
Epidemiological Characterization of Dengue in South Andaman Islands, India: An Update
*Corresponding Author(s):
Vijayachari PDepartment Of Medical Microbiology & Molecular Biology, ICMR-Regional Medical Research Centre, Port Blair -744103, Andaman And Nicobar Islands, India
Tel:+91 3192 251158 / +91 3192 251164,
Fax:+91 3192 25116
Email:directorrmrc@gmail.com
Abstract
Background: Dengue is a vector-borne viral infection and has become a significant tropical disease with increased recognition of atypical manifestations apart from the classical clinical features. A study was conducted to determine the dengue positivity rate among patients with clinical symptoms of dengue fever-like illness, visiting hospitals in South Andaman.
Methods: Hospital-based study was conducted in two tehsils of the South Andaman district, Andaman and Nicobar Islands, India, for four years, from 2018 to 2021. Serum samples from 10,313 suspected dengue cases were collected during the study period. Samples were subjected to NS1 and IgM ELISA tests to detect dengue antigens/ antibodies. The symptoms of all the cases were recorded and analyzed for their severity.
Results: Among 10,313 suspected patients screened, 679 (71%) were detected with dengue infection. Of these, 485 and 194 were positive for primary and secondary dengue respectively. Dengue haemorrhagic fever was observed in 10% of positive cases. Individuals in the age group of 21-30 years were more affected by dengue.
Conclusion: Dengue infection was observed to be high in South Andaman. In-depth epidemiological monitoring of dengue viral infection at regular intervals is essential to undertake effective control and management strategies.
Keywords
Dengue; Primary dengue; South Andaman; Virus
Introduction
Dengue outbreaks are known for their periodicity, and are the most important neglected viral disease of recent times, transmitted by mosquito vectors, and causing over one hundred million cases annually, especially in the tropical and sub-tropical countries [1]. Dengue fever has disseminated to more than 120 nations, making almost half of the world's population at risk. Dengue has now emerged as a public health problem due to its spread to more geographic areas, mainly through extensive human movement [2]. The severity of infection and transmission intensity has increased significantly in endemic areas and has undertaken major shifts in the present era which affects social, economic and health status of the society [3]. In the 1996 epidemic, approximately 16 000 cases and 545 deaths occurred throughout India [4]. Since 2010, the incidence of dengue has increased to about 15 per million people annually in different states. Every year more than 100 000 infections and 200–400 deaths occur throughout India [5].
As per World Health Organization, dengue fever can be suspected, based on the following symptoms, such as; acute febrile illness along with headache, rash, retro-orbital pain, muscle and joint pain, haemorrhagic manifestations and leukopenia. WHO case definition guidelines include; not less than two symptoms from the above-mentioned list of symptoms [6].
Dengue virus is an RNA virus belonging to the arbovirus family; Flaviviridae, genus Flavivirus, which has four distinct serotypes. The interaction of complex factors such as; viral, environmental and host determines the number of dengue cases, and also influences the signs and symptoms of the disease [7]. Dengue cases and fatalities have been reported more, both in urban and rural segments of the Asian continent. Infection is caused by one of the four serotypes and multiple sequential infections have been reported [8]. The primary infection of dengue is caused by one serotype, which induces antibodies corresponding to homologous serotypes, and it usually provides long-term protection. It also produces heterologous antibodies that provide short-term protection. However, infection with second serotype could cause secondary dengue, due to poor neutralizing antibodies and non-structural protein 1 (NS1), which play a major role in severe dengue infection [9]. WHO recommended a new approach in 2009 with the main purpose of enhancing triage and clinical care and recognising warning indicators of potentially severe dengue episodes. However, this classification is used only in few countries [10]. The need for epidemiological study of dengue focuses on the disease burden, vector epidemiology, serotypes circulated, economic concern of the disease and the status of vaccine development.
In Andaman & Nicobar Islands, there is a constant movement of people from mainland, India. In 1973, an outbreak of dengue occurred in most parts of India, but it was undetected and not reported in the Andaman Islands [11]. Until 2008, no instances of dengue cases were observed in these islands [6], and in 2009 few cases of dengue fever were reported. In the subsequent year (2010), cases of dengue haemorrhagic fever and dengue shock syndrome were reported, which are transmitted by the competent dengue vector; Aedes aegypti, with high-level of infestation reported within Port Blair, headquarter of the union territory. Since then, dengue cases have been increasing due to swift urbanization and continue to pose a serious public health problem. Dengue fever is a primary cause of hospitalisation and mortality in several countries. National passive dengue monitoring system aims to identify epidemic activity, rather than to quantify the burden of the disease. More precise estimates are needed to guide disease management programmes, allow efficient resource allocation, and evaluate the efficacy of innovative therapies such as dengue immunisation [12]. The present study was carried out to determine the epidemiological characteristics, viz; clinical profile of dengue, demographics, area-wise and seasonal association of dengue incidence, for a period of four years from 2018 to 2021.
Materials and Methods
Andaman and Nicobar Islands is a union territory of India and stretches over 700 km from north to south of Bay of Bengal. It consists of 572 islands, of which 38 has human settlements. According to 2011 census, the total population of the Islands is 380,581. Island has tropical climate, and precipitation is about 3,000 mm, which is obtained from southwest monsoon, during May to September, and tropical cyclones that follow in October and November.
A hospital-based study was carried out to investigate the characteristics of dengue infection in two tehsils of South Andaman district, viz; Port Blair and Ferrargunj, for a period of four years (2018-2021). Blood samples from 10,313 persons suspected of dengue fever were collected aseptically, after obtaining prior informed consent. Patients who were attending district hospitals and primary health care facilities and presenting with the symptoms of dengue (as per the case definition of WHO) were included in the study. The patient’s clinical history was recorded, which include, viz, onset of fever, symptoms, co-morbidity and demographic details such as age, sex, area, and recent travel history. The location of those who were confirmed of dengue infections were mapped using GIS. For mapping, ARC-GIS software was used to mark the hot spot areas of dengue.
Dengue was suspected when high fever (40°C/104°F) was recorded along with two of the symptoms during the febrile phase (2-7 days). Dengue cases were differentiated as mild and severe dengue based on the symptoms. Persons with severe headache, eye pain, muscular and joint problems, nausea, vomiting, swollen glands, and rashes was considered as mild dengue. Whereas, symptoms of plasma leakage, fluid accumulation, respiratory difficulties, severe bleeding, or organ dysfunction were considered as severe dengue case.
Laboratory Assays: Blood samples were collected and serum was used to perform NS1 antigen detection with Panbio® Dengue Early ELISA for patients reported of the duration of symptoms to be less than 5 days. Dengue specific IgM antibody detection was carried out on samples from patients reported with symptoms for more than 5 days, with IgM antibody capture enzyme-linked immunosorbent assay (MAC-ELISA). If the sample was positive for IgM antibody, it was further processed for IgG secondary antibody with Panbio® Dengue IgG capture ELISA.
A primary dengue case was defined as a laboratory-confirmed infection with NS1 antigen and/or IgM positive and IgG negative. While a secondary dengue case was defined as a laboratory-confirmed dengue infection with NS1 antigen and/or IgM positive along with positive IgG ELISA [13]. Data analysis was carried out for all relevant variables using descriptive statistics and the statistical difference in categorical variables between groups was evaluated with a chi-square test.
Results
Study Population and dengue infection
The study included 10,313 patients from the South Andaman district during the period 2018–2020, which included 5510 males and 4803 females, from two tehsils, viz, Port Blair and Ferrargunj (Figure 1). During the four-year study period, 679 patients (6.6%) tested positive for dengue infection. Among the total positives, maximum cases occurred in the year 2018 (38%), while 15% of the cases alone were recorded in 2020 (Table 1). In the lowest age group of 0-10 years, there were 108 positive individuals, which increased in subsequent age groups and reached a maximum of 203 cases in 21-30 years. Later, the number of cases decreased with increasing age, and 34 persons had infection above 60 years. Males were more affected than females. More cases were recorded from Port Blair tehsil, which is the State capital. There was no dengue related fatalities recorded during the study period.
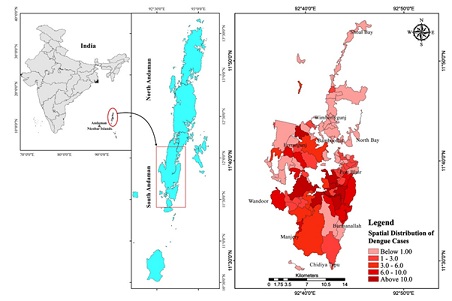 Figure 1: Spatial distribution of Dengue cases in South Andaman.
Figure 1: Spatial distribution of Dengue cases in South Andaman.
|
Characteristics |
Confirmed dengue (n= 679) |
Primary dengue (n = 485), n (%) |
Secondary dengue (n= 194), n (%) |
|
Age Groups (years) |
|
|
|
|
Median Age |
26 |
25 |
24.5 |
|
0-10 |
108 |
79(73) |
29(27) |
|
11-20 |
121 |
86(71) |
35(29) |
|
21-30 |
203 |
144(71) |
59(29) |
|
31-40 |
111 |
83(75) |
28(25) |
|
41-50 |
63 |
44(70) |
19(30) |
|
51-60 |
39 |
23(59) |
16(41) |
|
>60 |
34 |
26(76) |
8(23) |
|
Gender |
|
|
|
|
Male |
382 (56) |
284(59) |
98(51) |
|
Female |
297 (44) |
201(41) |
96(49) |
|
Tehsil |
|
|
|
|
Port Blair |
391 (58) |
298(61) |
93(48) |
|
Ferrargunj |
288 (42) |
187(39) |
101(52) |
|
Year |
|
|
|
|
2018 |
258 |
194(75) |
64(25) |
|
2019 |
179 |
113(63) |
66(37) |
|
2020 |
104 |
64(62) |
40(38) |
|
2021 |
138 |
114(83) |
24(17) |
Table 1: Demographic characteristics of primary and secondary dengue.
The seasonal trend showed that more cases occurred during the months, May to August, coinciding with the monsoon season. Almost 69 symptoms were reported in those diagnosed with dengue infection, and the common manifestations were fever (77.7%), Headache (48.9%), Bodyache (30.11%), Cough (29.5%), Arthralgia (20.4%), Chills (16.1%) and Giddiness (11.9%).
Mapping of cases using Geographic Information System (GIS)
In order to determine the hot spot areas of dengue in the two tehsils, spatial distribution of dengue cases in Pot Blair and Ferrargunj tehsils in south Andaman was mapped. Garacharama, Bathubasti, Terlabad, and Prothrapur in Port Blair Tehsil had the highest number of cases, while in Ferrargunj tehsil, more cases were found in the Manpur area.
Demographic characteristics of primary and secondary dengue
Among 679 individuals positive, primary dengue infection was found in 485 people whose serum samples tested positive for NS1 antigen by ELISA, and no anti-dengue IgG antibodies could be found. In 194 people, specific IgG antibodies were also found in the serum sample, along with antigens in the early serum sample, and were classified as secondary dengue as per the definition (Table 1). Port Blair recorded 298 primary cases, while 187 cases were recorded from Ferrargunj tehsil. In all the categories, one-fourth of positives were found with secondary dengue infection. Age group wise analysis of primary dengue infection showed similar trend as that of overall infection, with maximum number of cases observed in the age group of 21-30 years. The proportion of secondary dengue varied from 41% in 51-60 years to 23% in >60 years. Except in 51-60-year age group, all other groups showed more than 70% primary dengue. The median age of primary dengue cases was 25 year, and male to female ratio was 1.4:1. The median age of secondary dengue cases was 24.5 year (Table 1). There was no significant difference in the distribution of primary and secondary dengue with repect to gender (χ2= 3.64; P=0.05). While confirmed dengue infection did not show any difference between tehsils, while primary infection showed a preponderance in Port Blair tehsil as compared to Ferrargunj and was significant (χ2= 10.35; P=0.0013). This may be due to the widespread circulation of different serotypes in the capital, urban areas which exposes residents to a wider variety of strains. Among the four years studied, primary infection was high in 2018 which decreased in subsequent years. In the year 2021, secondary dengue was the lowest (17%) compared to previous three years. There was a significant difference in primary dengue infection between the four years (χ2= 21.2; P < 0.0001).
Dengue fever and dengue haemorrhagic fever
Among the 679 dengue positives, 609 (90%) were dengue fever (DF) and 70 were classified as dengue hemorrhagic fever (DHF) based on the definition of cases. All age groups were equally affected by symptomatic dengue positivity, but high number of cases was observed in the age group of 21 to 30 years, both for dengue fever and dengue haemorrhagic fever. Number of dengue fever cases increased from the lowest age group (101 in 0-10 years) to 180 in the 21-30 age group, which gradually declined further, reaching 34 in >60 years age group (Table 2). A similar trend was observed for dengue haemorrhagic fever (DHF). There have been no confirmed cases of DHF in people over the age of 50. There was a significant difference in dengue fever cases between the age groups (χ2= 15.2; P=0.019). Cases of DHF were characterised by thrombocytopenia, vertigo, hemoconcentration, hematochezia, thrombocytopenia, dehydration, and pleural effusion. There was 10% positivity rate for dengue haemorrhagic fever in individuals aged 0 to 50 years. DHF was found in 6-23% of the positives in different age groups. A significant proportion of males (71%) were positive for DHF compared to females and was significant (χ2= 7.2; P=0.006). Among the two tehsils, Port Blair had significantly higher number of DHF than Ferrargunj, but there was no significant difference observed. During the four years, viz, 2018 to 2021, the DHF incidence increased from 9% to 14%. However, mild dengue fever cases increased by 15% between 2018 and 2019, but decreased by 64% by 2021, indicating a significant positive trend.
|
Characteristics |
Confirmed dengue (n = 679) |
Dengue Fever (n = 609), n (90%) |
Dengue Haemorrhagic Fever (n = 70), n (10%) |
|
Age Groups (years) |
|
|
|
|
Median Age |
26 |
26 |
25 |
|
0-10 |
108 |
101(94) |
7(6) |
|
11-20 |
121 |
104(86) |
17(14) |
|
21-30 |
203 |
180(89) |
23(11) |
|
31-40 |
111 |
94(85) |
17(15) |
|
41-50 |
63 |
57(90) |
6(10) |
|
51-60 |
39 |
39(100) |
0 |
|
>60 |
34 |
34(100) |
0 |
|
Gender |
|
|
|
|
Male |
382 |
332(55) |
50(71) |
|
Female |
297 |
277(45) |
20(29) |
|
Tehsil |
|
|
|
|
Port Blair |
391 |
349(57) |
42(60) |
|
Ferrargunj |
288 |
260(43) |
28(40) |
|
Year |
|
|
|
|
2018 |
258 |
235(91) |
23(9) |
|
2019 |
179 |
164(92) |
15(8) |
|
2020 |
104 |
92(88) |
12(12) |
|
2021 |
138 |
118(86) |
20(14) |
Table 2: Characteristics of Dengue and Dengue Haemorrahagic fever.
Discussion
Dengue in South Andaman has infiltrated into urban areas of Port Blair, mainly due to the widespread distribution of Ae. aegypti, the vector mosquito. In the year 2011, positive dengue cases reported was 26 [14], has increased in number and severity and reached to 258 cases in 2018. Dengue fever exhibit a wide range of symptoms, from mild fever to severe dehydration and even death as in classical dengue fever, depending on the severity of the infection and the patient's exposure to the virus [12].
The occurrence of dengue and poverty has been interlinked in few areas with low-income communities, physical housing conditions and water supply which promote contact between host and the vector [15]. Owing to the association of housing facilities and dengue, area-wise distribution of dengue cases was evaluated in South Andaman but a sustained increase in dengue cases was consistent in two areas such as Port Blair and Ferrargunj tehsil during the four years of study period. Compared to Ferrargunj Tehsil, Port Blair had more cluster of cases, observed through GIS mapping. Garacharma, Terlabad, Bathubasthi, and Prothrapur are among the most endemic areas in Port Blair which are close to one another and has dense human habitation. Dengue fever is transmitted by a mosquito bite in the early morning hours of the day. It breeds in artificial or man-made stagnant water collections. Because of high population density and large number of water bodies in these urban/semi-urban locations, the residents inhabiting these areas are more vulnerable, and hence dengue prevalence was high [16,17]. Additionally, Port Blair is an important tourist destination and has rapid urbanization, due to which the virus gets imported from other endemic areas/regions.
A statistically significant difference was observed in dengue cases between male and female in the South Andaman District, especially DHF, with higher incidence in males. There was high dengue positivity rate in the age group of 21–30 years. This age group is involved in more daily outdoor activities, employment and travel, and are likely to get exposed more to the day biting Aedes mosquito vectors and thus acquire infection. Positivity in elderly was low, possibly due to their prior exposure to the virus in their younger days [18]. In Nicaragua, high morbidity and mortality was reported due to dengue, especially in children below 9 years of age [2]. In the present study, people of all age groups were affected by dengue and the youngest age group of 0-10 years showed 16% positivity.
In the Andaman Islands, the monsoon season begins in June and ends in September. Dengue positive cases has been reported to rise during monsoon period as compared to pre-and post-monsoon [12,17,18]. In Delhi, cold temperature was observed to an increase in vector abundance and thus transmission of the virus during the interepidemic period, especially after the monsoon period along with strong peaks from January to May, in Delhi and also in other places, such as Mumbai, Chennai and Kolkata [16]. Similarly, upsurge in dengue cases was reported in Uttar Pradesh during the post-monsoon period by 65%, from October to January [19]. In the present study, most of the dengue cases were reported from May through August (monsoon period).
A total of 10,313 dengue suspected cases were screened during the study period spanning four years in the South Andaman. Patients with fever, headache, bodily discomfort, giddiness, and a low platelet count were included in the study. Majority of patients had fever (77.7%), headache (48.9%) and body discomfort (30.11%), along with additional symptoms such as diarrhoea, weakness, mouth ulcer, sore throat, rhinorrhoea, neck stiffness and breathlessness ( < 10%). There were 679 dengue positive patients detected, including 310 NS1 positives and 369 IgM positives. Both NS1 and IgM positive individuals were screened for secondary antibody (IgG), to determine the prior infection [10,16]. Almost 485 people were positive for dengue primary infection, while 194 were positive for secondary dengue. Dengue fever has minor symptoms such as fever, headache, and body ache, while Dengue haemorrhagic fever (DHF) is manifested with severe symptoms such as haemorrhage, plasma leakage, and death [20]. In DHF, overproduction of IL-10 can suppress IFN signalling to increase DENV replication, resulting in an increase in viral titers [21]. Out of 679 dengue positive individuals, 609 were classified as dengue fever (DF) and had minor symptoms, while 70 had severe symptoms such as blood in their vomit and stool, a low platelet count, and were hospitalised for treatment, and hence were classified under DHF. Combined damage occurs to the coagulation system and to the capillaries which could lead to shock, haemorrhage and organ failure, as non-severe dengue could easily turn into severe dengue. Hence, the recommendation for early access to health care is essential with close monitoring for thrombocytopenia as well as the progression of the disease [8]. The present study had included all the clinical presentations of dengue patients all through the four-year study period with thorough monitoring of both mild and severe dengue patients. No specific antiviral drug is available against dengue, but several sulfated polysaccharides extracted from seaweeds have been studied, and high antiviral activity against DENV has been observed [22]. Glycyrrhizin and its derivatives or modified products induce antiviral activity by affecting the secretion of interferons against DENV and inhibit DENV protein transport and post-translational modifications [23].
Dengue infection can be controlled by closely monitoring the seasonal and climatic variations in endemic regions and raising public awareness. Further, major efforts should focus on high-positive zones, and stagnant water bodies should be removed by enhanced community awareness. Dengue serotypes 1 and 2 have already been identified in mainland India [18] and they are also present in the Andaman and Nicobar Islands. However, DENV 3 triggered an outbreak among visitors in Havelock Island in the south Andaman, in 2014 [24]. DENV4 was the most common among dengue positive individuals in the Andaman and Nicobar Islands in 2018 [25]. Thus, in A&N Islands, all the four dengue serotypes are prevalent. In addition, these Islands are famous tourist destinations for people from all over the world [14], and dengue fever has a high risk of dissemination during travel. Researchers should monitor the circulating serotype and conduct studies on the severity of dengue illness depending on the serotype [10,12,16,17]. Since all the four serotypes are reported, it is essential to monitor these serotypes throughout the Islands as a pilot study in the future. Additionally, screening of the suspected dengue cases in the Nicobar group of Islands is lacking, which needs in-depth investigation. Since the potential vectors are prevalent in both Andaman and Nicobar Islands [26], surveillance and monitoring of cases as well as the mosquito vectors of dengue virus is a pre-requisite in this archipelago.
Conclusion
Despite limited number of research articles on dengue in the Andaman Islands, this study could document, in detail, the positivity rate of dengue among 10,313 samples collected from 2018 to 2021. This paper has addressed the extent of distribution of dengue cases in South Andaman and shed light on the demographic characteristics, and distribution of dengue, area-wise and age groups. This epidemiological characteristic will initiate efficient and prompt measures in combating dengue and attain significant progress in the healthcare of the people of the Andaman Islands.
Conflict of interest statement
We declare no conflict of interest.
Acknowledgements
The authors are thankful to the Department of Health Research (DHR) for providing the funding for the SL-VRDL project. The authors are thankful to the Director of Health Services, Andaman and Nicobar administration for extending logistic support. The authors also thank the Medical Officer of CHC/PHC of South Andaman.
References
- https://iris.who.int/handle/10665/152781?&locale-attribute=es
- Sanyaolu A, Okorie C, Badaru O, Adetona K, Ahmed M, et al. (2017) Global epidemiology of dengue hemorrhagic fever: an update. J Hum Virol Retrovirol 5: 00179.
- Wilson ME, Chen LH (2015) Dengue: update on epidemiology. Curr Infect Dis Rep 17: 1-8.
- Mutheneni SR, Morse AP, Caminade C, Upadhyayula SM (2017) Dengue burden in India: recent trends and importance of climatic parameters. Emerg Microbes Infect 6: e70.
- https://ncvbdc.mohfw.gov.in/
- Chaaithanya IK, Bhattacharya D, Muruganandam N, Thamizhmani R, Babu BS, et al. (2012) Dengue: a newly emerging viral infection in Andaman and Nicobar Islands, India. Epidemiol Infect 140: 1920-1924.
- do Nascimento IDS, Pastor AF, Lopes TRR, Farias PCS, Gonçales JP, et al. (2020) Retrospective cross-sectional observational study on the epidemiological profile of dengue cases in Pernambuco state, Brazil, between 2015 and 2017. BMC Public Health 20: 1-10.
- Shet A, Kang G (2019) Dengue in India: Towards a better understanding of priorities and progress. Int J Infect Dis 84: S1-S3.
- Katzelnick LC, Gresh L, Halloran ME, Mercado JC, Kuan G, et al. (2017) Antibody-dependent enhancement of severe dengue disease in humans. Science 358: 929-932.
- Jing Q, Wang M (2019) Dengue epidemiology. Glob Health J 3: 37-45.
- Manimunda SP, Singh SS, Sugunan AP, Singh O, Roy S, et al. (2007) Chikungunya fever, Andaman and Nicobar Islands, India. Emerg Infect Dis13: 1259.
- Mistry M, Goswami Y, Chudasama RK, Thakkar D (2015) Epidemiological and demographic characteristics of dengue disease at a tertiary care centre in Saurashtra region during the year. J Vector Borne Dis 52: 299.
- Rao C, Kaur H, Gupta N, Sabeena SP, Ambica R, et al. (2019) Geographical distribution of primary & secondary dengue cases in India – 2017: A cross-sectional multicentric study. Indian J Med Res 149: 548-553.
- Vijayachari P, Singh SS, Sugunan AP, Shriram AN, Manimunda SP, et al. (2011) Emergence of dengue in Andaman & Nicobar archipelago: Eco-epidemiological perspective. Indian J Med Res 134: 235.
- Bavia L, Melanda FN, de Arruda TB, Mosimann ALP, Silveira GF, et al. (2020) Epidemiological study on dengue in southern Brazil under the perspective of climate and poverty. Sci Rep 10: 1-16.
- Vikram K, Nagpal BN, Pande V, Srivastava A, Saxena R, et al. (2015) Detection of dengue virus in individual Aedes aegypti mosquitoes in Delhi, India. J Vector Borne Dis 52: 129.
- Savargaonkar D, Sinha S, Srivastava B, Nagpal BN, Sinha A, et al. (2018) An epidemiological study of dengue and its coinfections in Delhi. Int J Infect Dis 74: 41-46.
- Ahmed NH, Broor S (2014) Comparison of NS1 antigen detection ELISA, real-time RT-PCR and virus isolation for rapid diagnosis of dengue infection in acute phase. J Vector Borne Dis 51: 194.
- Chakravarti A, Kumaria R (2005) Eco-epidemiological analysis of dengue infection during an outbreak of dengue fever, India. Virol J 2: 1-7.
- Gubler DJ (1998) Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 11: 480-496.
- Tsai TT, Chuang YJ, Lin YS, Wan SW, Chen CL, et al. (2013) An emerging role for the anti-inflammatory cytokine interleukin-10 in dengue virus infection. J Biomed Sci 20: 40.
- Damonte EB, Matulewicz MC, Cerezo AS (2004) Sulfated seaweed polysaccharides as antiviral agents. Curr Med Chem 11: 2399-2419.
- Baltina LA, Tasi YT, Huang SH, Lai HC, Baltina LA, et al. (2019) Glycyrrhizic acid derivatives as Dengue virus inhibitors. Bioorg Med Chem Lett 29: 126645.
- Kartick C, Bharathi GSJ, Surya P, Anwesh M, Arun S, et al. (2017) Outbreak investigation of fever mimicking dengue in Havelock Island, an important tourist destination in the Andaman & Nicobar Archipelago, 2014. Epidemiol Infect 145: 1437-1442.
- Alagarasu K, Kakade MB, Bachal RV, Bote M, Parashar D, et al. (2021) Use of whole blood over plasma enhances the detection of dengue virus RNA: possible utility in dengue vaccine trials. Arch Virol 166: 587-591.
- Sunish IP, Shriram AN, Sivan A, Vijayachari P (2014) Spatio-temporal distribution of Aedesmosquitoes in Car Nicobar Island: Implication in the transmission of arboviruses. J Asia-Pacific Entomol 17: 761-776.
Citation: Rajamani M, Samson R, Punnam Chander M, Sunish IP, Vijayachari P (2023) Epidemiological Characterization of Dengue in South Andaman Islands, India: An Update. J Community Med Public Health Care 10: 135
Copyright: © 2023 Rajamani M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

