
Evaluation of the Empiric Treatment of Peritoneal Dialysis-Related Peritonitis
*Corresponding Author(s):
Elaine ChengDepartment Of Pharmaceutical Sciences, Vancouver General Hospital, Vancouver, Canada
Tel:+1 6048754111 (extension 61661),
Email:Elaine.Cheng@vch.ca
Abstract
Aims: Peritonitis is a serious complication of Peritoneal Dialysis (PD). At our hospital, PD- related peritonitis is treated empirically with intraperitoneal cefazolin plus ceftazidime. Fungal peritonitis prophylaxis is prescribed at the nephrologist’s discretion. We aimed to evaluate the effectiveness of this empiric antibiotic regimen and to assess if routine fungal prophylaxis is indicated.
Materials and methods: A retrospective chart review of PD-related peritonitis episodes at our centre from 2013-2017 was done with documentation of the infecting pathogen(s), antimicrobial sensitivities, and relevant clinical outcomes.
Results: We identified 62 PD-related peritonitis episodes. Empiric cefazolin and ceftazidime were utilized in 48 (77%) and 51 (82%) episodes, respectively. The predominant organisms isolated included staphylococci (39%), gram negative bacteria (30%), streptococci (18%), and enterococci (8%). The isolated organism(s) was sensitive to empiric antibiotics in 55 (89%) episodes and resolution of infection occurred in 50 (81%) episodes. Fluconazole prophylaxis was prescribed in 15 (24%) episodes with no documented fungal peritonitis episodes.
Conclusion: Intraperitoneal cefazolin plus ceftazidime is appropriate for the empiric treatment of PD-related peritonitis at our hospital. Routine fluconazole prophylaxis is not indicated.
Keywords
Antibiotic resistance; Fungal peritonitis; Intraperitoneal antibiotic; Outcome of peritonitis; Peritonitis rate
Introduction
Peritoneal Dialysis (PD) patients are at risk of peritonitis, which can result in hospitalization, peritoneal membrane failure, conversion to hemodialysis or death [1-4]. The International Society of Peritoneal Dialysis (ISPD) recommends that patients presenting with signs and symptoms of peritonitis receive empiric intraperitoneal antibiotics as soon as possible once dialysate fluid samples have been obtained for cell count, culture and sensitivity [5].The ISPD recommend that either a first-generation cephalosporin or vancomycin be used for gram positive coverage, in combination with either a third-generation cephalosporin or aminoglycoside for gram negative coverage [5]. Intraperitoneal antibiotic administration has been shown to be superior to intravenous antibiotic administration [6]. However, no empiric antibiotic regimen has been shown to have superior efficacy [6]. Low quality evidence has suggested glycopeptide- based regimens may achieve higher rates of complete cure, and a proportional meta-analysis found glycopeptide (vancomycin) plus ceftazidime therapy to be associated with the highest cure rate [6,7]; however, no difference was found in the rate of treatment failure, catheter removal or relapse [6]. The ISPD recommend empiric antibiotic regimens be centre-specific based on the prevalence of resistant pathogens in each PD program [5]. Additionally, the most recent iteration of the ISPD guidelines recommend fungal peritonitis prophylaxis for all PD patients receiving antibiotics [5].
At our hospital, PD-related peritonitis is treated empirically with intraperitoneal cefazolin plus intraperitoneal ceftazidime unless the patient is allergic to cephalosporins or has a history of resistant infection. If an alternative antibiotic regimen is required, intraperitoneal vancomycin plus intraperitoneal tobramycin is prescribed. At our centre, fungal peritonitis prophylaxis is prescribed at the discretion of the nephrologist based on patient-specific risk factors for fungal peritonitis. Through a retrospective review we aimed to evaluate the effectiveness of this empiric antibiotic regimen based on the microbiology, resistance patterns and clinical outcomes ofperitonitis episodes at our centre. Additionally, we aimed to evaluate the need for routine fungal peritonitis prophylaxis based on the observed fungal peritonitis rate within our PD program.
Peritonitis episodes occurring at our hospital between January 1, 2013 and December 31, 2017 were retrospectively identified using the Patient Records and Outcome Management Information System (PROMIS) a province-wide integrated registry and clinical information system for kidney disease and transplant patients, which is maintained by British Columbia (BC) Renal. Peritonitis episodes were included only if the dialysis effluent was analyzed (cell count, differential, gram stain, culture and sensitivity) and empiric intraperitoneal antibiotic or antifungal therapy was administered. Based on the ISPD diagnostic criteria, peritonitis episodes were identified in patients who presented with at least two of the following characteristics: 1) clinical features consistent with peritonitis (abdominal pain and/or cloudy dialysate), 2) dialysis effluent white blood cell count >100/µL or > 0.1 x 109/L with > 50% polymorphonuclear cells, 3) positive dialysis effluent culture [5]. Episodes were excluded if it was determined to only be an exit site and/or tunnel infection or eosinophilic peritonitis. All patients included in the study were aged 18 years or older as our hospital offers health care services to adults only.
The annual peritonitis rate (number of episodes per patient year on PD) for the entire province of BC and our centre were obtained from PROMIS. Detailed review of the medical chart and electronic records was performed for all identified PD peritonitis cases. Data that was extracted included: patient demographics, empiric antibiotics administered, organisms isolated from dialysis effluent, culture and sensitivity of organisms isolated and peritonitis episode outcomes. The outcomes of interest were resolution of infection, refractory infection, relapse infection, recurrent infection, repeat infection and death. Resolution of infection was defined as the absence of signs and symptoms after 96 hours of antibiotic therapy without relapse in the 28days following initial therapy completion [5]. Refractory infection was defined as failure of the dialysis effluent to clear after 5 days of antibiotics [5]. Relapse infection was defined as an episode that occurs within 4 weeks of completion of therapy for a prior episode with the same organism [5]. Recurrent infection was defined as an episode that occurs within 4 weeks of completion of therapy for a prior episode with a different organism [5]. Repeat infection was defined as an episode that occurs more than 4 weeks after completion of therapy for a prior episode with the same organism [5]. Death due to peritonitis was defined as death with active peritonitis or within 4 weeks of a peritonitis episode, or any death attributed to peritonitis during hospitalization for a peritonitis episode [5]. Additional outcomes analyzed included PD catheter removal, transfer to hemodialysis and transfer back to peritoneal dialysis. Analysis was performed using descriptive statistics. Our study was deemed to be quality improvement and was thus exempt from review by the University of British Columbia Clinical Research Ethics Board. The study was conducted according to the Declaration of Helsinki.
Results
Annual peritonitis rates in BC for our study period of January 1, 2013 to December 31, 2017 95 were 0.38 (2013), 0.27 (2014), 0.25 (2015), 0.26 (2016) and 0.33 (2017) episodes per patient year on PD. At our hospital, annual peritonitis rates were 0.33 (2013), 0.17 (2014), 0.12 (2015), 97 0.13 (2016) and 0.09 (2017) episodes per patient year on PD.
A total of 42 patients (mean age 65 ± 13 years) developed 62 peritonitis episodes. Patient characteristics are shown in table 1. Peritonitis episode characteristics are shown in table 2. Continuous Cycling Peritoneal Dialysis (CCPD) was the PD modality in 47 (76%) episodes and Continuous Ambulatory Peritoneal Dialysis (CAPD) in 14 (23%) episodes. Concurrent exit site infections occurred in 10 (16%) episodes. Intraperitoneal cefazolin was prescribed as empiric gram positive therapy in 48 (77%) episodes and intraperitoneal vancomycin in 10 (16%) episodes. For empiric gram negative therapy, intraperitoneal ceftazidime was prescribed in 51 (82%) episodes and intraperitoneal tobramycin in 4 (7%) episodes. Additional antibiotics (eg.,piperacillin/tazobactam IV) were administered in 20 (32%) episodes. Fungal peritonitis prophylaxis with fluconazole was administered in 15 (24%) episodes.
|
Characteristicn(%) |
|
Mean Age(years) 65±13 |
|
Gender |
|
Male 18(43) |
|
Female 24(57) |
|
Ethnicity |
|
Caucasian 12(29) |
|
Filipino 12(29) |
|
East Asian 10(24) |
|
South Asian 3(7) |
|
Other 5(12) |
|
Primary Renal Disease |
|
Glomerulonephritis 20(48) |
|
Systemic Lupus Erythematosus 3(7) |
|
Focal Segmental Glomerulosclerosis 7(17) |
|
IgA Nephropathy 3(7) |
|
Unspecified 7(17) |
|
Diabetic nephropathy 12(29) |
|
Nephrosclerosis 3(7) |
|
Polycystic kidney disease 2(5) |
|
Amyloidosis 1(2) |
|
Obstructive Uropathy 1(2) |
|
Thrombotic Microangiopathy 1(2) |
|
Tuberculosis 1(2) |
|
Wegener's Granulomatosis 1(2) |
|
Comorbid Conditions |
|
Diabetes 17(41) |
|
Cardiovascular Disease 13(41) |
|
Cardiac Arrhythmias 5(12) |
|
Malignancy 4(10) |
|
Chronic Lung Disease 4(10) |
|
Congestive Heart Failure 2(5) |
Table 1: Patient Characteristics (N=42)
|
Characteristic n(%) |
|
Exit Site Antibiotics |
|
Mupirocin(Ointment) 30(48) |
|
Gentamicin (Cream or Ointment) 32(52) |
|
Dialysis Modality |
|
Continuous Cycling Peritoneal Dialysis (CCPD) 47(76) |
|
Continuous Ambulatory Peritoneal Dialysis (CAPD) 14(23) |
|
Hemodialysis (HD) 1(2) |
|
Median Duration Dialysis- Days 659 |
|
Resistant Organisms |
|
Methicillin-Resistant Staphylococcus Aureus (MRSA) 1(2) |
|
Vancomycin Resistant Enterococcus (VRE) 0(0) |
|
Immunosuppression 8(13) |
|
Antibiotics In the Past 3 months 15(24) |
|
Extraperitoneal Fungal Infection 3(5) |
|
Peritonitis Episode Number |
|
One 32(52) |
|
Two 17(27) |
|
Three 8(13) |
|
Four or greater 4(7) |
|
Concurrent Exit Site Infection 10(16) |
|
Hospitalization 35(57) |
|
Mean Length of Stay- Days 6.2 |
|
Empiric Gram Positive Coverage |
|
Cefazolin IP 48(77) |
|
Vancomycin IP 10(16) |
|
Pencillin Allergy 3(5) |
|
Prior Resistence 2(3) |
|
Unknown 5(8) |
|
None 4(7) |
|
Empiric Gram Negative Coverage |
|
Ceftazidime IP 51(82) |
|
Tobramycin IP 4(7) |
|
Ciprofloxacin PO 2(3) |
|
None 5(8) |
|
Mean Duration of Empiric Coverage- Days 2.8 |
|
Other Antibiotcs Administered 20(32) |
|
Fluconazole Prophylaxis 15(24) |
Table 2: Peritonitis Episode Characteristics (N=62)
|
Organism n(%) |
|
Staphylococci 29(39) |
|
Coagulase negative staphylococci 19(26) |
|
Staphylococcus epidermidis 11(15) |
|
Staphylococcus warneri 1(1) |
|
Not specified 7(10) |
|
Staphylococcus aureus 10(14) |
|
Streptococci 13(18) |
|
Streptococcus viridans group 2(3) |
|
Streptococcus salivarius 4(5) |
|
Streptococcus mitis 2(3) |
|
Streptococcuss parasanguis 1(1) |
|
Group B beta hemolytic streptococcus 2(3) |
|
Streptococcus sanguis 1(1) |
|
Streptococcus oralis 1(1) |
|
Enterococcus 6(8) |
|
Enterococcus faecalis 3(4) |
|
Enterococcus faecium 2(3) |
|
Vancomycin resistant enterococcus 1(1) |
|
Other Gram Positive 2(3) |
|
Rothia (Stomatococcus) mucilaginosa 1(1) |
|
Lactobacillus 1(1) |
|
Gram Negative 22(30) |
|
Escherichia coli 9(12) |
|
Acinetobacter 3(4) |
|
Burkholderia cepacia 1(1) |
|
Neisseria 2(3) |
|
Klebsiella pneumoniae 3(4) |
|
Pasteurella multocida 3(4) |
|
Leclerciaadecarboxylata 1(1) |
|
Anaerobic 2(3) |
|
Bacteriodes fragilis 1(1) |
|
Bacteriodes vulgatus 1(1) |
|
Culture Negative 6(10) |
Table 3: Organisms Isolated from Dialysis Effluent (N=74).
|
Organism Antibiotic Percent Sensitive (ns /nt) |
|
Staphylococci Cefazolin 92(24/26) |
|
(n=29) Vancomycin 100(5/5) |
|
Streptococci Penicillin G 77(10/13) |
|
(n=13) Vancomycin 100)13/13) |
|
Enterococci Vancomycin 83(5/6) |
|
(n=6) Gentamycin 67(4/6) |
|
Other Gram Positive Penicillin G 100(2/2) |
|
(n=2) Cefazolin 0(0/1) |
|
Vancomycin 50(1/2) |
|
Gram Negative Ceftazidime 100(12/12) |
|
(n=22) Ceftriaxone 100(18/18) |
|
Tobramycin 94(15/16) |
Table 4: Antibiotic Sensitivity of Organisms Isolated from Dialysate Effluent
A total of 74 organisms were isolated from the dialysate effluent. The organisms consisted of streptococci 13 (18%), staphylococci 29 (39%), enterococci 6 (8%), other gram positives 2 (3%), gram negatives 22 (30%) and anaerobic 2 (3%) (Table 3). No fungal isolates were identified. Culture negative peritonitis accounted for 6 (10%) episodes. The culture and sensitivity of the isolated organisms is shown in table 4. Isolated organisms were sensitive to the empiric antibiotic regimen in 89% of episodes.
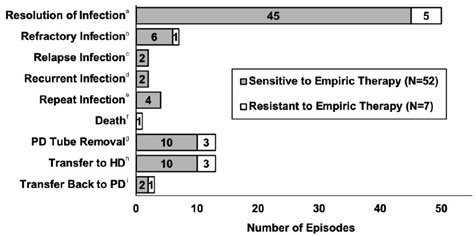
Figure 1: Peritonitis Episode Outcomes (N=62).
Overall resolution of infection occurred in 50 (81%) episodes (Figure 1). Other episode outcomes included refractory infection 7 (11%), relapse infection 2 (3%), recurrent infection 2 (3%), repeat infection 4 (7%) and death 1 (2%) (Figure 1). The PD catheter was removed in 13 (21%) episodes, and all of these patients transferred to hemodialysis. Transfer back to PD was achieved in 3 (5%) episodes.
Note: (a) Resolution of infection is no signs or symptoms of peritonitis after 5 days of antibiotics with no relapse for 4 weeks (b) Refractory infection is failure to clear PD effluent after 5 days of antibiotics (c) Relapse infection is an episode with the same organism < 4 weeks after antibiotics completed (d) Recurrent infection is an episode with different organism < 4 weeks after antibiotics completed (e) Repeat infection is episode with same organism > 4 weeks after antibiotics completed (f) Death due to peritonitis was defined as death with active peritonitis or within 4 weeks of a peritonitis episode, or any death attributed to peritonitis during hospitalization for a peritonitis episode.
Discussion
Peritonitis rates were lower at our centre compared to the rest of the province and were below the acceptable rate of 0.5 episodes per patient year on PD for all five years of the study period [5]. The incidence of peritonitis in the final year was 0.09 episodes per patient year, which is reduced from the rate of 0.33 episodes per patient year during the initial year of the study. The rate of peritonitis decreased over time at our centre, possibly due to strategies in place for the prevention of peritonitis. Prophylactic antibiotics prior to catheter placement have been shown to reduce the incidence of early peritonitis and are administered routinely to patients at our centre [5]. All patients received either mupirocin or gentamicin exit site antibiotics for the prevention of catheter and tunnel infections. Additionally, our PD training is facilitated by dedicated nursing staff who have been trained on the specific curriculum used to teach patients on how to perform the PD technique.
The most frequently isolated organisms were gram positive bacteria, with coagulase negative staphylococci being the most commonly isolated species. The literature suggests that coagulase negative staphylococci is the most frequently isolated organism in peritonitis episodes at most centres [8-10]. Among the gram-negative organisms isolated, Escherichia coli was the most common. Resistance rates were low among the species isolated. Staphylococci species were 92% sensitive to first generation cephalosporins. Almost all gram- positive isolates were sensitive to vancomycin, except for one isolate of enterococci. Gram negative organisms were 100% sensitive to third generation cephalosporins. Overall, organisms were sensitive to the empiric therapy prescribed in 89% of episodes. The culture negative rate was 10% over the past 5 years. Acceptable culture negative rates are typically around 10-20%, therefore our methods of dialysate effluent sample collection and inoculation are appropriate [10].
No episodes of fungal peritonitis were observed over the five-year study period. Risk factors for fungal peritonitis include administration of broad-spectrum antibiotics, immunosuppression, extraperitoneal fungal infection or HIV infection [11,12]. In the peritonitis episodes identified 13% were receiving immunosuppression, 24% had received antibiotics in the past 3 months and 5% had extraperitoneal fungal infections. Fungal prophylaxis was administered at the discretion of the nephrologist in 24% of peritonitis episodes. Although the most recent ISPD guidelines recommend that all patients receiving antibiotics be prescribed fungal peritonitis prophylaxis, the absence of fungal peritonitis at our centre over the past five years indicates that the current practice of selective prescribing based on risk factors is appropriate.
Resolution of infection was achieved in 81% of episodes. The literature reports resolution rates typically range from 75-86% depending on the antibiotic regimen selected [7,13]. The majority of refractory peritonitis episodes were gram positive or polymicrobial infections. Additionally, the majority of patients who had their PD catheter removed had either gram positive or polymicrobial peritonitis. However, the infection outcomes were similar regardless of whether the organism isolated was sensitive or resistant to the empiric therapy. This may be because the empiric therapy is narrowed to appropriate antibiotics quickly after culture and sensitivity data is obtained. The average duration of empiric coverage at our centre was ~3 days. Overall organisms were sensitive to empiric therapy in ~89% of episodes, therefore altering empiric antibiotic therapy is not likely to further optimize the rate of resolution of infection. Future research may consider other factors that may contribute to outcomes of refractory infection, relapse infection, PD catheter removal and transfer to hemodialysis.
Limitations include a small sample size which precluded the ability to associate patient characteristics with peritonitis episode outcomes. The number of peritonitis episodes may be under-reported due to missed or inappropriate PROMIS data entry which would lead to a falsely low peritonitis rate. Patients with multiple peritonitis episodes may impact the resistance patterns observed, however most patients in our study were experiencing their first or second episode of peritonitis. Finally, additional antibiotics (eg.,piperacillin/tazobactam) were administered in 32% of peritonitis episodes which may confound the interpretation of the effectiveness of our empiric antibiotic regimen.
In conclusion the peritonitis rate, incidence of antibiotic resistance and incidence of fungal peritonitis was low at our centre compared to reports from the literature. Intraperitoneal cefazolin plus intraperitoneal ceftazidime is an appropriate empiric antibiotic regimen for the treatment of peritoneal dialysis-related peritonitis at our hospital. There were no episodes of fungal peritonitis, therefore it is appropriate to continue our current practice of providing fluconazole prophylaxis at the discretion of the prescriber based on the patient’s risk factors for fungal peritonitis.
References
- Choi P, Nemati E, Banerjee A, Preston E, Levy J (2004) Peritoneal dialysis catheter removal for acute peritonitis: A retrospective analysis of factors associated with catheter removal and prolonged postoperative hospitalization. Am J Kidney Dis 43: 103-111.
- Fried LF, Bernardini J, Johnston JR, Piraino B (1996) Peritonitis influences mortality in peritoneal dialysis patients. J Am SocNephrol7: 2176-2182.
- Piraino B, Bernardini J, Sorkin M () Catheter infections as a factor in the transfer of continuous ambulatory peritoneal dialysis patients to hemodialysis. Am J Kidney Dis 13: 365-369.
- Ates K, KoçR, NergizogluG, ErtürkS, Keven K, et al. (2000) The longitudinal effect of a single peritonitis episode on peritoneal membrane transport in CAPD patients. Perit Dial Int20: 220-226.
- Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, et al. (2016) ISPD Peritonitis Recommendations: 2016 Update on Prevention and Treatment. Perit Dial Int36: 481-508.
- Ballinger AE, Palmer SC, Wiggins KJ, Craig JC, Johnson DW, et al. (2014) Treatment for peritoneal dialysis-associated peritonitis. Cochrane Database Syst Rev 26: CD005284.
- Barretti P, Doles JV, Pinotti DG, El Dib R (2014) Efficacy of antibiotic therapy for peritoneal dialysis-associated peritonitis: A proportional meta-analysis. BMC Infect Dis 14: 445.
- Munib S (2006) Continuous Ambulatory Peritoneal Dialysis (CAPD). Gomal J Med Sci. 4: 82-85.
- vonGraevenitz A, Amsterdam D (1992) Microbiological aspects of peritonitis associated with continuous ambulatory peritoneal dialysis. ClinMicrobiol Rev 4: 36-48.
- Mujais S (2006) Microbiology and outcomes of peritonitis in North America. Kidney IntSuppl: 55-62.
- Prasad N, Gupta A (2005) Fungal peritonitis in peritoneal dialysis patients. Perit Dial Int 25: 207-222.
- Bren A (1998) Fungal peritonitis in patients on continuous ambulatory peritoneal dialysis. Eur J ClinMicrobiol Infect Dis 17: 839-843.
- Xu R, Yang Z, Qu Z, Wang H, Tian X, et al. (2017) Intraperitoneal vancomycin plus either oral moxifloxacin or intraperitonealceftazidime for the treatment of peritoneal dialysis-related peritonitis: A randomized controlled pilot study. Am J Kidney Dis 70: 30-37.
Citation: Driver AS, Cheng E, Singh S (2021) Evaluation of the Empiric Treatment of Peritoneal Dialysis-Related Peritonitis. J Nephrol Renal Ther 7: 065.
Copyright: © 2021 Elaine Cheng, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

