enzymes is by lowering the enzyme loading rates or dosages. Additionally, application of pretreatment techniques prior to enzymatic saccharification can enhance the digestibility of the lignocellulosic fibers. Pretreatments should ideally use minimal amounts of energy and chemicals to limit the cost of the pretreatment process [4]. Mechanical process reduces the particle size and crystallinity by chipping, grinding, and milling to increase the surface area and reduce the degree of polymerization [5]. Milling alters the ultrastructure of lignocellulose and degree of crystallinity, causing cellulose to be more readily accessed and hydrolyzed by cellulase [6]. Liquid Hot Water (LHW) pretreatment is where the lignocellulosic biomass is saturated in water before it is heat treated under pressure to ensure the water stays in the liquid state and dissolves the hemicellulose fraction of the biomass into soluble oligosaccharides. Dilute acid pretreatment is where lignocellulose biomass is saturated with 0.2-2.5% w/w of acid and heat treated [7,8]. This process removes the hemicellulose and thus enhances the cellulose digestibility by providing the greater enzyme accessibility [9-12]. Dilute alkali pretreatment can be performed using low alkali concentration (i.e., 0.5-4%) and high temperatures [13,14]. This pretreatment will disrupt the lignin and xylan cross-linking ester bonds, increasing the porosity of the lignocellulose biomass [15]. This results in enriched fractions of cellulose and hemicellulose [16].
In this study, we analyzed the saccharification yields of the monosaccharides from pretreated DDGS using various commercial enzyme preparations and dosages. The goal was to screen various commercial enzymes at different dosages on pretreated and untreated DDGS to identify the best enzyme and lowest dosage needed to achieve the high amounts of monosaccharides from DDGS. Therefore, preliminary experiments were conducted to test the different commercial enzymes at various dosages on untreated DDGS. Once the enzyme and dosage were established, DDGS was pretreated by various physical and chemical treatments prior to enzyme saccharification.
MATERIAL AND METHODS
Enzymes
Enzymatic saccharification of untreated DDGS
Pretreatments
Enzymatic saccharification of the pretreated DDGS
Sugar analysis
Statistical analysis
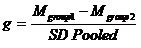
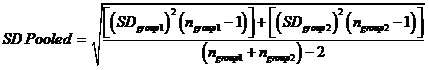
RESULTS
Preliminary screening of commercial enzymes on untreated DDGS
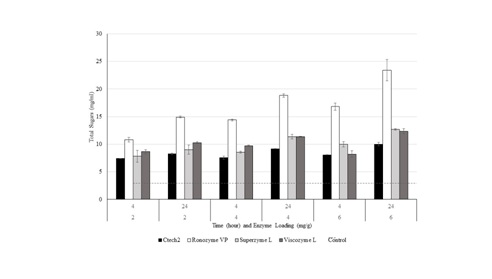
The control represents the DDGS without enzymes. Each data point represents the average of three replications and the error bars represent the standard deviation.
Enzymatic saccharification of pretreated DDGS
|
1Enzyme dosages for different pretreated DDGS sample |
Glucose (mg/ml) |
Total Sugars (mg/ml) |
|
Kady Mill |
||
|
0 |
1.87 ± 0.16nop |
7.60 ± 3.04lm |
|
0.125 |
6.88 ± 0.06hijk |
19.27 ± 3.81cdefgh |
|
0.25 |
11.93 ± 0.85cdef |
22.88 ± 2.45cdef |
|
0.5 |
12.11 ± 1.87cdef |
18.58 ± 1.03defghi |
|
0.75 |
12.05 ± 0.42cdef |
18.54 ± 2.78defghi |
|
1 |
13.39 ± 1.2bcd |
17.08 ± 0.56efghij |
|
Treated Kady Mill |
||
|
0 |
1.32 ± 0.9p |
9.38 ± 2.45klm |
|
0.125 |
6.48 ± 0.54ijkl |
15.93 ± 1.21ghijk |
|
0.25 |
8.55 ± 0.29fghi |
17.84 ± 0.06defghij |
|
0.5 |
12.25 ± 0.78cdef |
23.55 ± 1.39cdef |
|
0.75 |
13.64 ± 0.49bcde |
24.32 ± 0.84bcde |
|
1 |
14.2 ± 0.18bc |
23.58 ± 1.26bcdef |
|
0.5% Sulfuric Acid |
||
|
0 |
4.58 ± 0.17jklmno |
15.28 ± 2.37ghijk |
|
0.125 |
13.08 ± 0.56bcde |
35.42 ± 1.31a |
|
0.25 |
12.59 ± 0.41defg |
38.52 ± 4.18a |
|
0.5 |
12.38 ± 0.38cdef |
31.4 ± 1.13ab |
|
0.75 |
12.04 ± 0.61cdef |
25.77 ± 1.39bc |
|
1 |
17.4 ± 1.12a |
33.22 ± 3.32a |
|
Liquid Hot Water |
||
|
0 |
1.31 ± 0.19p |
11.78 ± 0.64jklm |
|
0.125 |
9.18 ± 2.0fghi |
24.57 ± 4.03bcd |
|
0.25 |
6.63 ± 0.82hijkl |
18.73 ± 2.49defgh |
|
0.5 |
7.67 ± 0.26ghij |
16.82 ± 3.55defghi |
|
0.75 |
9.86 ± 0.04efgh |
19.1 ± 1.25cdefgh |
|
1 |
15.88 ± 1.09ab |
22.38 ± 3.34cdefg |
|
4% Ammonium Hydroxide |
||
|
0 |
2.42 ± 0.12mnop |
5.63 ± 0.51m |
|
0.125 |
3.69 ± 0.22klmnop |
7.76 ± 0.92lm |
|
0.25 |
5.33 ± 0.77jklmn |
10.7 ± 1.28jklm |
|
0.5 |
5.65 ± 0.04jklm |
7.34 ± 1.1lm |
|
0.75 |
10.26 ± 0.22defg |
16.61 ± 0.81fghij |
|
1 |
11.24 ± 0.93cdef |
16.15 ± 0.1fghij |
|
1% Ammonium Hydroxide |
||
|
0 |
1.58 ± 0.06op |
5.32 ± 0.05m |
|
0.125 |
2.69 ± 0.14mnop |
6.97 ± 0.17m |
|
0.25 |
3.49 ± 0.14lmnop |
9.37 ± 0.26klm |
|
0.5 |
5.46 ± 0.42jklmn |
11.59 ± 2.72ijklm |
|
0.75 |
10.26 ± 0.61defg |
16.2 ± 1.11fghij |
|
1 |
9.17 ± 0.75fghi |
14 ± 2.04hijkl |
As seen in table 1, all the Viscozyme L dosages of 0.5% sulfuric acid total sugars were significantly different from the other pretreatments, but not between the different dosages. To determine the best dosage, glucose was compared from all pretreated DDGS. 0.5% sulfuric acid at 1 mg/g of Viscozyme L was significantly higher than all the other pretreatments.
DISCUSSION
Ronozyme VP performed the best out of all the enzymes tested. However, this enzyme is very low in protein content and is a powder feed enzyme that is meant to go on solid-state materials and not submerged-state materials. This was most prevalently seen as the powder had hydrophobic qualities, causing difficulties of completely dissolving the enzyme and evenly distributing it throughout the entire media. And since this was also a multienzyme complex, another enzyme was selected to be tested on the pretreated DDGS. Superzyme L and Viscozyme L were the next candidates and it was determined that Superzyme L did well at high dosages while Viscozyme L did well at low dosages. Viscozyme L also has known lignocellulose enzymes versus Superzyme L, which is a multienzyme complex.
Viscozyme L was continued on the pretreated DDGS with lower dosages. Overall, 0.5% sulfuric acid at 1 mg/g of Viscozyme L was the most effective when total sugars and glucose was taken into consideration. 0.5% sulfuric acid with Viscozyme L at 1 mg/g resulted in the total sugar yields of 33.22 ± 3.32 mg/ml while untreated DDGS at 2 mg/g produced 8.65 ± 0.36 mg/ml total sugars. This is a ~280% increase with a lower enzyme dosage. The pretreatment that did not have significant impact on total sugar yields was the dilute alkali pretreatment. One of the reason for this is monosaccharides, like galactose, mannose, and glucose, are rapidly destroyed by the hot aqueous alkaline solution used with dilute alkali pretreatment [23]. This was also reflected by the low amount of glucose on both the dilute alkali pretreated DDGS.
CONCLUSION
ACKNOWLEDGMENT
Funding support was provided by the South Dakota Agricultural Experiment Station under grant SD00H616.
REFERENCES
- Renewable Fuels Association (2017) Building Partnerships| Growing Markets: 2017 Ethanol Industry Outlook. Renewable Fuels Association, Washington, DC, USA.
- US Grains Council (2012) A guide to distiller's dried grains with solubles (DDGS). US Grains Council, Washington, USA.
- Fang X, Yano S, Inoue H, Sawayama S (2009) Strain improvement of Acremonium cellulolyticus for cellulase production by mutation. J Biosci Bioeng 107: 256-261.
- Mosier N, Wyman C, Dale B, Elander R, Lee YY, et al. (2005) Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol 96: 673-686.
- Tassinari T, Macy C, Spano L, Ryu DD (1980) Energy requirements and process design considerations in compression?milling pretreatment of cellulosic wastes for enzymatic hydrolysis. Biotechnology and Bioengineering 22: 1689-1705.
- Mais U, Esteghlalian AR, Saddler JN, Mansfield SD (2002) Enhancing Enhancing the enzymatic hydrolysis of cellulosic materials using simultaneous ball milling. Appl Biochem Biotechnol 98: 815-832.
- Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol Bioethanol production from agricultural wastes: An overview. Renewable Energy 37: 19-27.
- Cardona C, Quintero J, Paz I (2010) Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresource Technology 101: 4754-4766.
- Brownell HH, Saddler JN (1984) Steam-explosion pretreatment for enzymatic hydrolysis. Biotechnol Bioeng Symp 14.
- Converse AO, Grethlein HE (1985) Process for hydrolysis of biomass. US4556430A, Dartmouth College, USA.
- Grous WR, Converse AO, Grethlein HE (1986) Effect of steam explosion pretreatment on pore size and enzymatic hydrolysis of poplar. Enzyme and Microbial Technology 8: 274-280.
- Knappert D, Grethlein H, Converse A (1981) Partial Partial acid hydrolysis of poplar wood as a pretreatment for enzymatic hydrolysis. Biotechnol Bioeng Symp 11.
- Cheng KK, Zhang JA, Ping WX, Ge JP, Zhou YJ, et al. (2008) Sugarcane Sugarcane bagasse mild alkaline/oxidative pretreatment for ethanol production by alkaline recycle process. Appl Biochem Biotechnol 151: 43-50.
- Mirahmadi K, Kabir MM, Jeihanipour A, Karimi K, Taherzadeh M (2010) Alkaline pretreatment of spruce and birch to improve bioethanol and biogas production. BioResources 5.
- Tarkow H, Feist WC (1969) A mechanism for improving the digestibility of lignocellulosic materials with dilute alkali and liquid ammonia. Advances in Chemistry 95: 197-218.
- Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83: 1-11.
- Maher JM, Markey JC, Ebert-May D (2013) The other half of the story: effect size analysis in quantitative research. CBE Life Sci Educ 12: 345-351.
- Hedges LV (1981) Distribution theory for Glass's estimator of effect size and related estimators. Journal of Educational and Behavioral Statistics 6: 107-128.
- Cohen J (1969) Statistical power analysis for the behavioral sciences (2ndedn). Lawrence Erlbaum Associates, Inc., New York, USA.
- R Core Team (2011) R: A language and environment for statistical computing. Global Biodiversity Information Facility, Vienna, Austria.
- Felipe M (2013) Agricolae: statistical procedures for agricultural research. R package version 1:1-4.
- Freund R, Mohr DL, Wilson W (2010) Statistical Methods (3rdedn). Elsevier Publishing company, New York, USA.
- Kaye GI, Weber PB, Wetzel WM (2004) The alkaline hydrolysis process. ALN Magazine, USA.
Citation: Baldwin EL, Karki B, Iten L, Gibbons WR (2018) Enzymatic Hydrolysis of Various Pretreated Dried Distillers’ Grains with Solubles. Adv Ind Biotechnol 1: 004.
Copyright: © 2018 Emily L Baldwin, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

