
Journal of Translational Science and Research Category: Medical
Type: Research Article
Evaluation of the Cytotoxic Effect of Tuibur (Tobacco Brew) in Different Cultured Cell Lines
*Corresponding Author(s):
Ganesh Chandra JagetiaDepartment Of Zoology, Mizoram University, Maharana Pratap Colony, Sector-13, Hiran Magri, Udaipur-313002, India
Email:gc.jagetia@gmail.com
Received Date: Jul 23, 2019
Accepted Date: Aug 13, 2019
Published Date: Aug 20, 2019
Abstract
The consumption of tobacco and tobacco products is directly correlated to many human ailments including cancer. Tuibur.the brewed tobacco, is one of the forms of smokeless tobacco [1], which is frequently used by the inhabitants of Northeast India including Mizoram. The greater use of tobacco and its related products has led to increased frequency of cancer in this region of India. Therefore, it was decided to study the cytotoxicity of two grades of commonly used tuibur in cultured human peripheral blood lymphocytes (HPBLs), HeLa, V79 and Dalton’s lymphoma ascites (DLA) cells along with nicotine by MTT assay.Treatment of HPBLs, HeLa, V79 and DLA cells for 24 h with two grades of commercial tuibur and nicotine led to a concentration dependent rise in their cytotoxicity by MTT assay. Our results indicate that constant consumption of tuibur has a deleterious effect on cultured human peripheral blood lymphocytes, HeLa, V79 and DLA cells.
Keywords
DLA; Hela; Lymphocytes; Tobacco; Tuibur; V79 Cells
INTRODUCTION
Tobacco is known to contain more than eight thousand chemicals, out of which more than eighty are probable carcinogens [1-4]. Some of the common toxic chemicals present in tobacco include benzo[a]pyrene (B[a]P), N’-nitrosonornicotine(NNN), N’-nitrosoanatabine (NAT), N’-nitrosoanabasine (NAB), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK), N-Nitrosodimethylamine (NDMA), nitrite, cadmium, lead, arsenic, nickel, chromium, etc.[5,6]. The consumption of both smoked and smokeless tobacco is popular throughout the world and its detrimental effects could be observed from many medical records. Besides its deleterious consequence upon the pulmonary system, it has been linked to many forms of cancers. In fact, many studies suggested that almost all known cancers could be linked to tobacco use [7,8].
The form of tobacco used may vary considerably in different places and according to the choice of the user. Some individuals prefer smoking tobacco whereas others prefer to use smokeless tobacco, or both. However, there is no denying the fact that more than half of the tobacco users prefer smoking tobacco [9]. Tobacco smokers and those who use smokeless tobacco are exposed to thousands of chemicals present in the tobacco as well as tobacco smoke [10]. The smoking or combustion of tobacco generates additional chemicals which are carcinogens and therefore smokers as well as passive smokers are also at the higher risk of developing cancer (Baker et al[11]., 2004;Johnson et al., 2009) [11,12].
From time immemorial, Mizos have been using smokeless tobacco called tuibur (tobacco brew) in Mizoram and Hidhakphu in Manipur. It is used popularly throughout the state and is commercially available in the local market. Although there is no standard parameter for tuibur quality, it is usually categorized into two grades. The grading is mostly dependent on the amount of tobacco used in tuibur production. Tuibur is usually kept in the mouth by the individuals for roughly 5-10 minutes and then spitted out. The duration to keep tuibur in the mouth depends on its alkalinity and it is spitted out when it is no longer feels alkaline [13,14]. Therefore, the frequent use of tuibur in Mizoram stimulated us to investigate its cytotoxic effect on different cells in vitro.
The form of tobacco used may vary considerably in different places and according to the choice of the user. Some individuals prefer smoking tobacco whereas others prefer to use smokeless tobacco, or both. However, there is no denying the fact that more than half of the tobacco users prefer smoking tobacco [9]. Tobacco smokers and those who use smokeless tobacco are exposed to thousands of chemicals present in the tobacco as well as tobacco smoke [10]. The smoking or combustion of tobacco generates additional chemicals which are carcinogens and therefore smokers as well as passive smokers are also at the higher risk of developing cancer (Baker et al[11]., 2004;Johnson et al., 2009) [11,12].
From time immemorial, Mizos have been using smokeless tobacco called tuibur (tobacco brew) in Mizoram and Hidhakphu in Manipur. It is used popularly throughout the state and is commercially available in the local market. Although there is no standard parameter for tuibur quality, it is usually categorized into two grades. The grading is mostly dependent on the amount of tobacco used in tuibur production. Tuibur is usually kept in the mouth by the individuals for roughly 5-10 minutes and then spitted out. The duration to keep tuibur in the mouth depends on its alkalinity and it is spitted out when it is no longer feels alkaline [13,14]. Therefore, the frequent use of tuibur in Mizoram stimulated us to investigate its cytotoxic effect on different cells in vitro.
MATERIALS AND METHODS
Chemicals
Two grades of locally produced tuibur-A (prepared using more tobacco) and tuibur-B (prepared using lesser tobacco than A), were procured commercially from Aizawl market. Pure nicotine was purchased from Cayman Chemical Company, whereas MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], sodium dodecyl sulphate, RPMI-1640 media were purchased from Sigma Aldrich Chemical Co., Kolkata, India. Hydrochloric acid and isobutanol were obtained from SD Fine Chemicals, Mumbai, India.
Cell culture and treatment
All cell cultures were performed according to standard protocol (Jagetia et al., 2001) [15,16]. For human peripheral blood lymphocyte (HPBL) culture, peripheral blood was collected by venipuncture in a heparinized vacutainer from a 27-year-old healthy male volunteer, who had no known history of tobacco consumption. The blood was allowed to stand for half an hour and the buffy coat containing lymphocytes was separated and used for culture. HeLa (human cervical cancer cell), V79 (Chinese hamster cell)and DLA (Dalton’s lymphoma ascites) were procured from National Centre for Cell Science, Pune, India.
Experimental
Each cell culture was separately divided into four treatment groups namely control group, tuibur-A group, tuibur-B group and nicotine group. The control group from each cell cultures did not receive any treatment and served as control. The HPBLs, HeLa, V79 and DLA cells of tuibur-A and tuibur-B group each were treated with 10, 20, 40, 60, 80, and 100 µl/ml of tuibur-A or tuibur-B, whereas the HPBLs, HeLa, V79 and DLA cells of nicotine group were exposed to 10, 20, 40, 60, 80, and 100 µg/ml nicotine, respectively. The nicotine group served as a positive control.
MTT assay
The MTT assay was performed according to the standard protocol [17], where 5000 HPBLs, HeLa, V79 and DLA cells were seeded into several individual wells of a 96 well microplate for each cell line and exposed to different doses of tuibur-A or B or nicotine as the case may be. The cell cultures were incubated at 37°C for 48 h in a CO2 incubator in an atmosphere of 5% CO2 in air and 95% humidity. After 48 h of incubation, 20 µl of MTT was added to each well and the cells were further incubated for 2-4 h. After the formation of formazan crystal, 100 µl of MTT lysis buffer was added to each well to dissolve the crystals. The cultures were further incubated overnight and the OD was taken at 570 nm in a microplate spectrophotometer (SpectraMax M2, Molecular Devices, San Jose, CA, USA). The survival of the cells has been expressed as percentage. Usually four wells were used for each concentration in each group and the experiment was repeated for confirmation.The cytotoxicity was calculated by the formula: Absorbance of Treatment/ Absorbance of ControlX100.
Statistical analyses
All statistical analyses were performed using OriginPro-8 (OriginLab Corporation, Northampton, USA) and Microsoft excel 2013. Student’s t-test was employed to determine significant difference among the treatment groups. Correlation coefficient was performed to determine relationship between different treatment concentrations and cytotoxicity within a group. The test of homogeneity was applied between the original and repeat experiments and no statistical difference was reported between the two experiments. Since the difference between the data of two experiment was non-significant the data were combined.
RESULTS
The results are shown in (Table 1) and (Figures 1-4) as mean±standard error of the mean (SEM). The evaluation of the two grades of tobacco brew A, B and nicotine showed concentration dependent increase in cytotoxicity in all the cell lines tested as well as HPBLs. Comparison between the control group (0 dose) with the different treatment groups showed significant (p<0.05) increase in the cytotoxicity in HPBLs, HeLa, V79 and DLA cells. Despite this fact the differences among tuibur A and B groups were not statistically significant. The effect of tuibur A treatment showed higher toxicity than tuibur B and nicotine in HPBLs whereas the nicotine treatment exerted higher toxicity in HeLa, V79 and DLA cells. Pearson’s correlation showed that all treatment groups demonstrated strong positive correlation between treatment concentrations and cytotoxicity.
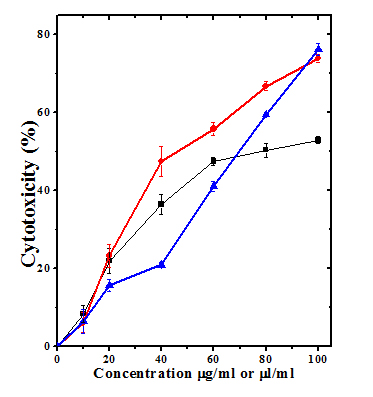 Figure 1: Cytotoxic effect of different concentrations of tuibur-A, tuibur-B and nicotine on human peripheral blood lymphocytes. Squares: Tuibur-A; Circles: Tuibur-B; Triangles: Nicotine. p
Figure 1: Cytotoxic effect of different concentrations of tuibur-A, tuibur-B and nicotine on human peripheral blood lymphocytes. Squares: Tuibur-A; Circles: Tuibur-B; Triangles: Nicotine. p 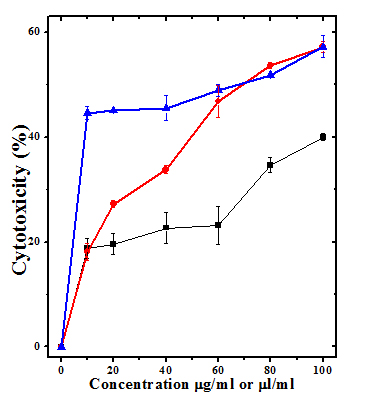 Figure 2: Cytotoxic effect of different concentrations of tuibur-A, tuibur-B and nicotine on HeLa cells. Squares: Tuibur-A; Circles: Tuibur-B; Triangles: Nicotine. p
Figure 2: Cytotoxic effect of different concentrations of tuibur-A, tuibur-B and nicotine on HeLa cells. Squares: Tuibur-A; Circles: Tuibur-B; Triangles: Nicotine. p 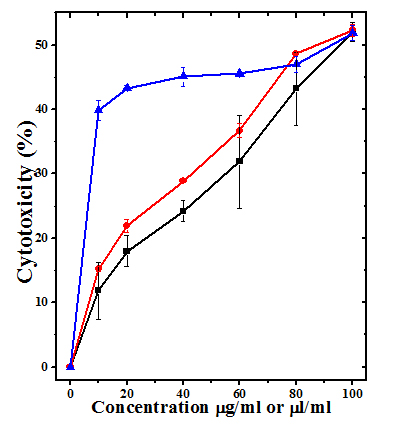 Figure 3: Cytotoxic effect of different concentrations of tuibur-A, tuibur-B and nicotine on V79 cells. Squares: Tuibur-A; Circles: Tuibur-B; Triangles: Nicotine. p
Figure 3: Cytotoxic effect of different concentrations of tuibur-A, tuibur-B and nicotine on V79 cells. Squares: Tuibur-A; Circles: Tuibur-B; Triangles: Nicotine. p 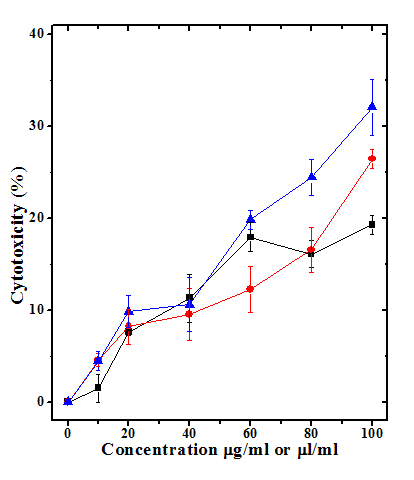 Figure 4: Cytotoxic effect of different concentrations of tuibur-A, tuibur-B and nicotine on Dalton’s lymphoma ascites (DLA). Squares: Tuibur-A; Circles: Tuibur-B; Triangles: Nicotine. p
Figure 4: Cytotoxic effect of different concentrations of tuibur-A, tuibur-B and nicotine on Dalton’s lymphoma ascites (DLA). Squares: Tuibur-A; Circles: Tuibur-B; Triangles: Nicotine. p |
Cells |
Treatment |
||
|
Tuibur A |
Tuibur B |
Nicotine |
|
|
HPBL |
0.937* |
0.962* |
0.990* |
|
HeLa |
0.946* |
0.983* |
0.956* |
|
V79 |
0.997* |
0.993* |
0.948* |
|
DLA |
0.923* |
0.954* |
0.986* |
Table 1: Pearson’s correlation between cytotoxicity in various cells treated with different concentrations of Tuibur A, Tuibur B and nicotine groups.
*Correlation is significant at p
DISCUSSION
Tobacco has been used by humans since a long time however its adverse effects came to be known in the last century. Tobacco contains numerous chemicals and many of which have been reported toinduce cancer and other diseases [2,18,19]. Most studies, if not all, reported the use of tobacco in any form only has negative health impact on the users [19,20]. There has been only a handful of literature on the scientific investigation of tuibur. Therefore, the present study was designed to study the viability and cytotoxic effect of different grades of tuiburA and B on HPBLs, HeLa, V79 and DLA cells.
A preliminary report on the chemical composition of tuibur showed the presence of polyaromatic hydrocarbons and carbonyl compounds in the tar phase [14]. The epidemiological study among the Mizos showed that tuibur users are at a higher risk of developing gastric cancer. Similary, use of tuibur accompanied with smoking, betel (paan), and sahdah consumption have been reported to increase the risk of gastric cancer [21,22]. Besides the occurrence of gastric cancer in Mizoram, tuibur consumers were found to have a variety of mtDNA D-loop region mutations and polymorphisms. Individuals with Arg/Pro genotype, GSTM1 null genotype and GSTT1 non-null genotype were also suggested to have a higher risk of gastric cancer if they consume tuibur and smoke tobacco [23, 24].The induction of cytotoxicity by tuibur in a concentration dependent manner in all the cell types in the present study indicates that tuibur exerted detrimental effect on all cells and the highest toxicity was observed on the HPBLs followed by V79 cells. The DLA cells were least effective. Similarly, onion bulbs treated with tuibur showed reduced root growth, reduced mitotic index, and formation of micronuclei, lagging chromosomes, and c-mitosis [1]. A study on seven smokeless tobacco aqueous extracts showed concentration-dependent inhibition on the growth and viability of oral bacteria cultured under anaerobic conditions [25]. Nicotine one of the active constituents of tobacco has been reported to inhibit cell proliferation and decrease protein synthesis in a dose dependent manner in cultured periodontal ligament fibroblast [26]. We also report here the deleterious effect of nicotine in different cells. Nicotine has been reported to stimulate endothelial cell DNA synthesis and cell proliferation at concentrations lower than <10-8 M and it was cytotoxic at a concentration>10-6M [27]. Likewise, cigarette smoke extract has been reported to induce cytotoxicity in orbital fibroblasts [28].
The exact mechanism of action of the cytotoxic effect of tuibur on different cells is not known. It may have used multiple pathways to exert its cytotoxic effect. Since one of the major components of tobacco is nicotine, the effects observed in the present study may be due to nicotine. Tobacco contains NNN, and NNK apart from nicotine that have been found to trigger cytotoxicity [29]. The induction of DNA damage in different cells may be the most important mechanisms of cell death. Nicotine has been reported to induce DNA damage in human tonsillar, lymphocytes and respiratory tract cells [30,31]. Chewing tobacco has been reported to induce, lipid peroxidation, DNA fragmentation and DNA ladders [32], which may have contributed to death of various cells in our study. The induction of oxidative stress that induce DNA damage may be another mechanism of induction of cytotoxicity. Nicotine has been reported to reduce antioxidant enzymes including superoxide dismutase, catalase, glutathione-s-transferase, and glutathione reductase, and increase lipid peroxidation [33]. Therefore, increased oxidative stress may be one of important mechanisms that induced cytotoxicity in the present study. Cigarette smoke extract has been reported to induce reactive oxygen species (ROS) in cultured fibroblasts [28]. At molecular level tuibur may have triggered the activation of NF-κB, COX-2 and reduced Nrf-2 activation causing higher oxidative stress and cell death. The smoking of tobacco has been reported to activate NF-κB, COX-2 and survivin and reduce Nrf2 in earlier studies [34-36].
A preliminary report on the chemical composition of tuibur showed the presence of polyaromatic hydrocarbons and carbonyl compounds in the tar phase [14]. The epidemiological study among the Mizos showed that tuibur users are at a higher risk of developing gastric cancer. Similary, use of tuibur accompanied with smoking, betel (paan), and sahdah consumption have been reported to increase the risk of gastric cancer [21,22]. Besides the occurrence of gastric cancer in Mizoram, tuibur consumers were found to have a variety of mtDNA D-loop region mutations and polymorphisms. Individuals with Arg/Pro genotype, GSTM1 null genotype and GSTT1 non-null genotype were also suggested to have a higher risk of gastric cancer if they consume tuibur and smoke tobacco [23, 24].The induction of cytotoxicity by tuibur in a concentration dependent manner in all the cell types in the present study indicates that tuibur exerted detrimental effect on all cells and the highest toxicity was observed on the HPBLs followed by V79 cells. The DLA cells were least effective. Similarly, onion bulbs treated with tuibur showed reduced root growth, reduced mitotic index, and formation of micronuclei, lagging chromosomes, and c-mitosis [1]. A study on seven smokeless tobacco aqueous extracts showed concentration-dependent inhibition on the growth and viability of oral bacteria cultured under anaerobic conditions [25]. Nicotine one of the active constituents of tobacco has been reported to inhibit cell proliferation and decrease protein synthesis in a dose dependent manner in cultured periodontal ligament fibroblast [26]. We also report here the deleterious effect of nicotine in different cells. Nicotine has been reported to stimulate endothelial cell DNA synthesis and cell proliferation at concentrations lower than <10-8 M and it was cytotoxic at a concentration>10-6M [27]. Likewise, cigarette smoke extract has been reported to induce cytotoxicity in orbital fibroblasts [28].
The exact mechanism of action of the cytotoxic effect of tuibur on different cells is not known. It may have used multiple pathways to exert its cytotoxic effect. Since one of the major components of tobacco is nicotine, the effects observed in the present study may be due to nicotine. Tobacco contains NNN, and NNK apart from nicotine that have been found to trigger cytotoxicity [29]. The induction of DNA damage in different cells may be the most important mechanisms of cell death. Nicotine has been reported to induce DNA damage in human tonsillar, lymphocytes and respiratory tract cells [30,31]. Chewing tobacco has been reported to induce, lipid peroxidation, DNA fragmentation and DNA ladders [32], which may have contributed to death of various cells in our study. The induction of oxidative stress that induce DNA damage may be another mechanism of induction of cytotoxicity. Nicotine has been reported to reduce antioxidant enzymes including superoxide dismutase, catalase, glutathione-s-transferase, and glutathione reductase, and increase lipid peroxidation [33]. Therefore, increased oxidative stress may be one of important mechanisms that induced cytotoxicity in the present study. Cigarette smoke extract has been reported to induce reactive oxygen species (ROS) in cultured fibroblasts [28]. At molecular level tuibur may have triggered the activation of NF-κB, COX-2 and reduced Nrf-2 activation causing higher oxidative stress and cell death. The smoking of tobacco has been reported to activate NF-κB, COX-2 and survivin and reduce Nrf2 in earlier studies [34-36].
CONCLUSION
The tuibur A, B and nicotine treatment induced cytotoxicity in a concentration dependent manner in HPBLs, HeLa, V79 and DLA cells. The cytotoxic effect may be due to induction of DNA damage, DNA adducts and lipid peroxidation.
ACKNOWLEDGEMENT
The above study was carried out under the grant sanctioned by the University Grants Commission, Government of India, New Delhi to GCJ.
REFERENCES
- Bhisey RB (2012) Chemistry and toxicology of smokeless tobacco. Indian J Cancer 4: 364-372.
- Arimilli S, Damratoski BE, Bombick B, Borgerding MF, Prasad GL (2012) Evaluation of cytotoxicity of different tobacco product preparations. Regul Toxicol Pharmacol 3: 350-360.
- Ding YS, Zhang L, Jain RB, Jain J, Wang RY, et al. (2008) Levels of tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons in mainstream smoke from different tobacco varieties. Cancer Epidemiol Biomarkers Prev 12: 3366-3371.
- Cooper RG (2006) Effect of tobacco smoking on renal function. Indian J Med Res 3: 261-268.
- Stepanov I, Hecht SS (2005) Tobacco-specific nitrosamines and their pyridine-N-glucuronides in the urine of smokers and smokeless tobacco users. Cancer Epidemiol Biomarkers Prev 4: 885-891.
- Borgerding MF, Bodnar JA, Curtin GM, Swauger JE (2012) The chemical composition of smokeless tobacco: A survey of products sold in the United States in 2006 and 2007. Regul Toxicol Pharmacol 3: 367-387.
- Musk AW, De Klerk NH (2003) History of tobacco and health. Respirology 8: 286-290.
- Elmasry S, Asfour S, Vaccari JPR, Travascio F (2015) Effects of tobacco smoking on the degeneration of the intervertebral disc: A finite element study. Plos One Page No: 1-22.
- WHO global report on trends in prevalence of tobacco smoking 2015. WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland, Page No: 4-359.
- Jha P, Ranson MK, Nguyen SN &Yach D (2002) Estimates of global and regional smoking prevalence in 1995, by age and sex. Am J Public Health 6: 1002-1006.
- Baker RR, Massey ED, Smith G (2004) An overview of the effect of tobacco on smoke chemistry and toxicity. Food ChemToxicol Page No: 53-83.
- Johnson MD, Schilz J, Djordjevic MV, Rice JR, Shields PG (2009) Evaluation of in vitroiassays for assessing the toxicity of cigarette smoke and smokeless tobacco. Cancer Epidemiol Biomarkers Prev 12: 3263-3304.
- Lalpawimawha, Lalruatfela B, Chenkual S, Ralte Z, Zomuana T, et al. (2015) Association of tobacco use, betel consumption and gastric cancer in Mizoram. Sci Vis 2: 59-67.
- Lalmuanpuii R, Muthukumaran RB (2016) Fourier transform-infrared spectroscopic characterization of the particulate phase of commercial tuibur. Sci Vis 2: 68-73.
- Freshney R (1987) Culture of Animal Cells: A Manual of Basic Technique, Alan R. Liss, Inc., New York, Page No: 117.
- Jagetia GC, Jayakrishnan A, Fernandes D &Vidyasagar MS (2001) Evaluation of micronuclei frequency in the cultured peripheral blood lymphocytes of cancer patients before and after radiation treatment. Mutat Res 2: 9-16.
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods 2: 55-63.
- Stepanov I, Hecht SS, Ramakrishnan S, Gupta PC (2005) Tobacco-specific nitrosamines in smokeless tobacco products marketed in India. Int J Cancer 1: 16-19.
- Hecht SS, Szabo E (2014) Fifty years of tobacco carcinogenesis research: from mechanisms to early detection and prevention of lung cancer. Cancer Prev Res 1: 1-8.
- West R (2017) Tobacco smoking: Health impact, prevalence, correlates and interventions. Psychol Hlth 8: 1018-1036.
- Phukan RK, Zomawi E, Hazarika NC, Mahanta J (2005) Tobacco use and stomach cancer in Mizoram, India; Cancer Epidemiol Biomarkers Prev 8: 1892-1896.
- Lalmuanpuii R, Ghatak S, Pautu JL, Lallawmzuali D, Muthukumaran RB, et al. (2015) Mutation profiling in mitochondrial D-loop associated with stomach cancer and tobacco consumers. J Clin Med Genom 3: 1-5.
- Malakar M, Devi KR, Phukan RP, Kaur T, Deka M, et al. (2012) Genetic polymorphism of glutathione S-transferase M1 and T1, tobacco habits and risk of stomach cancer in Mizoram, India. Asian Pacific J Cancer Prev 9: 4725-4732.
- Malakar M, Devi KR, Phukan RP, Kaur T, Deka M, et al (2014) p53 codon 72 polymorphism interactions with dietary and tobacco related habits and risk of stomach cancer in Mizoram, India. Asian Pacific J Cancer Prev 2: 717-723.
- Liu M, Jin J, Pan H, Feng J, Cerniglia CE, et al. (2016) Effect of smokeless tobacco products on human oral bacterial growth and viability. Anaerobe 42: 152-161.
- Chang YC, Huang FM, Tai KW, Yang LC, Chou MY (2002) Mechanism of cytotoxicity of nicotine in human periodontal ligament fibroblast cultures in vitro. J Periodont Res 4: 279-285.
- Villablanca AC (1998) Nicotine stimulates DNA synthesis and proliferation in vascular endothelial cells in vitro. J ApplPhysiol 6: 2089-2098.
- Kau HC, Wu SB, Tsai CC, Liu CJ, Wei YH (2016) Cigarette smoke extract-induced oxidative stress and fibrosis-related genes expression in orbital fibroblasts from patients with graves’ ophthalmopathy Oxid Med Cell Longev 2016:4676289.
- Moghbel N, Ryu B, Cabot PJ, Steadman KJ (2016) In vitro cytotoxicity of Nicotianagosseileaves, used in the Australian aboriginal smokeless tobacco known as pituri or mingkulpa. Toxicol Lett Page No: 45-51.
- Kleinsasser NH, Sassen AW, Semmler MP, Harréus UA, Licht AK, et al. (2005) The tobacco alkaloid nicotine demonstrates genotoxicity in human tonsillar tissue and lymphocytes. Toxicol Sci 2: 309-317.
- Ginzkey C, Stueber T, Friehs G, Koehler C, Hackenberg S, et al. (2012) Analysis of nicotine-induced DNA damage in cells of the human respiratory tract. Toxicol Lett 1: 23-29.
- Bagchi M, Balmoori J, Bagchi D, Stohs SJ, Chakrabarti J, et al. (2002) Role of reactive oxygen species in the development of cytotoxicity with various forms of chewing tobacco and pan masala. Toxicol 3: 247-255.
- Das S, Chakraborty SP, Roy S, Roy S (2012) Nicotine induced pro-oxidant and antioxidant imbalance in rat lymphocytes: in vivo dose and time dependent approaches. Toxicol Mech Methods 9: 711-720.
- Hasnis E, Bar-Shai M, Burbea Z, Reznick AZ (2007) Mechanisms underlying cigarette smoke-induced NF-kappa B activation in human lymphocytes: the role of reactive nitrogen species. J PhysiolPharmacol 5: 275-287.
- Garbin U, FrattaPasini A, Stranieri C, Cominacini M, Pasini A, et al. (2009) Cigarette Smoking Blocks the Protective Expression of Nrf2/ARE Pathway in Peripheral Mononuclear Cells of Young Heavy Smokers Favouring Inflammation. PLos One 12: e8225.
- Yang M, Chen P, Peng H, Zhang H, Chen Y, et al. (2015) Cigarette smoke extract induces aberrant cytochrome-c oxidase subunit II methylation and apoptosis in human umbilical vascular endothelial cells. Am J Physiol-Cell Physiology 5: 378-384.
Citation: Lalruatfela B , Zoremsiami J, Jagetia GC (2019) Evaluation Of The Cytotoxic Effect Of Tuibur (Tobacco Brew) In Different Cultured Cell Lines. J Transl Sci Res 2: 005.
Copyright: © 2019 Lalruatfela B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
© 2026, Copyrights Herald Scholarly Open Access. All Rights Reserved!

