
Evaluation and Utilization of Dicarboxylic Acids (DCA) as an Alternative to Strong Mineral Acids for Selective Extraction of C5-Sugars in an Integrated Biorefinery
*Corresponding Author(s):
Jagannadh SatyavoluConn Center For Renewable Energy Research, University Of Louisville, Louisville, KY 40292, United States
Tel:1 502 654-0738,
Email:jagannadh.satyavolu@louisville.edu
Abstract
Keywords
INTRODUCTION
Hemicellulose Hydrolysis and Xylose Decomposition
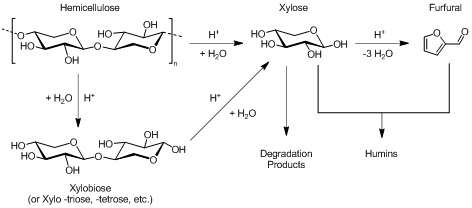
Figure 1: Scheme showing the mechanism of xylan conversion to xylose [26].
This clearly shows that the release of xylose as well as its degradation requires H+, indicating that solutions with higher hydronium concentrations should facilitate the release of the xylose more readily, but could also lead to degradation of xylose to furfural and humins.
DICARBOXYLIC ACIDS (DCA)
|
Acid |
Acid Costs |
Reactor Costs |
Selectivity to C5 Sugars |
C5 Yields |
Potential Recyclability |
|
|
Sulfuric Acid |
Inexpensive |
Higher, requires corrosion resistant materials |
Higher Furfural yields (1.0 g/L) |
High yields >90% |
Need caustic to neutralize |
|
|
DCA |
Expensive |
Lower, lesser need for corrosion resistance |
Lower Furfural yields, approx. 33% of sulfuric yields |
High yields >90% |
Potential for separation and recycle |
Table 1: Benefits and disadvantages of using sulfuric and Di-Carboxylic Acids (DCA) in hydrolysis processes.
Oxalic and maleic acids have been the most studied DCA in the selective removal of hemicellulose from biomass material [27,33-39]. Previous work focused on the use of DCA as a pretreatment to remove hemicellulose for the production of ethanol from residual C6 sugar fermentation (via enzymatic hydrolysis of cellulose) and the synthesis of furfural via acid catalyzed dehydration of C5 sugars in hydrolyzate in some instances [27,33-40]. However, the prohibitively high cost of DCA compared to mineral acid requires that 1) the DCA are recovered from the hydrolyzate and then recycled in order to reduce chemical costs; and 2) the removed hemicelluloses are utilized to convert to value added bioproducts.
Ultimately, the implementation of a less corrosive, controllable, selective and potentially recyclable dicarboxylic acids to perform hydrolysis of hemicellulose in biomass would be invaluable towards making an integrated biorefinery economically viable. The objective of this work is to evaluate a chosen set of DCA for selectivity towards C5-sugars (primarily xylose) in order to obtain a hydrolyzate solution that is rich in C5 saccharides (xylose) with minimal degradation products. The intent is to reduce the overall processing cost for an integrated C5-based refinery leveraging the potential benefits using DCA compared to strong mineral acids for use in hemicellulose hydrolysis.MATERIALS AND METHODS
Materials
Figure 2: Acids investigated for C5-hydrolysis during this study.
D-glucose; L-arabinose; D-xylose; ethanol; the acids - sulfuric (H2SO4), oxalic (H2Ox), succinic (H2Su), fumaric (H2Fu), adipic (H2Ad), maleic (H2Ma) and p-Toluenesulfonic (PTSA); 2-furfuraldehyde (Ff); 5-hydroxymethylfurfuraldehyde (5-HMF); glycerol; and acetic acid were purchased from VWR (≥ 99% purity) and were used as received without further purification. Ff and 5-HMF was used as received and stored in a fridge (40C).
The Brown-Forman Corporation supplied the corn based Dried Distiller’s Grains (DDG) used in this study. The DDG samples were dried to (<10% moisture) and manually sifted through a 1 mm screen. Moisture balance readings for each biomass were performed on 1 g samples using an Intelligent Weighing Technologies Model IL-50.001 moisture balance before use.
Methods
Proton concentration calculations
Where, the pH was the measured pH value at the start of each of the runs. To calculate the theoretical [H+], the pKa from literature was treated in the same manner as the pH (equation 2) and then set up with the dissociation equilibrium equation (equation 3).
In equation 3, “A” represents the anion associated with the acid. From equation 3, an equilibrium table was set up and the resulting quadratic equation was solved for [H+]. Sulfuric acid, PTSA and the DCA were also compared using their combined severity factor [26,44].
Combined Severity Factor (CSF)
Where, t is the time of reaction, Tr is the reaction temperature, Tref is the reference temperature (1000C) and pH is the measured pH value of the acid solution before the reaction starts.
RESULTS AND DISCUSSION
Selective hemicellulose hydrolysis using DCA
|
Acid |
Xylose (g/l) |
Glucose (g/l) |
Arabinose (g/l) |
Total Sugars(g/l) |
|
Sulfuric |
18.8 ± 0.4 |
2.9 ± 0.2 |
10.28 ± 0.4 |
31.99 ± 1.00 |
|
PTSA |
12.5 ± 0.3 |
0.63 ± 0.3 |
8.6 ± 0.1 |
21.67 ± 0.7 |
|
Oxalic |
13.7 ± 1.5 |
0.92 ± 0.4 |
8.55 ± 0.1 |
23.18 ± 2.0 |
|
Maleic |
7.7 ± 0.9 |
2.66 ± 1.7 |
7.51 ± 0.1 |
17.84 ± 2.7 |
|
Fumaric |
3.4 ± 0.1 |
0.07 ± 0.0 |
6.49 ± 0.1 |
9.94 ± 0.2 |
|
Succinic |
1.6 ±0.1 |
1.61 ± 0.9 |
5.02 ± 0.2 |
7.5 ± 0.1 |
|
Adipic |
1.34 ± 0.2 |
1.43 ± 0.3 |
4.19 ± 0.4 |
6.95 ± 0.9 |
|
Sugar selectivity (%) |
||||
|
Sulfuric |
58.79 ± 0.2 |
9.08 ± 0.7 |
32.13 ± 0.5 |
- |
|
PTSA |
57.46 ± 0.5 |
2.01 ± 0.0 |
40.53 ± 0.5 |
- |
|
Oxalic |
58.99 ± 1.8 |
3.85 ±1.6 |
37.16 ± 3.0 |
- |
|
Maleic |
43.25 ± 6.8 |
14.54 ± 9.2 |
42.21 ± 2.4 |
- |
|
Fumaric |
34.1 ± 0.4 |
0.65 ± 0.1 |
65.25 ± 0.6 |
- |
|
Succinic |
21.74 ± 2.2 |
10.13 ± 10.0 |
68.13 ± 7.7 |
- |
|
Adipic |
19.29 ± 0.1 |
20.32 ± 1.5 |
60.39 ± 1.4 |
- |
Table 2 shows that in the case of the sulfuric, PTSA, oxalic and maleic acids, the xylose concentration is higher than the arabinose concentration, with sulfuric being the highest overall xylose concentration. Further, table 2 reveals that the oxalic acid and sulfuric acid have the highest selectivity for xylose (comparable to that from sulfuric acid), a value 59% for each. Maleic acid, forming lower amounts of xylose and a comparable amount of arabinose would discount its use in the processes mentioned previously. Table 2 also shows that the other DCA (adipic, succinic and fumaric) remove higher amounts of arabinose compared to xylose. As a result, they have low xylose selctivities of 19, 21 and 34% respectively. Since oxalic acid has the best performance as a DCA for xylose release and less corrosive than sulfuric acid, it has the best chance at replacing sulfuric acid for xylose hydrolysis.
Something else to consider is the potential to use the arabinose selective acids (adipic, succinic and fumaric) as a pre-treatment in a two-step hydrolysis. These particular acids show an affinity for arabinose removal and could be used initialy to remove arabinose from the biomass before the biomass is hydrolyzed with one of the xylose selective acids. This could improve overall yields and selectivity of both sugars.
Lu et al., reported xylose yields close to 100% for both sulfuric acid and maleic acid at a cost over loading of 40g/l in the reactor with significantly lower yields at higher DDG loadings (62.5% and 55.2% respectively at 150g/l loading) [27]. The yield values are comparable to the values reported in our current work using DDG. Scordia et al., reported a near complete monomer liberation from oxalic acid using 4:1 liquid to solid weight ratio (comparable to 25g/l) using similar acid concentrations for giant reed [35]. Low yields from oxalic acid in our study (72% C5 sugars yield) could be due to using different type of biomass (DDG vs giant reed) or due to differences in the type of reactors used (percolating reactor vs stirred type reactor).
DEGRADATION PRODUCT FORMATION
|
Acid |
Furfural (g/l) |
Acetic Acid (g/l) |
|
Sulfuric |
0.97 ± 0.3 |
1.9 ± 1.5 |
|
PTSA |
0.38 ± 0.2 |
0.9 ± 0.1 |
|
Oxalic |
0.34 ± 0.4 |
1.0 ± 0.2 |
|
Maleic |
0.29 ± 0.2 |
0.5 ± 0.1 |
|
Fumaric |
0.49 ± 0.0 |
7.4 ± 0.2 |
|
Succinic |
0.30 ± 0.0 |
0.3 ± 0.0 |
|
Adipic |
0.21 ± 0.0 |
11.1 ± 0.4 |
The data presented shows that the highest ratio of furfural formation using a DCA compared to that using the sulfuric acid was with fumaric acid (0.505), which also delivered the lowest yield of xylose as presented in table 2. The ratio for furfural was at or below 0.39 for the other DCA used in this study. In the case of the other degradation product - acetic acid, both the fumaric and adipic acids led to a much higher concentration of acetic acid compared to the other acids, reaching concentrations more than five times as high as sulfuric. This would indicate that the structures of these DCA are more effective at catalyzing xylose degradation to acetic acid than the remaining acids. This result could be due to thermal degradation of xylose or xylan to acetic acids prior to formation of more stable dehydrated furfural. However, as illustrated in figure 1, total furfural or acetic acid concentrations cannot account for the entire loss (degradation) of xylose; possibly polymerization to humin can also happen simultaneously.
INVESTIGATING THE FACTORS INFLUENCING THE HYDROLYSIS PRODUCT YIELDS
pKa and hydronium ion concentration
|
Acid |
pKa, 1 |
pKa, 2 |
pH (Theoretical) |
Combined Severity Factor (CSF) |
Solubility (g/ml at 200C)g |
|
H2SO4 |
-3 |
1.99 |
1.3 |
1.66 |
Miscible |
|
PTSA |
-2.8 |
- |
1.3 |
1.66 |
90 |
|
H2Ox |
1.23 |
4.14 |
1.8 |
1.16 |
100 |
|
H2Ma |
1.98 |
6.07 |
1.57 |
1.39 |
4.3 |
|
H2Fu |
3.03 |
4.44 |
2.78 |
0.18 |
6.9 |
|
H2Su |
4.19 |
5.6 |
2.71 |
0.25 |
478 |
|
H2Ad |
4.42 |
5.41 |
2.76 |
0.2 |
67 |
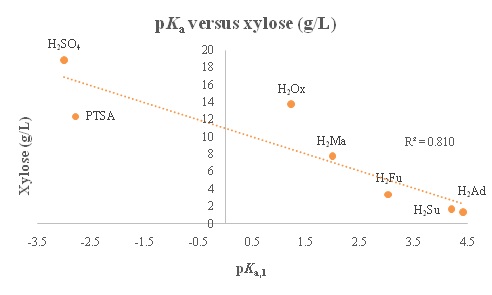
Dissociation is very dependent on the physical structure of the molecule. For example, maleic acid has a lower pKa than fumaric acid because an intramolecular hydrogen bond is formed after H2Ma loses the initial proton, making the conjugate base of maleic acid more stable than that of the conjugate base of the fumaric acid. In the current study, sulfuric acid is the strongest acid with a pKa of -3, and adipic acid is the weakest with a pKa of 4.42. Therefore, since this reaction is catalyzed by the hydronium ion, the more acidic the solution, the better the xylose release should be. A plot of the pKa values vs xylose concentrations is presented in figure 3 (a).
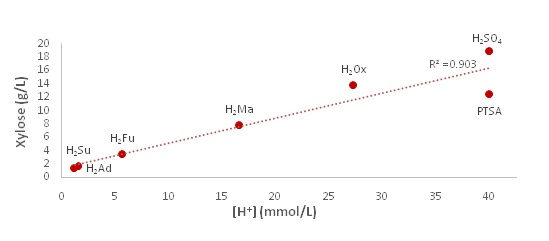
As can be seen from the figure 3(a), the xylose concentration follows an inverse trend with the pKa,1 value, as would be expected. The R2 value of 0.8103 shows a relatively good correlation between the pKaand the xylose release. However, the oxalic acid shows that xylose can be removed effectively even with a more positive pKa. This indicates that there is more to the process than just acid dissociation. The theoretical concentration of [H+] in solution compared to xylose concentration is presented in figure3 (b). As shown in the figure, the xylose concentration has a strong (R2 = 0.9037) positive correlation with the hydronium ion concentration in the acid solution. This would indicate that the experimental data follow predictions made based on acid strength, showing evidence that a strong acid would be the best for xylose release on the whole. However, PTSA seems not to fit this trend as it has the same ion concentration as sulfuric acid, but a much lower xylose release value compared to sulfuric acid. This indicates a possible significance of the second proton. Absence of second proton for PTSA seems to hinder the xylose yield even though its pKa is a comparable to that of sulfuric. In figure 4, pKa,1 and furfural concentration are plotted to determine the relationship between pKa and degradation products.
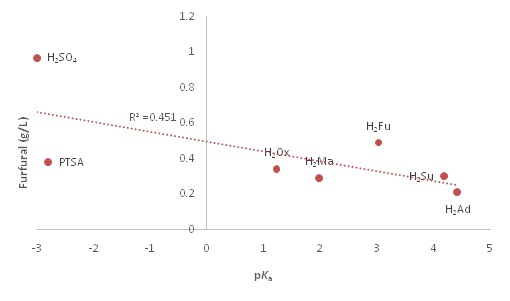
From the plot, it is seen that, in general, the lower the pKa, the higher the furfural concentration. However, the trend is loose (R2 = 0.451), as all of the acids except the sulfuric have similar Ff values. This is indicative that the degradation product is not dependent on the hydronium concentration completely. This plot also rejects the idea that acids with a second proton would produce higher degradation product concentrations as all of the diprotic DCA have almost the same [Ff] as PTSA. In this data set, sulfuric acid is essentially an outlier due to its high concentration of Ff around 1.0 g/l. This experimentally confirms that certain DCA (e.g., oxalic) can be suitable to replace sulfuric acid in C5 hydrolysis reactions. It also shows that the remaining acids would all be effective at reducing furfural amounts, even if the xylose concentrations produced are comparable to sulfuric acid. This is further illustrated by plotting the xylose concentrations against the corresponding degradation product concentrations figure 5.
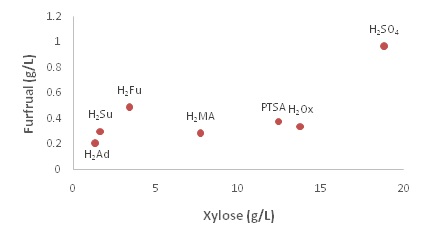
These plots can help determine the most suitable DCA that can deliver a high xylose value and a low degradation product value. Figure 5 (a) shows that the most effective acid for minimal furfural production is oxalic acid with sulfuric showing a much higher furfural concentration and the other acids not able to produce enough xylose. Figure 5 (b) shows that, again the best acid for hydrolysis optimization would be the oxalic, with the exception of sulfuric. However, if sulfuric acid’s affinity for fufural production is considered, then oxalic acid remains the desirable acid for C5-hydrolysis of biomass.

Combined Severity Facor (CSF)
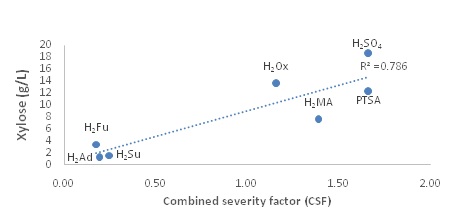
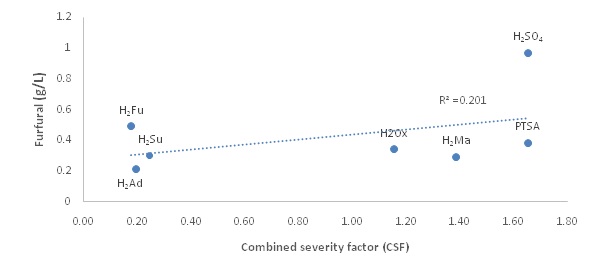
Kinetic studies on xylose release using oxalic acid, maleic acid and PTSA
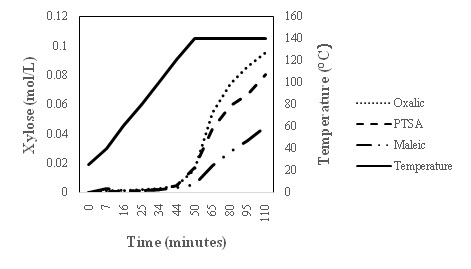
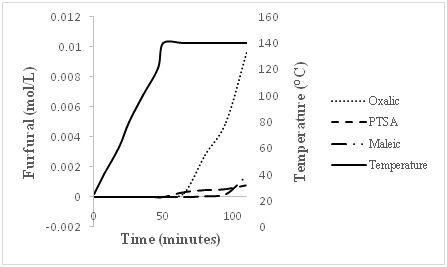
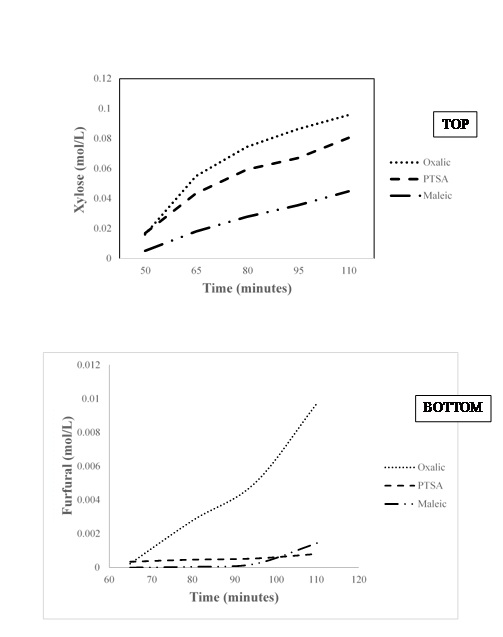
CONCLUSION
ACKNOWLEDGEMENT
REFERENCES
- Bozell JJ, Patel MK (2006) Feedstocks for the Future: Renewables for the Production of Chemicals and Materials. ACS Symposium Series 921.
- Sanders J, Scott E, Weusthuis R, Mooibroek H (2007) Bio-refinery as the bio-inspired process to bulk chemicals. Macromol Biosci 7: 105-117.
- van Haveren J, Scott EL, Sanders J (2007) Bulk chemicals from biomass. Biofuels Bioprod Biorefin 2 : 41-57.
- Alonso DM, Bond JQ, Dumesic JA (2010) Catalytic conversion of biomass to biofuels. Green Chemistry 12: 1493-1513.
- Rinaldi R, Schüth F (2009) Acid hydrolysis of cellulose as the entry point into biorefinery schemes. ChemSusChem 2: 1096-1107.
- Alonso DM, Wettstein SG, Mellmer MA, Gurbuz EI, Dumesic JA (2013) Integrated conversion of hemicellulose and cellulose from lignocellulosic biomass. Energy Environ Sci 6: 76-80.
- Zhang GS, Zhu M-M, Zhang Q, Liu Y-M, He H-Y, et al. (2016) Towards quantitative and scalable transformation of furfural to cyclopentanone with supported gold catalysts. Green Chem 18: 2155-2164.
- Uchiyama T, Shishikura K, Ogawa K, Ohshima Y, Miyairi S (2016) An efficient method for the preparation of 1,5-anhydroalditol from unprotected carbohydrates via glycopyranosyl iodide. Tetrathedron Letters 57: 5294-
- Wang W, Li N, Li G, Li S, Wang W, et al. (2017) Synthesis of Renewable High-Density Fuel with Cyclopentanone Derived from Hemicellulose. ACS Sustainable Chem Eng 5: 1812-
- Yang J, Li N, Li G, Wang W, Wang A, et al. (2014) Synthesis of renewable high-density fuels using cyclopentanone derived from lignocellulose. Chem Commun 50: 2572-
- Werpy T, Petersen G (2004) Top value added chemicals from biomass: Results of Screening for Potential Candidates from Sugars and Synthesis Gas. US Department of Energy, National Renewable Energy Laboratory, Denver W Pkwy, Golden, CO, USA.
- Fonseca DA, Lupitskyy R, Timmons D, Gupta M, Satyavolu J (2014) Towards integrated biorefinery from dried distillers grains: Selective extraction of pentoses using dilute acid hydrolysis. Biomass and Biotechnology 71: 178-186.
- Noureddini H, Byun J (2010) Dilute-acid pretreatment of distillers’ grains and corn fiber. Bioresour Technol 101: 1060-1067.
- Balat M, Balat H, Oz C (2008) Progress in bioethanol processing. Prog Energy Combust Sci 34: 551-573.
- Bensah EC, Mensah M (2013) Chemical pretreatment methods for the production of Cellulosic ethanol: technologies and innovations. Int J of Chem. Eng 179607:1-22.
- Alireza E, Hashimoto AG, Fenske JJ, Penner MH (1997) Modeling and Optimization of the Dilute-sulfuric-acid Pretreatment of Corn Stover, Poplar and Switchgrass. Bioresource Technology2-3: 129-36.
- Danon B, Marcotulio G, de Jong W (2014) Mechanistic and kinetic aspects of pentose dehydration towards furfural in aqueous mediaemploying homogeneous catalysis. Green Chem 16: 39-54.
- Vinueza NR, Kim ES, Gallardo VA, Mosier NS, Abu-Omar MM, et al. (2015) Tandem mass spectrometric characterization of the conversion of xylose to furfural. Biomass and Bioenergy 74: 1-5.
- Feather MS (1970) The conversion of D-xylose and D-glucuronic acid to 2-furaldehyde. Tetrahedron Lett 11: 4143-4145.
- Feather MS, Harris DW, Nichols SB (1972) Routes of conversion of D-xylose, hexuronic acids, and L-ascorbic acid to 2-furaldehyde. J Org Chem 37: 1606-1608.
- Harris DW, Feather MS (1973) Evidence for C2 to C1 intramolecular hydrogen transfering during the acid-catalized isomerization of D-glucose to D-fructose. Carbohydr Res 30: 359-365.
- Hurd CD, Isenhour LL (1932) Pentose reactions: I furfural formation. J Am Chem Soc 54: 317-330.
- Antal MJ, Leesomboon T, Mok WS, Richards GN (1991) Mechanism of formation of 2-furaldehyde from D-xylose. Carbohydr Res 217: 71-85.
- Zeittsch KJ (2000) The Chemistry and Technology of Furfural and its Many By-Products (1stedn). Elsevier, USA. Pg no:
- Enslow KR, Bell AT (2012) The kinetics of Brønsted acid-catalyzed hydrolysis of hemicellulose dissolved in 1-ethyl-3-methylimidazolium chloride. RSC Adv 2: 10028-10036.
- Chum HL, Johnson DK, Black SK, Overend RP (1990) Pretreatment-Catalyst effects and the combined severity parameter. Appl Biochem Biotechnol 24: 1-14.
- Lu YL, Mosier NS (2007) Biomimetic catalysis for hemicellulose hydrolysis in corn stover. Biotechnol Prog 23: 116-123.
- Attan? EC, Doumani TF (1949) Solubilities of Aliphatic Dicarboxylic Acids in Water. Industrial and Engineering Chemistry 41: 2015-2017.
- Gaivoronskii AN, Grazhan VA (2005) Solubility of adipic acid in organic solvents and water. Russian Journal of Applied Chemistry 78: 404-408.
- Hayes DJM (2013) Second-generation biofuels: why they are taking so long. Energy and Environment 2: 304-334.
- Moreas LdS, Kronemberger FdA, Ferraz HC, Habert AC (2015) Liquid-liquid extraction of succinic acid using a hollow fiber membrane contactor. Journal of Industrial and Engineering Chemistry 21: 206-211.
- Grzenia DL, Schell DJ, Wickramasinghe SR (2008) Membrane extraction for removal of acetic acid from biomass hydrolysates. Journal of Membrane Science 322:189-195.
- Lu Y, Mosier NS (2008) Kinetic modeling analysis of maleic acid-catalyzed hemicellulose hydrolysis in corn stover. Biotech and Bioeng 101: 1170-1181.
- Kootstra AMJ, Mosier NS, Scott EL, Beeftink HH, Sanders JPM (2009) Differential effects of mineral and organic acids on the kinetics of arabinose degradation under lignocellulose pretreatment conditions. Biochemical Engineering 43: 92-97.
- Scordia D, Cosentino SL, Lee JW, Jefferies TW (2011) Dilute oxalic acid pretreatment for biorefining giant reed (Arundo donax L.). Biomass and Bioenergy 35: 3018-3024.
- Kootstra AMJ, Beeftink HH, Scott EL, Sanders JMP (2009) Comparison of dilute mineral and organic acid pretreatment for enzymatic hydrolysis of wheat straw. Biochemical Engineering 46: 126-131.
- vom Stein T, Grande PM, Kayser H, Sibilia F, Leitner W, de Maria PD (2011) From biomass to feedstock: one-step fractionation of lignocellulose components by the selective organic acid-catalyzed depolymerization of hemicellulose in a biphasic system. Green Chemistry 7: 1772-1777.
- Lee JW, Rodrigues RC, Kim HJ, Choi IG, Jeffries TW (2010) The roles of xylan and lignin in oxalic acid pretreated corncob during separate enzymatic hydrolysis and ethanol fermentation. Bioresour Technol 101: 4379-4385.
- Kim ES, Liu S, Abu-Omar MM, Mosier NS (2012) Selective Conversion of Biomass Hemicellulose to Furfural Using Maleic Acid with Microwave Heating. Energy & Fuels 26: 1298-1304.
- Riera FA, Alvarez R, Coca J (1991) Production of furfural by acid hydrolysis of corncobs. J Chem Tech Biotech 50: 149-155.
- Dang L, Du W, Black S, Wei H (2009) Solubility of fumaric acid in propan-2-ol, ethanol, acetone, propan-1-ol, and water. Journal of Engineering Data 54: 3112-3113.
- Yang B, Wyman CE (2008) Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels, Bioproducts & Biorefining 2: 26-40.
- Belyea RL, Rausch KD, Tumbleson ME (2004) Composition of corn and distillers dried grains with solubles from dry grind ethanol processing. Bioresource Technology 94: 293-298.
- Lee JW, Jefferies TW (2011) Efficiencies of acid catalysts in the hydrolysis of lignocellulosic biomass over a range of combined severity factors. Bioresour Technol 102: 5884-5890.
- Taherzadeh MJ, Karimi K (2007) Acid-based hydrolysis processes for ethanol from lignocellulosic materials: a review. BioResources 2: 472-499.
- Gutherie JP (1978) Hydrolysis of esters of oxy acids: pKa values for strong acids; Brønsted relationship for attack of water at methyl; free energies of hydrolysis of esters of oxy acids; and a linear relationship between free energy of hydrolysis and pKa holding over a range of 20 pKCanadian Journal of Chemistry 56: 2342-2354.
- Dippy JFJ, Hughes SRC, Rozanski A (1959) The dissociation constants of some symmetrically disubstituted succinic acids . J. Chem. Soc: 2492-2498.
Citation: Kim ES, Herde ZD, Thilakaratne R, Burns CT, Satyavolu J (2018) Evaluation and Utilization of Dicarboxylic Acids (DCA) as an Alternative to Strong Mineral Acids for Selective Extraction of C5-Sugars in an Integrated Biorefinery. Adv Ind Biotechnol 1: 001
Copyright: © 2018 Eurick S Kim, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

