
Evaluation of a Natural Polymer for the Development of Colon Specific Drug Delivery System
*Corresponding Author(s):
S MundadaDepartment Of Pharmaceutics, SNJB’s SSDJ College Of Pharmacy, Neminagar, Chandwad, Nashik, India
Tel:+91-9224360591,
Email:atishmundada@rediffmail.com
Abstract
Objective: Polysaccharide gums represent one of the most abundant industrial raw materials and have been the subject of intensive research due to their sustainability, biodegradability and safety. The purpose of present study was to evaluate a novel polymer, Anogeissus latifolia, as a carrier in the development of colon targeted drug delivery system.
Method: The matrix tablets of Diclofenac sodium were prepared using a 32 randomized full factorial design. The amount of natural polymer (X1) and amount of HPMC K100M (X2) were selected as independent variables. While the percentage cumulative drug release in lag time (Y1) and percentage cumulative drug release at 12 hours (Y2) were selected as dependent variables. The developed formulations were evaluated for Hardness, Friability, Weight variation, Drug content and in vitro drug release behaviour.
Results: The outcomes of physicochemical evaluation of developed formulations showed that all formulations showed optimum hardness, friability within limit and uniform weight and drug content. The formulation containing 40 %w/v natural polymer and 10 %w/v HPMC K100M was found to be optimized in terms of minimum drug release in lag time (≤ 25%) and % cumulative drug release in 12 h ( >95%).
Conclusion: The developed system exhibited a promising targeting behaviour proving Anogeissus latifolia gum to be an excellent carrier for colon targeting and thus it can be exploited commercially.
Keywords
INTRODUCTION
Targeted drug delivery into the colon is highly desirable for local treatment of bowel diseases such as ulcerative colitis, Chrohn’s disease, amoebiasis, colon cancer and also for systemic delivery of protein and peptide drugs because of less digestive enzymatic activity compared to stomach and small intestine. To deliver the compounds in non degraded form to lower part of the Gastrointestinal Tract (GIT), they must first of all pass through the stomach, the upper part of intestine and must use the characteristics of the colon to specifically release the drugs in this part of digestive tract [1] .Various approaches have been developed for colon targeting including pH dependent systems, Time dependent system and Microbially triggered delivery system. The pH dependent delivery system protects the formulation in the stomach and proximal part of small intestine but it may start to dissolve in the lower part of small intestine and thus it shows poor site specificity [2]. Similarly time dependent delivery systems are not able to sense any variation in upper GIT transit time [3,4]. Colonic bacteria possess ability to degrade a variety of polysaccharides present in the diet that are not degraded either in stomach or small intestine. Hence, the use of biodegradable polymers holds great promise for drug delivery to colon [1].
Gums and mucilages are used as inert pharmaceutical excipients and also as medicinal agent. They are widely used in food and pharmaceutical industry as thickening, stabilizing emulsifying and disintegrating agents [5]. The polysaccharide gums represent one of the most abundant industrial raw materials and have been the subject of intensive research over comparable synthetic materials due to their sustainability, biodegradability and safety [6]. The most of the natural polymers are obtained from trees in the forms of gums as tree exudates. Tree exudates gum are polysaccharides with high molecular weight mainly composed of monosaccharide unit, uronic acid and to some extent proteins and fibers. The purpose of this study was to evaluate novel polymer derived from the tree exudates of Anogeissus latifolia (Combretaceae, Myrtales) as a colon specific drug delivery carrier. It is a large deciduous tree found in dry areas of India. It is also known as Gum ghatti or Indian gum. This plant exudates has been in use for a long time and its name is derived from the word “Ghat” which means a mountain pass because of its ancient mountain transportation routes. It is approved as a food additive in Japan and has Generally Recognized as Safe (GRAS) in United States.
Gum ghatti is an extremely complex polysaccharide that occurs in nature as mixed calcium and magnesium salts of uronic acid and/or ghattic acids and has also been reported to contain approximately 3% protein. It consists of L-arabinose, D-galactose, D-mannose, D-xylose and D-glucuronic acid in a 48:29:10:5:10 molar ratio and < 1% of rhamnose, which is present as nonproducing end groups. Gum contains alternating 4-O-substituted and 2-O-substituted μ-D-mannopyranose units and chains of 1 → 6 linked β-D galactopyranose units with side chains of L-arabinose units. This gum has been reported to be an excellent emulsifier, thickener and binder. The gum is comprised of around 80% soluble dietary fiber, acts as a prebiotic by supplying the matrix required to sustain the bacterial flora of the human colon. This hydrocolloid is resistant to gastrointestinal enzymes and known to be degraded enzymatically only by the specific microflora of the colon such as Bifidobacterium longum [6,7,9-11]. In this investigation, gum ghatti in the form of matrix tablets have been evaluated for its ability to deliver formulation to the colon using Diclofenac Sodium (DS) as a model drug.
EXPERIMENTAL
Materials
| Coded levels | Actual value in percent | |
| X1 | X2 | |
| -1 | 20 | 10 |
| 0 | 30 | 20 |
| 1 | 40 | 30 |
RESULTS AND DISCUSSION
Characterization of drug
Solubility: DS is a slightly soluble drug in distilled water and 0.1 N HCl. The solubility of drug was found to be 0.0010 mg/ml and 0.64 mg/ml in 0.1N HCl and phosphate buffer pH- 6.8 respectively.
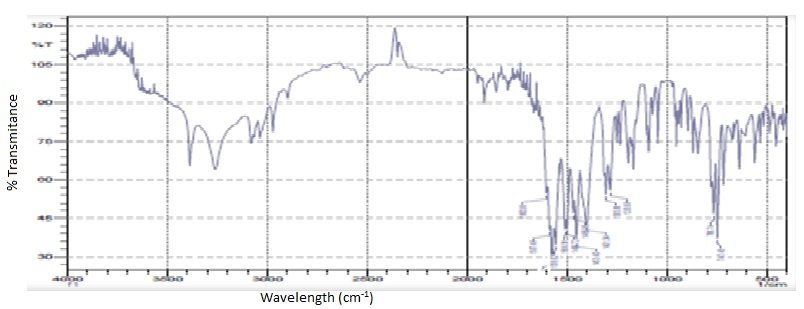
FTIR spectrum of diclofenac sodium: The IR spectra of DS (Figure 1a) exhibited principal peaks at wavenumbers 1573, 756, 1509, 775, 1283, 1305 cm−1.
| Parameter | Results |
| Solubility | Soluble in hot water, forming a viscous solution; practically insoluble in chloroform, Acetone, Methanol Also polymer swells in 0.1 N HCl and phosphate buffer and shows good gelling properties. |
| Moisture content (%) | 3% |
| Ash Value | 4% |
| Acid Insoluble ash | 1.60% |
| Water soluble ash | 1.40% |
| Viscosity 10% solution (Cps) | 400 |
| Swelling Index (%) | 150 |
| Heavy metals | Not more than 10 ppm |
| Ethanol soluble Extractive(%w/v) | 0.8% w/v |
| Density (g/ml) | Bulk- 0.9 ± 0.010 |
| Tapped- 0.984 ± 0.011 | |
| Carr’s index (%) | 8.54 ± 0.78 |
| Hausner’s ratio | 1.09 ± 0.055 |
| Angle of repose (θ) | 29.340 ± 0.53 |
The gum powder exhibited insolubility in common solvents except distilled water. The moisture content, ash value and all other parameters were within the limits as specified in official compendium. The values of angle of repose, Carr’s index, Hausner’s ratio indicated that the polymer has good flow property and compressibility.
FTIR spectrum of natural polymer: IR spectrum of natural polymer (Figure 1b) showed broad peaks at 3408.4 cm-1 (O-H stretching of carbohydrates), 2928, 1406, 1234 cm-1 (-CH2 asymmetric stretching, scissoring and twisting and rocking vibration of methylene groups, 1445.35 cm-1 (-CH and -CH2 in plane bending in carbohydrates), 1089.51cm-1 (-C-O stretching region as complex bands resulting from C-O and C-O-C stretching vibrations) and 643.71 cm-1 (pyranose ring).


Physicochemical evaluation: The physicochemical evaluation of developmental batches F1-F9 showed good flow property, uniformity of weight & drug content in powder blend as well as optimum hardness and friability limit specified for tablet formulations. The results are depicted in table 3 and table 4.
| Test Batch | Bulk Density | Tapped density | Carr’s index | Hausner’s Ratio | Angle of repose |
| F1 | 0.73±0.034 | 0.79±0.003 | 7.59±1.1 | 1.08±0.0023 | 31.36±0.21 |
| F2 | 0.69±0.065 | 0.75±0.003 | 8.01±0.51 | 1.07±0.005 | 31.99±0.25 |
| F3 | 0.7±0.025 | 0.75±0.03 | 6.66±1.07 | 1.07±0.001 | 31.07±0.18 |
| F4 | 0.74±0.005 | 0.81±0.001 | 8.02±0.66 | 1.08±0.005 | 32.61±0.04 |
| F5 | 0.74±0.005 | 0.84±0.01 | 11.9±0.72 | 1.08±0.007 | 32.64±0.37 |
| F6 | 0.68±0.04 | 0.73±0.02 | 6.84±0.61 | 1.07±0.007 | 30.81±0.22 |
| F7 | 0.75±0.03 | 0.82±0.04 | 8.53±0.4 | 1.08±0.004 | 32.81±0.08 |
| F8 | 0.82±0.042 | 0.88±0.056 | 6.81±0.56 | 1.09±0.005 | 33.16±0.22 |
| F9 | 0.81±0.005 | 0.87±0.041 | 6.89±1.07 | 1.076±0.011 | 33.62±0.05 |
| Test | Tablet weight (mg) | Hardness (Kg/cm2) | Friability (%) | Drug Content (%) |
| Batch | ||||
| F1 | 300.75±3.38 | 4.33±0.28 | 0.32±0.16 | 102.9±1.98 |
| F2 | 298.25±4.6 | 5.13±0.32 | 0.44±0.19 | 100.5±0.57 |
| F3 | 300.7±3.04 | 6.01±0.5 | 0.58±0.13 | 99.26±2.50 |
| F4 | 302.9±4.16 | 6.50±0.5 | 0.24±0.15 | 96.60±2.75 |
| F5 | 300.5±4.17 | 6.50±0.5 | 0.26±0.15 | 96.30±1.13 |
| F6 | 299.9±4.16 | 6.00±0.45 | 0.66±0.10 | 98.96±1.15 |
| F7 | 299.6±4.07 | 6.33±0.5 | 0.20±0.10 | 102.0±1.26 |
| F8 | 301.0±3.62 | 5.83±0.28 | 0.21±0.16 | 96.63±2.56 |
| F9 | 301.1±4.63 | 6.00±0.5 | 0.56±0.20 | 97.03±1.91 |
The precompression parameters of all batches were found to be in acceptable range indicating good flow behaviour.
The weight variation and friability was not more than 5% and 1% respectively. Good drug content uniformity was found among different batches of the tablets.
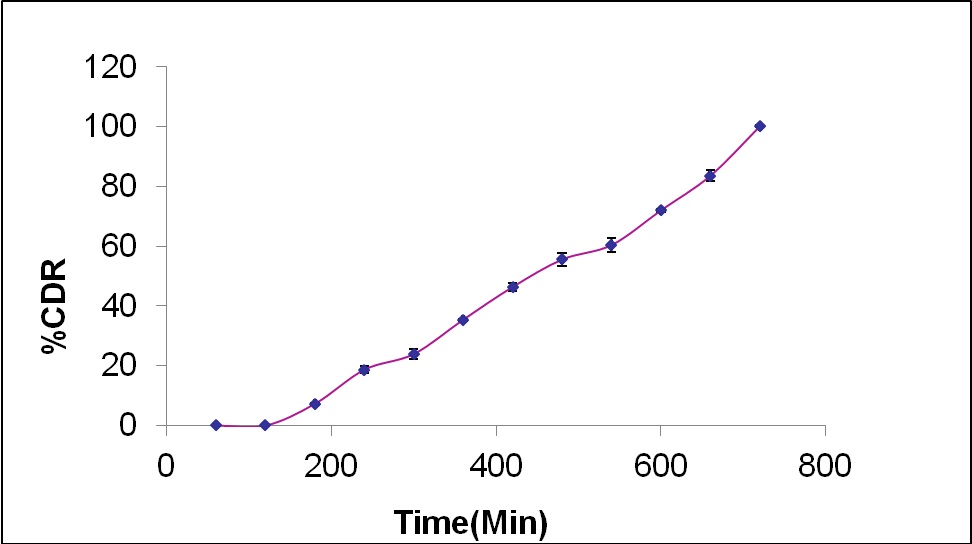
In vitro drug release study: The result of in vitro drug release is depicted in figure 2. All formulations (F1 - F9) were able to retain tablet in intact form up to 12 hrs and also sustained drug release up to 12 hrs. All the developed batches were capable of preventing drug from being released completely in the physiological environment of stomach but showed certain drug release in the small intestine. The formulations F3 and F6 were able to minimize the drug release up to 25% in the lag time of 5 hrs. All the developed batches showed cumulative drug release more than 90% after 12 hrs.
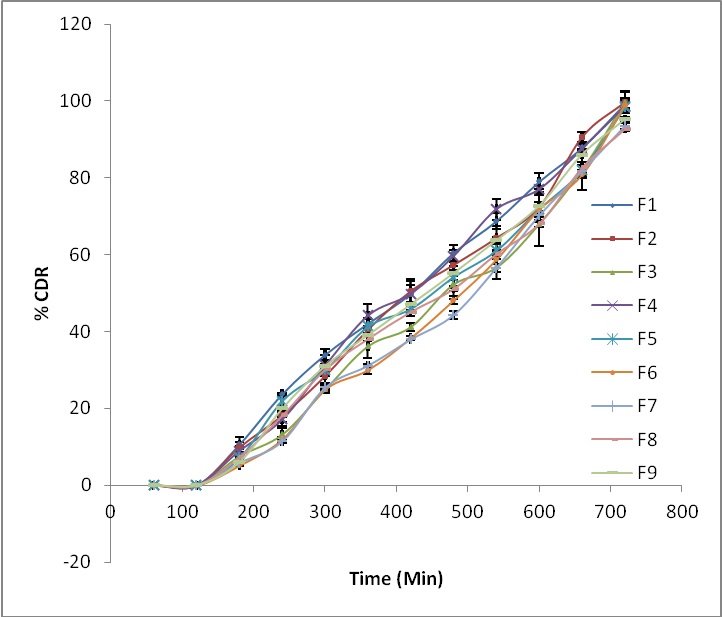
Figure 2: in vitro drug release profile of developmental batches (F1-F9).
The developed batches were able to prevent the drug release in acidic environment of stomach due the acid resistant property of natural polymer as well as low solubility of drug in acidic medium i.e., in first 2 hrs but unable to prevent the drug release in intestinal pH. Thus minimum drug release in lag time (5 hrs) i.e., drug release in between 20-25% was the basis of selection of optimized batch. The batches with higher concentration of natural polymer with low and intermediate concentration of HPMC K100M respectively showed minimum drug release i.e., ≤ 25% in lag time of 5 hrs. This can be explained on the basis that on increase in natural polymer concentration, hardness of tablets was increased and porosity was decreased which reduced the drug release in lag time. But the batch with higher concentration of both natural polymer and HPMC K100M showed more swelling in initial hours so unable to maintain drug release below 25% in lag time.
Optimization data analysis: The data obtained after putting all values for % CDR in lag time and % CDR in 12 hrs of all 9 batches analysed to get the optimum formulation based on the desired objective. Polynomial models including interaction and quadratic terms were generated for the entire response variables using Multiple Linear Regression Analysis (MLRA) approach. The general form of the MLRA model is represented in the Equation

Whereas, the B0 is the arithmetic average of all the quantitative outcomes of nine runs and B1 and B2 are the coefficients computed from the observed experimental values of Y. X1 and X2 are the coded levels of independent variables. The interaction terms (X1 and X2) shows how the response values changes when the two factors are simultaneously changed. The polynomial equations can be used to draw conclusion after considering the magnitude coefficient and the mathematical sign that the coefficient carries. A high positive or negative value in the equation represent that by making a minor change in the setting of that factor one may obtain a significant change in the dependent variable. Statistical validity of the polynomials was established on the basis of Analysis of Variance (ANOVA) provision in the Design Expert software. Level of significance was considered at p < 0.05. The best-fitting mathematical model was selected based on the comparison of several statistical parameters, including the Coefficient of Variation (CV), the multiple correlation coefficient(R2), the adjusted multiple correlation coefficient (adjusted R2) and the Predicted Residual Sum of Squares (PRESS), provided by the software. PRESS indicates how well the model fits the data, and for the chosen model, it should be small relative to the other models under consideration. The 3-D response surface graphs and the 2-D contour plots also generated by the Design Expert software. These plots are very useful to see interaction effects of the factors on responses.
%Drug release in lag time = +57.38222-0.93533(X1)-1.11092(X2)+0.036625(X1)(X2)
X1=Proportion of natural polymer (Gum Ghatti); X2=Proportion of. Of HPMC K 100
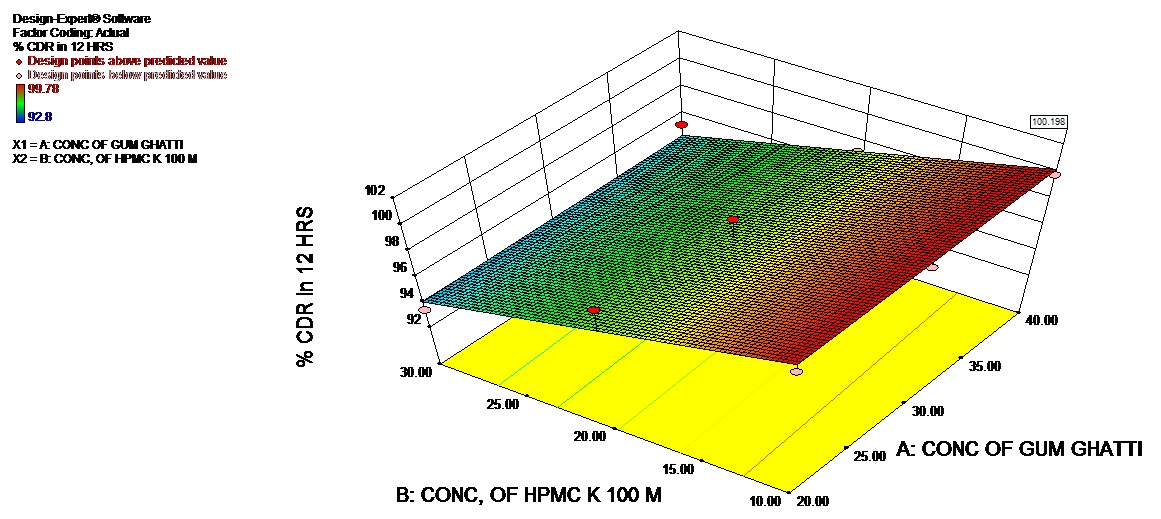
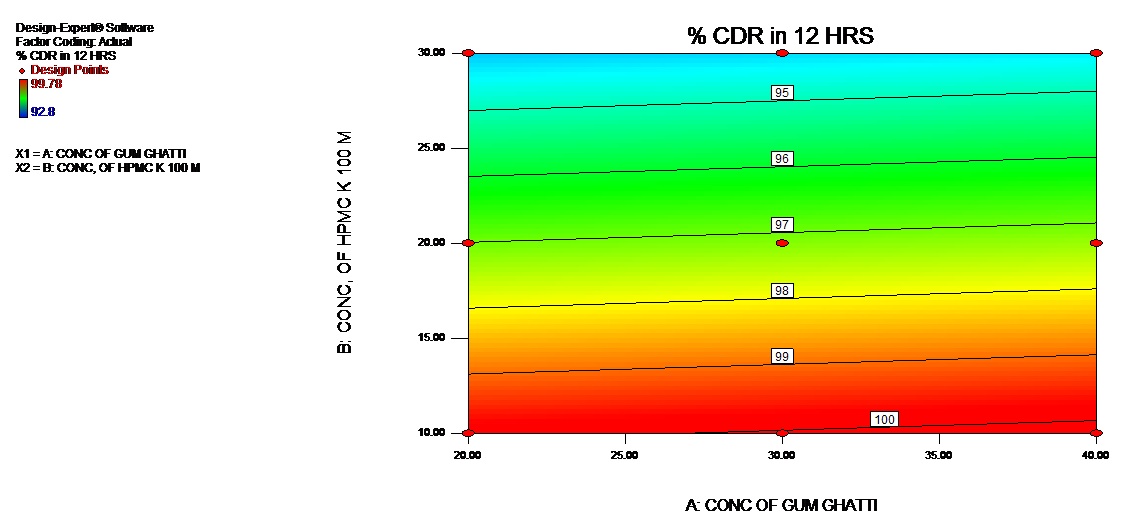
The Model F-value of 6.34 (Table 5) implies the model is significant. There is only a 3.72% chance that a “Model F-Value” this large could occur due to noise. Values of “Prob > F” less than 0.0500 indicate model terms are significant. In this case AB is significant model terms. Values greater than 0.1000 indicate the model terms are not significant. If there are many insignificant model terms (not counting those required to support hierarchy), model reduction may improve your model. The 3D response and contour plot is depicted in figures 3a and 3b.
| Source | Sum of | Df | Mean | F-value | P-value | Significance |
| squares | Square | Probe>F | ||||
| Model | 50.07 | 2 | 25.03 | 18.23 | 0.0028 | Significant |
| Conc. Of Natural polymer | 0.13 | 1 | 0.13 | 0.094 | 0.7695 | - |
| Conc. Of HPMC K100 M | 49.94 | 1 | 49.94 | 36.36 | 0.0009 | Significant |
| Residual | 8.24 | 6 | 1.37 | |||
| Core total | 58.31 | 8 |
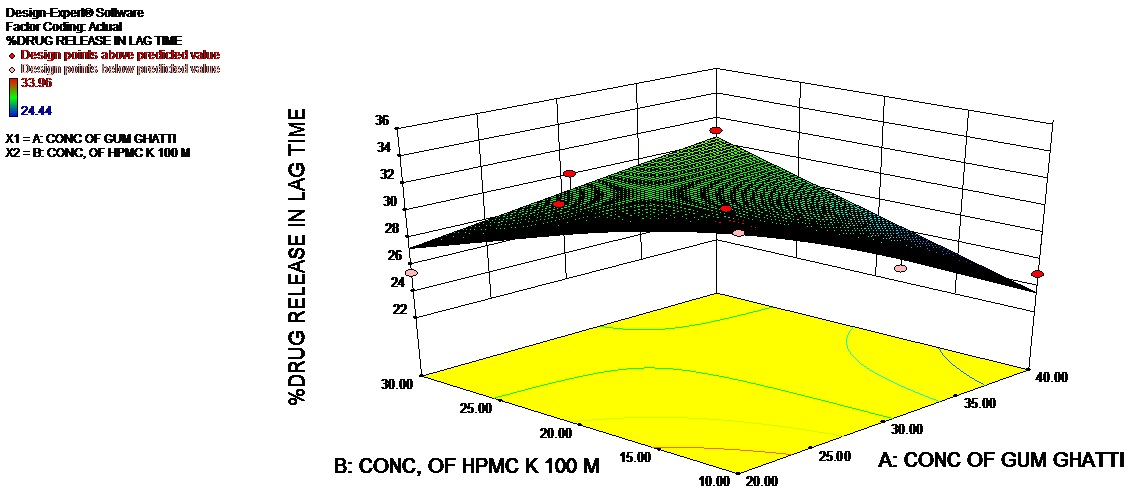
Figure 3a: 3D Response plot of %CDR in lag time.
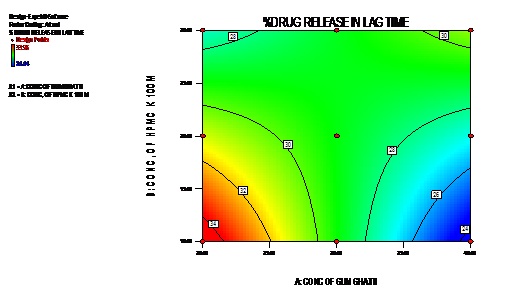
Figure 3b: Contour plot of % CDR in lag time.
X1=Conc. of natural polymer (Gum Ghatti); X2=Conc. of HPMC k 100 M
The Model F-value of 18.23 (Table 6) implies the model is significant. There is only a 0.28% chance that a “Model F-Value” this large could occur due to noise. Values of “Prob > F” less than 0.0500 indicate model terms are significant. In this case B is significant model terms. Values greater than 0.1000 indicate the model terms are not significant. If there are many insignificant model terms (not counting those required to support hierarchy), model reduction may improve your model. The 3D response and contour plot is depicted in figures 4a and 4b.
| source | Sum of | Df | Mean | F-value | P-value | Significance |
| squares | Square | Probe>F | ||||
| Model | 78.43 | 3 | 26.14 | 6.34 | 0.0372 | Significant |
| Conc. Of Natural Polymer | 24.68 | 1 | 24.68 | 5.98 | 0.0582 | - |
| Conc. HPMC K100 M | 0.089 | 1 | 0.089 | 0.022 | 0.8891 | - |
| AB | 53.66 | 1 | 53.66 | 13.01 | 0.0154 | Significant |
| Residual | 20.62 | 5 | 4.12 | |||
| Core total | 99.05 | 8 |
| Sr. No. | Amount of natural polymer (%w/w) | Amount of HPMC K 100M | % CDR in 12 hrs | % CDR in lag time | Desirability |
| 1 | 40% | 10% | 100.1 | 23.5 | 1 |
The optimized batch was then developed and evaluated for various physicochemical properties and the results of physicochemical evaluation and in vitro drug release study for optimized formulation have been depicted in table 8 and figure 5 respectively.
| Sr. No. | Evaluation Parameters | Result |
| 1. | Weight Variation | 300.6±2.78 |
| 2. | Hardness | 5.83±0.28 |
| 3. | Friability (%) | 0.46±0.13 |
| 4. | Drug content | 100.26±0.5 |
| 5. | % CDR in lag time (5 h) | 23.92±1.77 |
| 6. | %CDR (12h) | 100.08±0.31 |

Where, Mt=is the amount of drug released in time t; M∞=amount of drug release at infinite time K is constant, and n represents the release exponent indicative of mechanism of drug release.
When n=0.5 means Fickian diffusion, 0.5>n
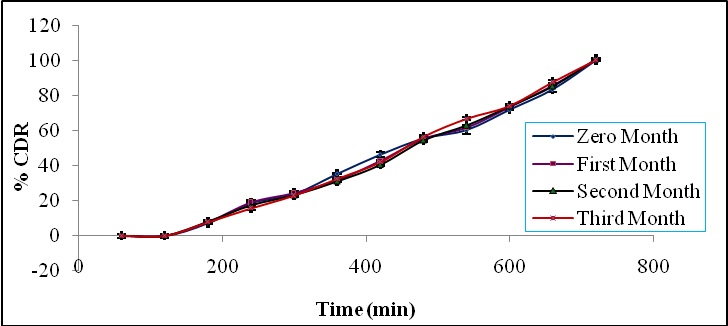
Stability study: The stability studies were carried out for the optimized formulation. The optimized formulation did not show any significant change in hardness, friability and drug content when kept at accelerated conditions of temperature and humidity. Also no significant difference in values of in vitro drug release profile observed during the stability studies (Figure 6).
CONCLUSION
This study exhibited that it is possible to control the release rate of Diclofenac sodium over a wide time scale using Anogeissus latifolia as a sustained release polymer. The combination of both natural and synthetic polymers can be a better choice in comparison to costly synthetic sustained release polymers to achieve desired sustained release effect of formulation. The present study provides a new alternative which is cheaper, having lesser side effects & biocompatible formulation of Diclofenac sodium in the treatment of IBD. Thus this polymer proved to be an excellent carrier for colon targeting and can be exploited commercially.
ACKNOWLEDGEMENT
AUTHOR(S)’ STATEMENT(S)
REFERENCES
- Jain A, Gupta Y, Jain SK (2007) Perspective of biodegradable natural polysaccharides for site-specific drug delivery to colon. J Pharm Pharm Sci 10: 86-128.
- Ashford M, Fell JT, Attwood D, Woodhead P (1993) An in vitro investigation into the suitability of pH-dependent polymers for colonic targeting. Int J Pharm. 91: 241-245.
- Fukui E, Miyamura N, Kobayashi M. (2001) An in vitro investigation of the suitability of press-coated tablets with hydroxypropylmethylcellulose acetate succinate (HPMCAS) and hydrophobicn additives in the outer shell for colon targeting. J Control Release 70: 97-107.
- Ashford M, Fell JT, Attwood D, Sharma H, Woodhead P (1994) Studies on pectin formulations for colonic drug delivery. Journal Controlled Release 30: 225-232.
- Jani GK, Shah DP, Prajapati VD, Jain VC (2009) Gums and mucilages: versatile excipients for pharmaceutical formulations. Asian J Pharm. 4: 308-322.
- Deshmukh AS, Setty CM, Badiger AM, Muralikrishna KS (2012) Gum ghatti: A promising polysaccharide for pharmaceutical applications. Carbohydrate Polym. 87: 980-986.
- Kaur L, Singh J, Singh H (2009) Characterisation of gum ghatti (Anogeissus latifolia): A structural and rheological approach. J Food Sci. 74: 328-332.
- Cartensen JT, Rhodes CT (2005) Preformulation. In: Cartensen JT, Rhodes CT (eds.). Drug Stability principles and practices (3rd edn)., Marcel Dekker Inc., New York, USA.
- Kora AJ, Beedu SR, Jayaraman A. (2012) Size controlled green synthesis of silver nanoparticles mediated by gum ghatti (Anogeissus latifolia) and its biological activity. Org Med Chem Lett 2: 17.
- Salyers AA, Vercelotti JR, West SE, Wilkins TD (1977) Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. App Environ Micro 33: 319-322.
- Crociani F, Alessandrini A, Mucci MM, Biavati B (1994) Degradation of complex carbohydrates by Bifidobacterium spp. Int J Food Micro 24: 199-210.
- Ministry of Health and Family Welfare (2007) Indian Pharmacopeia (5thedn). Indian Pharmacopeia Commission Ghaziabad, India.
- Reddy KJ, Gaikwad SB (2011) Preliminary phytochemical standardisation of tree exudates from India: Gum kondagogu and gum ghatti. Res J Pharm Bio Chem Sci 2: 1023-1034.
- British Pharmacopoeia (2009) British Pharmacopoeia (Vol III). The stationary office, London, UK.
- United States Pharmacopeial Convention (2007) United States Pharmacopoeia. United States Pharmacopeial Convention, Rockville, USA.
- Jain NK (2004) Response Surface optimization of drug delivery system. In: Jain NK (ed.). Progress in controlled and novel drug delivery system, CBS Publishers and Distributors, New Delhi, India.
- Dash S, Murthy P, Nath L, Chowdhury P (2010) Kinetic modelling on drug release from controlled drug delivery systems. Acta Pol Pharm 67: 217-223.
- Cartensen JT, Rhodes CT (2005) Preformulation. In: Cartensen JT, Rhodes CT (eds.). Drug Stability principles and practices (3rdedn.) Marcel Dekker Inc., New York, USA.
Citation: Pandit AH, Mundada AS (2017) Evaluation of a Natural Polymer for the Development of Colon Specific Drug Delivery System. J Pharmacol Pharmaceut Pharmacovigil 1: 003.
Copyright: © 2017 Ashish H Pandit, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

