Department Of Internal Medicine, Nephrology Division, King Fahd Hospital Of The University, Al-Khobar, Saudi Arabia
Abstract
The aim of this study was to look at the overall risk of developing peritonitis in end stage renal disease patients who are maintained on peritoneal dialysis and underwent colonoscopy due to various indications and to find if there is any correlation with intraperitoneal prophylactic ceftazidime or not prior to colonoscopy.
Patients and Methods: A total of 103 patients out of 144 patients on Automated Peritoneal Dialysis (APD) undergoing diagnostic colonoscopy were enrolled in a prospective randomized study. The study was extended from January 2012 until January 2018. Patients were randomized into two age and sex matched groups; group A (51 patients) who had prophylaxis ceftazidime prior to colonoscopy and group B (52 patients) who had colonoscopy without prophylactic antibiotics. The following parameters: age, gender, duration on dialysis, duration on APD, diabetic status, use of antibiotics before the procedure, and indications for and findings of colonoscopy were studied. Prophylactic antibiotics were given for prevention of peritonitis according to the 2016 ISPD (International Society of Peritoneal Dialysis) guidelines recommendation which is a class C weak suggestion.
Results: Of all colonoscopies 63.1% showed normal findings, 17.2% with colonic polyps at different sites, 12.9% with angiodysplastic-like lesions, 5.4% with colonic ulcer (s), 3.2% with diverticulae without diverticulitis and 1.1% had transverse colon stricture. Post-colonoscopy peritonitis was documented in 4 (7.8%) and 5 (5.8%) patients in groups A and B respectively (p> 0.05); the causative organisms were mainly gram-negative bacteria. All peritonitis cases resolved with treatment and one of the patients in group A required catheter removal because of fungal peritonitis. Complications other than peritonitis were 0.0% in both groups.By multiple logistic regression analysis, diabetes mellitus was the only independent variable that entered into the best predictive equation over the development of post-colonoscopy peritonitis but not antibiotic use.
Conclusion: The overall risk of peritonitis in the studied population is 8.7%. It seems that there is no correlation between the group who received intraperitoneal prophylactic ceftazidime before the procedure of colonoscopy and the group who had not received it in terms of protection against peritonitis post colonoscopy. Only old age, diabetes mellitus and low serum albumin appear to be of significance. Polypectomy; partial or complete did not increase peritonitis episodes in our study population.
Keywords
Antibiotic prophylaxis; APD; Colonoscopy; Diabetes; ESRD; Peritonitis; Polypectomy
INTRODUCTION
The expansion of peritoneal dialysis uses in the late years as an option for Renal Replacement Therapy (RRT) for End Stage Renal Disease (ESRD) patients and the development of Automated Peritoneal Dialysis (APD) have led to improved quality of life as well as increased survival among those patients. Maintaining patients on peritoneal dialysis for many years, however, has its own challenges that face nephrologists. These challenges based on the fact that most of ESRD patients are suffering from different comorbidities. Such challenges may require the need for other services namely cardiology, endocrinology, gastroenterology and others, each of which has its own diagnostic procedures that might harm patients if done without proper preparation. Colonoscopy is one of these procedures that may be required for both screening for and diagnosis of colon cancer as indicated. Colorectal cancer is still the fourth leading cause of cancer related death in the world [1]. Although controversial, the overall incidence of cancer is reported to be higher in patients with ESRD than in the general population [2-8]. Unfortunately, no studies have documented the rate of colorectal cancer among patients with end stage renal disease [5]. There are, however, no reported data concerning the prevalence of the disease amongst Saudi ESRD patients who are subjected to dialysis. The association between the risk of neoplasia and the severity of Chronic Kidney Disease (CKD) has been noted [9]. One study has linked ESRD to the increased prevalence of colorectal lesions in patients with positive faecal occult blood [10]. Variable factors may influence the prevalence of colorectal lesions in this population in particularly diabetes, type and length on dialysis, statin use, immunosuppressive drugs and obesity. The current recommendations for colonoscopy screening in ESRD patients before renal transplantation are the same as those for the general population as detecting colorectal cancer may exclude patients from the transplant list for at least five years after clinical remission. These recommendations, however, are for average risk patients with average life expectancies or for screening in higher risk populations. However, there has been an undercurrent that all patients should have the right to screening, not to do so is an error of omission, and quality of care can be measured by examining screening rates [11]. Wong et al., [12] examine the net benefit and cost-effectiveness of colorectal cancer screening in dialysis patients not on the wait list, dialysis patients wait-listed for a kidney transplant and kidney transplant recipients. The benefits of screening were estimated to be 2.6 added days of life for each dialysis patient, 6.9 days for wait-listed patients and 12 days for those transplanted. Polyps of adenomatous origin are not a contraindication to renal transplantation and their prevalence among ESRD patients is not defined yet. One concerned study [13] did not reveal a statistically significant increased risk of colorectal polyps in this population. The study was, however, criticized because of its small sample size. Cost effectiveness studies showed that routine CRC screening in the ESRD population in USA would exceed general thresholds for cost effectiveness [14] but considering the improved life expectancy of ESRD patients on dialysis, cancer screening for potential transplant candidates is consistent with the recommendations for general population. Additionally; dialysis patients are at risk for different GI disorders with bleeding complications. Current guidelines recommend screening for average risk people between ages 50 and 75 years but there is no consensus for dialysis population [15] and the need for colonoscopy is left for the clinical judgement for evaluating patients' pre- renal transplantation or if there are indications such as positive occult blood, chronic altered bowel habits or significant loss of weight in considered active patients.
In Saudi Arabia, colorectal cancer is the most common cancer among men and the third commonest among women. In a retrospective analysis of all cases of Colorectal Cancer (CRC) from the Saudi cancer registry between January 2001 and December 2006, the incidence of CRC in Saudi Arabia was found to be on a constant rise, and the age at the time of diagnosis was lower when compared with results from developed countries [16]. ESRD patients are more likely to develop this notorious disease and particularly those on Peritoneal Dialysis (PD) who have long life expectancy are good candidates for colon cancer screening prerenal transplantation or even just screening as the general population.
Our PD unit is one of the biggest units in Saudi Arabia where more than 200 PD patients are taken care of; the clear majority of them are on APD. Our protocol includes colonoscopy for those with unexplained anaemia, weight loss, positive occult blood and chronic constipation or diarrhoea and as part of the prerenal transplantation protocol. The fear of developing peritonitis after colonoscopy is unjustified as few cases have been reported in the literature on post colonoscopy peritonitis in PD patients [17-21]. A retrospective study [22] found that the risk of peritonitis after colonoscopy without antibiotic prophylaxis was 6.3%; colonic biopsy or polypectomy did not appear to further increase the risk and no peritonitis was observed in patients that received prophylactic antibiotics although the difference was not statistically significant. Few cases have been reported on the incidence of peritonitis following colonoscopy in Continuous Ambulatory Peritoneal Dialysis (CAPD) patients. Those reports claimed that instrumental diagnostic procedures such as colonoscopy may play a significant role in the development of gram negative peritonitis in CAPD patients [23,24]. Similar results were reported by Yip et al., [25]. All reported cases about peritonitis following colonoscopy were on CAPD and there were no case reports in APD patients. The recent guidelines of the International Society of Peritoneal Dialysis (ISPD) showed evidence 2-C favoring the use of prophylactic antibiotics prior to colonoscopy; however there have been no controlled randomized studies to support these recommendations.
Hypothesis
In view of these challenges, our study aimed at investigating the need of prophylactic antibiotics prior to colonoscopy in APD patients undergoing this procedure to prevent peritonitis.
PATIENTS AND METHODS
This prospective randomized study of patients with ESRD on APD and undergoing colonoscopy was performed according to the Declaration of Helsinki at King Fahd University Hospital, Al-Khobar, Saudi Arabia. The study was conducted from January 2012 throughout January 2018 with prior approval by King Fahd Hospital Human Ethical committee. All patients were above 18 years of age and written informed consents were obtained from every patient after full explanation of the aim of the study, the complications of colonoscopy and the expected outcomes.
Between January 2012 throughout January 2018, 103 patients, (75 males, 28 females) were included in this study. Patients were randomized and allocated to either group A or B using 1:1 ratio in an alternating fashion, one patient is allocated to group A and the other one goes to group B based on their scheduled date of colonoscopy; Group A: 51 patients on APD with prophylactic antibiotic therapy before the flexible colonoscopy, Group B: 52 patients on APD without prophylactic antibiotics (Table 1). Exclusion criteria were: history of colonic or rectal resection, neurologic deficit, pregnancy, ongoing sepsis, valvular or chronic heart disease, urinary tract infections, chronic liver disease, exit-site or tunnel infections, pneumonia or pulmonary tuberculosis, peritonitis or history of peritonitis for the last one year and unwillingness to give informed consent (Figure 1). All flexible colonoscopy examinations were performed by trained gastroenterology consultants. All Staff in the endoscopy unit were aware of the potential hazard of cross-infection and assiduous mechanical cleaning followed by disinfection was done. The following parameters: age, gender, duration on dialysis, diabetic state, use of antibiotics before the procedure, and indications for and findings of colonoscopy were studied. APD peritonitis episodes occurring within 1 week after colonoscopy, culture results and outcomes of peritonitis were recorded. At our center, the colonoscopy bowel preparation protocol included a low residue diet 2 days before the examination and patients are instructed to take a fluid diet the day before the procedure. Oral electrolyte lavage solutions such as polyethylene glycol but not aqueous sodium phosphate solution were used as laxative for bowel preparation as sodium phosphate solution is not desirable in end stage renal disease patients. Peritoneal Dialysis Effluent (PDE) was drained and the patient’s abdomen was kept empty before the procedure. Prophylactic antibiotics were given for prevention of peritonitis as suggested by the 2016 ISPD guidelines [15]. Prophylactic antibiotics for APD peritonitis prevention were not a routine at our center. Peritonitis was diagnosed when abdominal pain and cloudy fluid occurred with or without fever, and when peritoneal fluid White Blood Cell (WBC) count was >100/mm3, with >50% neutrophils. Episodes with peritoneal eosinophilia but negative bacterial culture were excluded. The PDE was sent for hematological and microbiological examination when patients complained of abdominal pain or if the PDE was turbid. For the microbiological tests, 50 ml peritoneal fluid was centrifuged at 5000g for 15 minutes. The deposit was inoculated on 5% sheep blood agar, MacConkey agar, Sabouraud agar and incubated aerobically at 35°C for up to 72 hours. All isolates were identified by standard biochemical methods and the identity of the isolates was confirmed using the Vitek Automicrobic System (bioMerieux, Vitek, Hazelwood, Missouri, USA). Antimicrobial susceptibility was tested by the Kirby–Bauer disk diffusion method and results interpreted according to the National Committee for Clinical Laboratory Standards criteria. Reappearance of signs of infection with the same organism(s) isolated in the dialysate within two weeks after the completion of antibiotic treatment was classified as relapse, and not as a new episode.
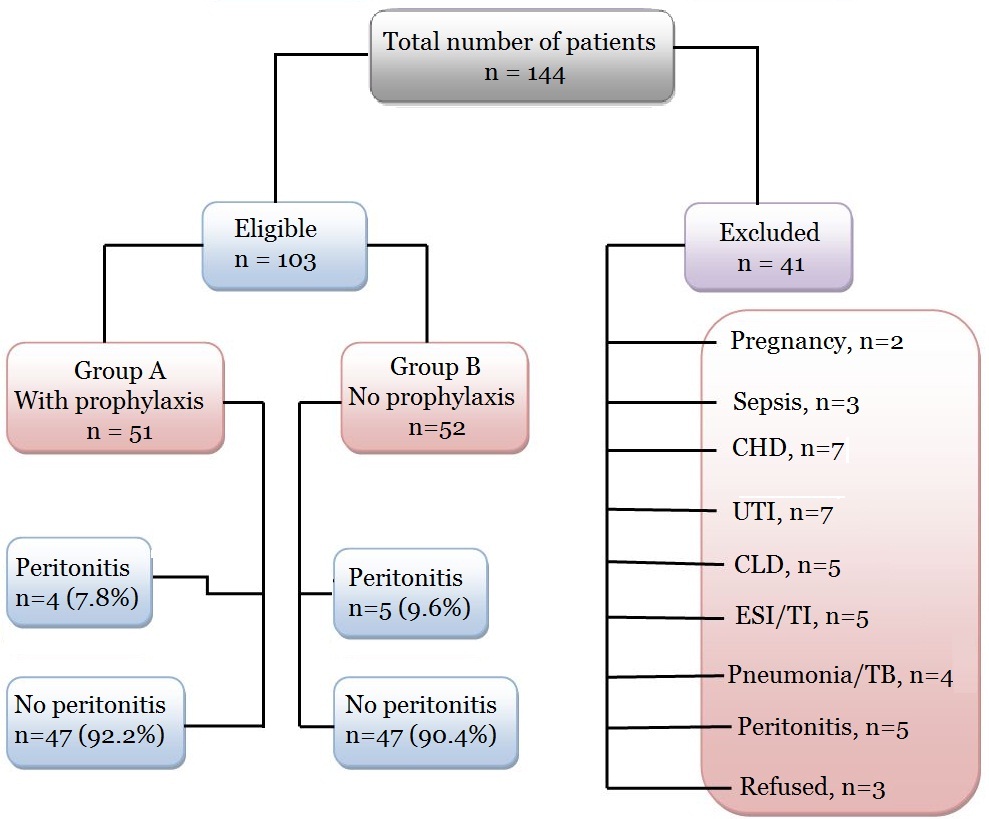 Figure 1:
Figure 1: Consort diagram demonstrating study design and patients' progress.
CHD: Chronic or valvular Heart Disease, UTI: Urinary Tract Infection, CLD: Chronic Liver Disease, peritonitis: ongoing or previous.
|
|
Group A
(n =51)
|
Group B
(n = 52)
|
p
|
|
Age (years), mean + SD
|
61+ 12.5
|
63+ 11.8
|
> 0.05
|
|
Female/Male (female %)
|
13/37 (35.1)
|
15/38 (39.4)
|
> o.o5
|
|
Smokers (%)
|
21.7
|
19.1
|
> 0.05
|
|
Hypertension, n (%)
|
38 (82.6)
|
36 (76.6)
|
> 0.05
|
|
BMI at beginning, mean + SD
|
29.1 + 4.1
|
29.3 + 3.8
|
> 0.05
|
|
Diabetes mellitus, n (%)
|
16(34.8)?
|
18 (38.3)
|
> 0.05
|
|
Duration of diabetes, (years), mean + SD
|
18.8+ 10.7
|
20.5+ 10.2
|
> 0.05
|
|
Duration on APD, months (mean + SD)
|
31.1+11.8
|
30.6+ 12.5
|
> 0.05
|
|
Overall FBS in diabetics, mmol/L (mean + SD)
|
8.6 + 1.3
|
8.4 + 1.4
|
> 0.05
|
|
Overall Hgb A1C % in diabetics (mean + SD)
|
7.1% + 0.5
|
6.8 + 0.8.
|
> 0.05
|
|
Hgb at colonoscopy, gm/dl (mean + SD)
|
10.16 ± 2.25
|
10.32+ 2.77
|
> 0.05
|
|
BUN at colonoscopy, mg/dl (mean + SD)
|
44.18 +10.23
|
46.12 + 9.81
|
> 0.05
|
|
Serum Cr. at colonoscopy, mg/dl (mean + SD)
|
8.28 + 2.55
|
8.33 + 1.87
|
> 0.05
|
|
Serum K+ (mEq/L)
|
4.1 + 1.9
|
3.9 + 2.1
|
> 0.05
|
|
Serum albumin (gm/l)
|
3.8+ 2.0
|
3.7 + 1.8
|
> 0.05
|
|
Renal Cr Cl. ml/m (mean + SD)
|
6.3 + 2.1
|
6.1 + 2.8
|
> 0.05
|
Table 1: Demographic characteristics of the study population.
BMI: Body Mass Index; APD: Automated Peritoneal Dialysis; FBS: Fasting Blood Sugar; Hgb: Hemoglobin; BUN: Blood Urea Nitrogen; Cr: Creatinine; K+: Potassium; Cr Cl: Creatinine Clearance.
All Patients were on Automated Peritoneal Dialysis (APD) and their dialytic prescription consisted of 1.36% and 2.27% glucose-based solutions Dianeal® over 9-10 hours night dwell and 7.5% icodextrin (Extraneal®, Baxter Castlebar, Ireland) 2 liters as the last fill for the day dwell. Total daily PD volume ranged between 10-12 liters with a fill volume ranging between 2.0-2.5 liters/cycle.
COLONOSCOPY PROCEDURE
In the procedure room, all patients were given supplemental oxygen (4 L/min) through a nasal cannula, and a 3-lead electrocardiogram, pulse oximetry, and blood pressure were monitored. Only the anesthesiologist certified in advanced life support and who completed a structured training program were permitted to administer propofol under the guidance of the endoscopist. The anesthesiologist who administered the sedative medications and physicians were present for the entire period of sedation and examination. The anesthesiologist attempted to achieve a level of sedation that allowed the patient to tolerate the procedure with minimal to mild pain while maintaining adequate cardiorespiratory function. Propofol induction of sedation was begun with an initial 40 mg bolus (20-30 mg for elderly and smaller patients at the discretion of the endoscopist and anesthesiologist) administered intravenously followed by titration with 10–20 mg boluses. After an initial bolus infusion of propofol, the patient was observed for 30-60 seconds before deciding to administer the next bolus. Fentanyl was administered intravenously in 12.5- or 25 g boluses and midazolam as 0.5–1.0 mg boluses. Additional medication was titrated at 1-3 minute intervals to achieve or maintain the desired level of sedation. An endoscopy technician was available to assist the colonoscopy operator with technical maneuvers. This staffing pattern has been used in our endoscopy suite for all sedated procedures for several years and was not changed for the study. The following time points were recorded: initiation of sedation, full sedation (when the nurse and endoscopist mutually agreed the patient was sedated sufficiently to begin the procedure), colonoscope insertion, intubation of the cecum, and colonoscope removal from the anus. Interventional procedures like polypectomy were performed when indicated with disposable polypectomy snare G-Flex. Post polypectomy bleeding (if any) was managed by epinephrine injection, hemoclip and heat probe.
Biopsies were taken when indicated by disposable biopsy forceps (Endow by Olympus).
After the procedure, both the physician and the nurse completed a questionnaire that assessed the patient’s level of sedation, pain, and ability to cooperate. Any complications (decline in oxygen saturation to less than 85%, heart rate less than 50 beats per minute, blood pressure less than 90/50 mm Hg, or need for mechanical ventilation) were recorded.
Prophylactic antibiotic therapy
At our center we use either intraperitoneal ceftriaxone or ceftazidime for prophylaxis but for this study we used only ceftazidime. After complete emptying of the abdomen of peritoneal dialysis fluid, antibiotic prophylaxis with 1.0 g ceftazidime in 100 ml of normal saline 0.9% was given intraperitoneally one hour prior to colonoscopy and kept for 7 hours to allow for adequate absorption after the procedure.
Peritonitis therapy
Peritonitis episodes were treated with our center’s standard antibiotic protocol, which has been changed systematically over time. The first-line antibiotic regimen for APD peritonitis was first- or second-generation cephalosporin plus gentamicin (loading dose 60 mg i.v. + 4-5 mg/L intraperitoneal). Cefazolin or cefoxitin (2 gi.v. + 50 mg/L intraperitoneal) combined with ceftazidime (2 g i.v + 1 g intraperitoneal) was also used in our PD unit since the year 2010 according to the ISPD peritonitis guidelines [15]. Vancomycin was used as a second-line therapy for primary nonresponding patients. Antibiotic regimens for individual patients were modified when culture results became available. Treatment usually lasted for either 2 weeks or at least 7 more days after normalization of the effluent WBC count, whichever was longer. Requirement of cessation of peritoneal dialysis, temporarily or permanently, and death during peritonitis, were defined as treatment failure. Heparin administration (500-1000 IU/L of dialysis fluid) and exchange of tubing was performed routinely in all cases of peritonitis. The indications for catheter removal included peritonitis caused by Pseudomonas species, peritonitis caused by fungi, cases with prolonged course or multiple recurrences, and episodes with suspected bowel perforation.
STATISTICAL METHODS
Continuous variables are expressed as mean ± SD and categorical variables are expressed as percentage. Student t-test or Mann-Whitney test was used to compare the means of continuous variables. Chi-square test was used to compare the percentages of discrete variables. Multiple logistic regression analyses were used to establish the best determinants over the development of at least 1 episode of peritonitis of enteral origin (dependent variable). The predictive variables included in the model were: age, gender, diabetic versus non diabetic, intestinal abnormalities, time on APD, hemoglobin and albumin levels and prophylactic antibiotic use. P values were not adjusted for multiple testing and therefore should be considered descriptive. The statistical analyses were limited to data regarding only the first episode of peritonitis, unless otherwise noted. Statistical significance was accepted at p<0.05. The statistical analysis was performed using SPSS for windows version 20 (IBM).
RESULTS
In a total of 144 APD patients included during the study period of 6 years, 106 colonoscopies were performed in 103 APD patients. Indications for repeating the procedure were inadequate preparation in one and partial or incomplete resection of a sessile polyp in two patients. Mean age was 62.3 ± 9.4 years and duration of dialysis was 35.2 ± 10.6 months; 34(36.6%) patients were diabetics.Demographic characteristics of patients are summarized in table 1. The two groups were age and sex matching. Diabetes mellitus was present in 34.8% and 38.3% and hypertension in 82.6% and 76.6% in the two groups respectively (p> 0.05). Mean duration of diabetes mellitus and the duration on APD was 18.8 ± 10.7 years and 20.5 ± 10.2 years, 31.1 ±11.8 months and 30.6 ± 12.5 months in the groups A and B respectively (p> 0.05).The difference in overall Fasting Blood Sugar (FBS) and Hemoglobin A1-C (Hgb A1-C) was not statistically significant between the two groups (p> 0.05). At the time of colonoscopy the mean Blood Urea Nitrogen (BUN), serum creatinine and renal creatinine clearance were 44.18 ± 10.23 mg/dl and 46.12 ± 9.81 mg/dl; 8.28 ± 2.55 mg/dl and 8.33 ± 1.87 mg/dl; 6.3 ± 2.1 and 6.1 ± 2.8 ml/min in groups A and B respectively (p> 0.05). Mean hemoglobin level, serum potassium (K+) and serum albumin were similar in both groups at the time of the procedure (p> 0.05) (Table 1). Indications for and findings of colonoscopy are summarized in table 2 and figure 2. Of all colonoscopies 60.2% showed normal findings, 17.2% with colonic polyps at different sites, 12.9% with angiodysplastic-like lesions, 5.4% with colonic ulcer(s), 3.2% with diverticulae without diverticulitis and 1.1% had transverse colon stricture which was managed with stent insertion. Inflammatory bowel disease in the three patients was inactive for more than one year. Findings at colonoscopy are shown in figure 2. Post-colonoscopy peritonitis was documented in 2 (3.9%) and 3 (5.8%) patients in groups A and B respectively (p> 0.05); the causative organisms were mainly gram negative bacteria (4 out of 5 cases were gram negative bacteria and one with Candida albicans) (Table 3). Peritonitis episodes were not documented in any patient with diverticulosis or biopsied colonic polyps. All peritonitis cases resolved with treatment and one of the patients in group A required catheter removal because of fungal peritonitis. Complications other than peritonitis were 0.0% in both groups. Different variables were analyzed to demonstrate its relation with peritonitis episodes (Table 4). No significant difference in serum BUN or serum creatinine was observed between those who developed peritonitis and those who did not in the two groups (p> 0.05). Seven factors met the criteria for inclusion in the univariate analysis: age (≥ 60) (Odds Ratio [OR]=1.41, 95% Confidence Interval [95% CI]=1.11-1.6, P=0.0336), male sex (OR=0.79, 95% CI= 0.66-0.93, P= 0.0462), diabetes mellitus (OR=1.23, 95% CI=1.15–1.62, P,0.0389), duration on APD (OR=1.58, 95% CI=1.24–1.81, P=0.0308), hemoglobin level (OR=0.89, 95% CI=0.83-1.1, P=0.0430). By multiple logistic regression analysis, the presence of diabetes mellitus was the only independent variable that entered into the best predictive equation over the development of enteric peritonitis (log likelihood ratio = -25.072, odds ratio = 17; 95% CI odds ratio: 2-151).
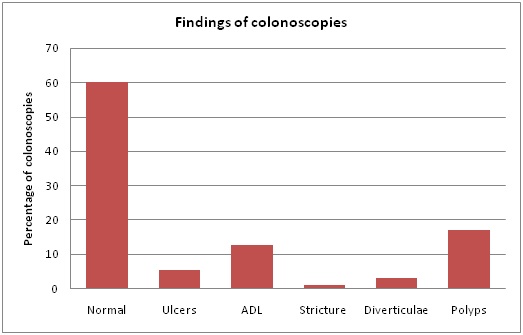
Figure 2: Finding of colonoscopies in the study population.
|
Number (%)
|
Indication
|
Findings (number)
|
Action (number)
|
|
19 (18.4)
|
Screening for colonic Cancer
|
Normal (15)
Transverse and descending colon polyps (4)
|
None (15)
Biopsies and removal (4)
|
|
18(17.4)
|
Investigation for iron deficiency anemia
|
Normal (14)
Angiodysplastic like lesions (4)
|
None (14)
Biopsies & bleeding protocol (4)
|
|
14 (15.1)
|
Altered bowel habits (chronic diarrhea or chronic constipation)
|
Normal (11)
Diverticulae (3)
Transverse colon polyps (2)
|
None (11)
None (3)
Biopsies and removal (2)
|
|
12 (12.9)
|
Positive fecal occult blood testing without overt rectal bleeding
|
Normal (7)
Angiodysplastic-like lesions (4)
Descending colon polyp (3)
|
None (7)
Biopsies & bleeding protocol (4)
Biopsies and removal (3)
|
|
9 (9.7)
|
Overt rectal bleeding
|
Normal (1)
Transverse or descending colon ulcers (2)
Angiodysplastic-like lesions (3)
Ascending & transverse colon polyp (3)
|
None (1)
Biopsies & bleeding protocol (2)
Biopsies & bleeding protocol (3)
Biopsies and removal (3)
|
|
8 (8.6)
|
Finding of polyp (s) during sigmoidoscopy
|
Normal (5)
Descending colon polyps (2)
Angiodysplastic-like lesions (1)
|
None (5)
Biopsies and removal (2)
Biopsies & bleeding protocol (1)
|
|
11 (1o.6)
|
Bloody effluent
|
Normal (10)
Transverse colon polyp (1)
|
None (10)
Biopsies and removal (1)
|
|
9 (8.7)
|
Family history of colon cancer or polyps
|
Normal (7)
Ascending colon polyp (1)
Descending colon ulcer (1)
|
None (7)
Biopsies and removal (1)
Biopsies (1)
|
|
3 (3.2)
|
Inflammatory bowel disease
|
Transverse and/or descending colon ulcers (2)
Transverse colon stricture (1)
|
Biopsies (2)
Stent (1)
|
Table 2: Indications for and findings of colonoscopy.
|
Patient’s No#
|
Group A (4 cases) Microorganisms
|
Patient’s No#
|
Group B (5 cases) Microorganisms
|
|
12
|
E. coli + Enterococcus faecalis
|
5
|
E. coli
|
|
34
|
Candida albicans
|
22
29
|
Klebsiella species
Negative culture
|
|
36
42
|
Klebsiella
S. aureus
|
40
48
|
Enterococcus
Negative culture
|
Table 3: Microorganisms responsible for peritonitis.
|
|
Group A
Peritonitis No peritonitis
|
p
|
Group B
Peritonitis No peritonitis
|
p
|
|
Number (%)
|
4 (7.8)47 (92.2%)
|
|
5 (5.8) 47 (90.4%)
|
0.1742
|
|
Age (year)
|
66.5 + 10.8 54.3 +11.2
|
0.0370
|
65.1 + 12.1 55.2 + 10.5
|
0.0342
|
|
Sex (M/F)
|
3/0 36/12
|
0.0461
|
3/13 6/12
|
0.0432
|
|
Diabetes, n (%)
|
2/2 (100) 14/44 (31.8)
|
0.0412
|
3/3 (100) 15/44 (34.1)
|
0.0358
|
|
Duration on APD, month, (mean)
|
33.4 + 9.62 1.1 +11.3
|
0.0281
|
31.6 + 7.7 23.5 + 9.2
|
0.0320
|
|
BUN, mg/dl (mean)
|
44.8 + 10.7 43.3 + 11.2
|
0.5804
|
46.4 +8.8 44.8 + 9.1
|
0.6213
|
|
Creatinine, mg/dl (mean)
|
8.5 + 2.2 8.8 +2.5
|
0.4036
|
7.8 + 2.6 9.0 +2.1
|
0.3892
|
|
Hemoglobin, gm/dl (mean)
|
7.5 + 3.4 9.6 +2.2
|
0.0447
|
7.4 + 2.7 10.1 + 1.4
|
0.0355
|
|
Serum K+, mEq/l (mean)
|
3.8 + 2.1 3.8 +1.8
|
0.4982
|
4.0 + 2.3 3.7 +2.6
|
0.4432
|
|
Serum albumin, gm/dl (mean)
|
2.3 + 2.0 4.3 +0.8
|
0.0283
|
2.4 + 1.9 4.1 +1.2
|
0.0311
|
Table 4: Comparison of characteristics of patients with and without peritonitis after colonoscopy.
DISCUSSION
Peritonitis remains the most serious complication of peritoneal dialysis. Around 18% of the infection-related mortality in PD patients is the result of peritonitis. Although less than 4% of peritonitis episodes result in death, peritonitis continues to be a leading factor to death in 16% of deaths on PD [16]. In addition, peritonitis is probably the most common cause of technique failure in PD, and it remains a major cause of patients discontinuing PD and switching to hemodialysis. Therefore, the PD community continues to focus attention on prevention and treatment of PD-related infections [16-24]. Peritonitis caused by enteral microorganisms is relatively infrequent in PD patients [25-27]. The source of contamination in those cases not associated with catheter exit-site or tunnel infections is thought to be transmural [1,25]. Microorganisms can gain access to the peritoneum from the intestinal lumen or through genital organs [28,29]. Diagnostic instrumental procedures, such as colonoscopy, have been implicated in the development of these peritonitis episodes [12,13]. However, in many cases there is no evidence that links peritonitis to colonoscopy as a risk factor [27,28]. The recommendations concerned with colonoscopy in PD patients are not based solely on randomized controlled trials because such studies in PD patients are limited, where there is no definitive evidence but the group feels there is sufficient experience to suggest a certain approach, this is indicated as “opinion” based. The recommendations are not meant to be implemented in every situation but are recommendations only. Each center should examine its own pattern of infection, causative organisms, and sensitivities and adapt the protocols as necessary for local conditions [17]. Post colonoscopy peritonitis in patients undergoing PD can result from translocation microorganisms across the bowel wall [30] and it has been alleged that gastrointestinal endoscopic procedures in those patients can lead to peritonitis [31]. Contrary to Yip et al., who, in selected cohort of PD patients with indications of colonic examinations, suggested that diverticulosis, may be a risk factor for the development of enteric peritonitis, we did not encounter peritonitis episodes in our 3 diverticulosis patients. Colonic diverticulosis did not appear to affect the outcome of colonoscopy in our patients. Supporting our findings was the report by Toda et al., [32] who studied 317 PD candidate patients over approximately 4 years and concluded that asymptomatic diverticulosis identified by computed tomography was not a risk factor for enteric peritonitis in their study population. A retrospective study by Tip et al., [33] found that the risk of peritonitis after colonoscopy without antibiotic prophylaxis was 6.3%. The authors however, indicated that it lacks statistical significance compared with prior antibiotic prophylaxis [33]. Colon biopsy or polypectomy did not appear to further increase the risk of peritonitis in our cohort [34]. Interestingly, the International Society for Peritoneal Dialysis recommended antibiotic prophylaxis before any procedure involving the abdomen or pelvis, including colonoscopy [14]. Again, it is important to notice that these recommendations were based only on observational studies and case reports. The 2005 and the 2016 ISPD guidelines suggested empirical 1 gram ampicillin or aminoglycoside with or without metronidazole before colonoscopy [14,35]. These guidelines recommend antibiotic prophylaxis for CAPD patients undergoing colonoscopy with polypectomy; however, there has been little literature to support these recommendations. Studies on these guidelines are rare, and randomized controlled studies to support this recommendation are lacking. Moreover, these new guidelines clearly stated that the optimal antibiotic regimen has not been determined by clinical study yet [14]. Similar suggestions were made by the Dutch Federation of Nephrology with the addition of dialysate drainage before the procedure [36]. However, these suggestions have not gained wide acceptance. Contrary to the suggestions above, the American Society for Gastrointestinal Endoscopy and the British Society of Gastroenterology do not suggest prophylactic antibiotics before colonoscopy [37,38]. There exists a lack of consensus on this issue. There have been few case reports in the literature on peritonitis following colonoscopy in peritoneal dialysis patients [6,7,12-15]. These reports suggested that instrumental procedures such as colonoscopy may precipitate gram negative peritonitis in PD patients. On the other hand, some literature reported bacterial peritonitis following endoscopic polypectomy in peritoneal dialysis patients despite antibiotics prophylaxis [39]. So far there are no strong data demonstrating a causal association between endoscopic procedures and bacteremia or that antibiotic prophylaxis prior to endoscopic procedures protects against bacteremia. Much of the existing data reflects estimated risk associated with conventional endoscopic techniques. There are no results available that confidently quantify bacteremia rates with newer endoscopic procedures such as per oral endoscopic myotomy, endoscopic submucosal dissection, flexible colonoscopy or polypectomy [34]. We studied APD patients with and without antibiotic prophylaxis before flexible sigmoidoscopy. The difference in peritonitis episodes in our study between the two groups was not statistically significant (3.9% vs. 5.8%, p> 0.05). Surprisingly, none of the post-polypectomy and none of our patients with diverticular disease (without diverticulitis) had post-procedure peritonitis in our cohort; finding that correlates with the report of Yip et al., [34] who stated that colonic biopsy or polypectomy was not associated with a higher risk of peritonitis in their CAPD patients. In addition, we did not encounter serious complications of colonoscopy i.e., perforation or hemorrhage. Transient bacteremia occurs frequently during routine daily activity, often at rates exceeding those associated with endoscopic procedures. Brushing and flossing of teeth has been associated with rates of bacteremia of 20% to 68%, use of toothpicks with rates of 20% to 40%, and even activity that might be considered entirely physiologic, such as chewing food, with rates ranging from 7% to 51% [40]. One patient from the group of those who received prophylactic antibiotics had Candida species in peritoneal fluid culture. Although we could not prove the relation between antibiotic prophylaxis and the development of this unexpected growth, it is not unreasonable to speculate that antibiotic administration may have favored intestinal non-bacterial overgrowth (Candida in our case) which, potentially, may have conditioned the pathogenicity of these organisms in that patient. The human colonic microflora ecosystem, its metabolic functions, and its colonization resistance are vital for the well-being of the host, production of vital metabolites, and prevention of infection. In a study by Edlund and Nord [41] marked ecological disturbances were seen in the intestinal microflora during antibiotic treatment. The numbers of enterococci, enterobacteria (except E. coli) and peptostreptococci increased significantly during treatment. Eight patients became newly colonized by Klebsiella spp. and Citrobacter freundii during treatment. The number of patients colonized with yeasts (mostly C. albicansi ) increased from zero to nine during treatment; two patients were still colonized with yeasts after treatment. Sullivan et al., [42,43] reported that administration of antimicrobial agents, therapeutically or as prophylaxis, causes disturbances in the ecological balance between the host and the normal intestinal microflora and that by using antimicrobial agents, the risk of emergence and spread of resistant strains between patients and dissemination of resistant microorganisms increases significantly. In a study concerned with a similar matter, Berg [44] concluded that the colonic microflora appears to stimulate the host immune system to respond rapidly to pathogen challenges. Although the cells of the intestinal tract coexist with the normal commensal microflora, they recognize and clear invading pathogens before returning to homeostasis with the commensal bacteria. The colonic microflora provides a number of benefits, including contributing to the host’s nutrition and protecting the host from infection. In most cases of antimicrobial prophylaxis or therapy, the bacterial populations in some genera are reduced in numbers while those in other genera increase. In some cases, the increased numbers of certain bacteria are accompanied by resistant strains of bacteria or overgrowth by fungi. Treatment with antimicrobial combinations does not necessarily prevent resistance development. It may even result in fungal overgrowth and appearance of bacteria with resistance to all of the drugs in the combination [45]. Given the notorious possibility of resistant strains’ development and the relative rarity with which most PD patients undergo colonoscopy procedures, the frequency and risk of colonoscopy-related bacteremia, as we demonstrated in our study, is trivial compared with the frequency of bacteremia encountered with routine daily activity. This provides a strong rationale against routine administration of antibiotic prophylaxis prior to all endoscopic procedures.
There are some limitations, however, in our study. First, this study was conducted in a single tertiary medical center, and endoscopy-associated complications may vary in different hospitals. Second, the study was conducted on a selected group of APD patients after applying strict exclusion criteria. Third, the study recorded only 93 endoscopic procedures and may have underestimated the importance of antibiotic prophylaxis. Therefore, larger randomized trials are required to explore the necessity of antibiotic prophylaxis in the prevention of postcoloscopic PD peritonitis. Nevertheless, our study has the strength of being the first prospective randomized study in this field.
CONCLUSION
There is no strong correlation between colonoscopy and risk of peritonitis in end stage renal disease patients on peritoneal dialysis and apparently the overall risk is low 8.7%, (less than 10.0 %). In addition there was no correlation between the risk of peritonitis and intraperitoneal prophylactic ceftazidime. Neither polypectomy; partial or complete nor diverticulosis were associated with increased incidence of post-colonoscopy peritonitis. The study, however, the study recorded limited number of patients and may have underestimated the importance of antibiotic prophylaxis. Therefore, largerprospective randomized trialsareneeded.
REFERENCES
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, et al. (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66: 683-691.
- Kumar S, Abcarian H, Prasad ML, Lakshmanan S (1982) Bacteremia associated with lower gastrointestinal endoscopy, fact or fiction? I. Colonoscopy. Dis Colon Rectum 25: 131-134.
- Shorvon PJ, Eykyn SJ, Cotton PB (1983) Gastrointestinal instrumentation, bacteraemia, and endocarditis. Gut 24: 1078-1093.
- Macrae FA, Tan KG, Williams CB (1983) Towards safer colonoscopy: A report on the complications of 5000 diagnostic or therapeutic colonoscopies. Gut 24: 376-383.
- Carlos CA, McCulloch CE, Hsu CY, Grimes B, Pavkov ME, et al. (2017) Colon Cancer Screening among Patients Receiving Dialysis in the United States: Are We Choosing Wisely? J Am Soc Nephrol 28: 2521-2528.
- Ray SM, Piraino B, Holley J (1990) Peritonitis following colonoscopy in a peritoneal dialysis patient. Perit Dial Int 10: 97-98.
- Bac DJ, van Blankenstein M, de Marie S, Fieren MW (1994) Peritonitis following endoscopic polypectomy in a peritoneal dialysis patient: the need for antibiotic prophylaxis. Infection 22: 220-221.
- Fried L, Bernardini J, Piraino B (2000) Iatrogenic peritonitis: the need for prophylaxis. Perit Dial Int 20: 343-345.
- Poortvliet W, Selten HP, Raasveld MH, Klemt-Kropp M (2010) CAPD peritonitis after colonoscopy: follow the guidelines. Neth J Med 68: 377-378.
- Petersen JH, Weesner RE, Giannella RA (1987) Escherichia coli peritonitis after left-sided colonoscopy in a patient on continuous ambulatory peritoneal dialysis. Am J Gastroenterol 82: 171-172.
- Holley J, Seibert D, Moss A (1987) Peritonitis following colonoscopy and polypectomy: a need for prophylaxis. Perit Dial Bull 7:105.
- Verger C, Danne O, Vuillemin F (1987) Colonoscopy and continuous ambulatory peritoneal dialysis. Gastrointest Endosc 33: 334-335.
- Sprenger R, Neyer U (1987) Enterococcus-peritonitis after endoscopic polypectomy: need for prophylactic antibi-otics. Perit Dial Bull 7:263.
- Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, et al. (2016) ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int 36: 481-508.
- Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, et al. (2010) Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 30: 393-423.
- Aljumah AA, Aljebreen AM (2017) Policy of screening for colorectal cancer in Saudi Arabia: A prospective analysis. Saudi J Gastroenterol 23: 161-168.
- Woodrow G, Turney JH, Brownjohn AM (1997) Technique failure in peritoneal dialysis and its impact on patient survival. Perit Dial Int 17: 360-364.
- Choi P, Nemati E, Banerjee A, Preston E, Levy J, et al. (2004) Peritoneal dialysis catheter removal for acute peritonitis: a retrospective analysis of factors associated with catheter removal and prolonged postoperative hospitalization. Am J Kidney Dis 43: 103-111.
- Szeto CC, Chow KM, Wong TY, Leung CB, Li PK (2002) Conservative management of polymicrobial peritonitis complicating peritoneal dialysis--a series of 140 consecutive cases. Am J Med 113: 728-733.
- Bayston R, Andrews M, Rigg K, Shelton A (1999) Recurrent infection and catheter loss in patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 19: 550-555.
- Bunke CM, Brier ME, Golper TA (1997) Outcomes of single organism peritonitis in peritoneal dialysis: gram negatives versus gram positives in the Network 9 Peritonitis Study. Kidney Int 52: 524-529.
- Piraino B, Bernardini J, Sorkin M (1986) The influence of peritoneal catheter exit-site infections on peritonitis, tunnel infections, and catheter loss in patients on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 8: 436-440.
- Piraino B, Bernardini J, Sorkin M (1989) Catheter infections as a factor in the transfer of continuous ambulatory peritoneal dialysis patients to hemodialysis. Am J Kidney Dis 13: 365-369.
- Johnson DW, Dent H, Hawley CM, McDonald SP, Rosman JB, et al. (2009) Associations of dialysis modality and infectious mortality in incident dialysis patients in Australia and New Zealand. Am J Kidney Dis 53: 290-297.
- Tranaeus A, Heimbürger O, Lindholm B (1989) Peritonitis in continuous ambulatory peritoneal dialysis (CAPD): diagnostic findings, therapeutic outcome and complications. Perit Dial Int 9: 179-190.
- Piraino B, Bernardini J (1997) The high risk of gram-negative peritonitis. Perit Dial Int 17: 36.
- Nebel M, Gerding W, Finka K, Weber M (1997) Enteric peritonitis in PD patients. Perit Dial Int 17: 35.
- Coward RA, Gokal R, Wise M, Mallick NP, Warrell D (1982) Peritonitis associated with vaginal leakage of dialysis fluid in continuous ambulatory peritoneal dialysis. Br Med J (Clin Res Ed) 284: 1529.
- Swartz RD, Campbell DA, Stone D, Dickinson C (1983) Recurrent polymicrobial peritonitis from a gynecologic source as a complication of CAPO. Perit Dial Bull 3: 32-33.
- Piraino B, Bernardini J, Brown E, Figueiredo A, Johnson DW, et al. (2011) ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int 31: 614-630.
- Poortvliet W, Selten HP, Raasveld MH, Klemt-Kropp M (2010) CAPD peritonitis after colonoscopy: follow the guidelines. Neth J Med 68: 377-378.
- Toda S, Ito Y, Mizuno M, Suzuki Y, Ito I, et al. (2012) Asymptomatic diverticulosis identified by computed tomography is not a risk factor for enteric peritonitis. Nephrol Dial Transplant 27: 2511-2516.
- Yip T, Tse KC, Lam MF, Cheng SW, Lui SL, et al. (2010) Colonic diverticulosis as a risk factor for peritonitis in Chinese peritoneal dialysis patients. Perit Dial Int 30: 187-191.
- Yip T, Tse KC, Lam MF, Cheng SW, Lui SL, et al. (2007) Risks and outcomes of peritonitis after flexible colonoscopy in CAPD patients. Perit Dial Int 27: 560-564.
- Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, et al. (2005) Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 25: 107-131.
- Sipahi S, Güngör Ö, Kircelli F, Aydin P, Ülker EA, et al. ( 2012) Peritonitis after Colonoscopy in a Peritoneal Dialysis Patient. Turk Neph Dial Transpl 21: 105-106.
- ASGE Standards of Practice Committee, Khashab MA, Chithadi KV, Acosta RD, Bruining DH, et al. (2015) Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc 81: 81-89.
- Allison MC, Sandoe JAT, Tighe R, Simpson IA, Hall RJ, et al. (2009) Antibiotic prophylaxis in gastrointestinal endoscopy. Gut 58: 869-880.
- Katsanos KH, Tsianos EV (2010) Bacterial peritonitis following multiple endoscopic polypectomy in a peritoneal dialysis patient despite antibiotic prophylaxis. Annals of Gastroenterology 23: 211-212.
- Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, et al. (2007) Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 116: 1736-1754.
- Edlund C, Nord CE (1999) Effect of quinolones on intestinal ecology. Drugs 58: 65-70.
- Sullivan A, Edlund C, Nord CE (2001a) Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect Dis 1: 101-114.
- Sullivan A, Edlund C, Svenungsson B, Emtestam L, Nord CE (2001b) Effect of perorally administered pivmecillinam on the normal oropharyngeal, intestinal and skin microflora. J Chemother 13: 299-308.
- Berg RD (1996) The indigenous gastrointestinal microflora. Trends Microbiol 4: 430-435.
- Rafii F, Sutherland JB, Cerniglia CE (2008) Effects of treatment with antimicrobial agents on the human colonic microflora. Ther Clin Risk Manag 4: 1343-1358.




