
Experience about Improving the Histological Detection of Gastric Helicobacter pylori
*Corresponding Author(s):
Chi-Min ShihDepartment Of Pathology, Chia-Yi Hospital, Ministry Of Health And Welfare, Chiayi, Taiwan
Tel:+886 963023378,
Email:sgmluca@gmail.com
Chien-Kuo Liu
Department Of Gastroenterology, St. Joseph’s Hospital, Yunlin, Taiwan
Tel:+886 56337333,
Email:Liu2004888@yahoo.com.tw
Abstract
Histological detection of Helicobacter pylori dwelling in gastric mucous film remains the routine practice for examination of gastric specimens by pathologists. However, lower specificity compared to sensitivity has been reported in a few articles. To improve its specificity, to prevent unnecessary antibiotic use leading to drug resistance and to confirm the availability of other tests for H. pylori detection are important. We have found that the infection rates of H. pylori among three hospitals (from one suburban and two urban areas in Taiwan) vary, ranging from 13.5% (23/170, Giemsa stain) to 21.1% (19/90 cases, immunohistochemical stain) and 24.6% (27/110, Giemsa stain), respectively. We have made serial reviews at an attempt to correct an over diagnosis of H. pylori infection from 41.8% to 24.6%, which is caused by a forceful hint of mistakenly high H. pylori infection rate. The presence of neutrophils in gastric mucosa with/without mucosal erosion is related to medium or high H. pylori density significantly (P=0.03), but the significant difference disappears if trace and low H. pylori densities are included. Furthermore, the infection rates of H. pylori of the gastric specimens taken by 4 gastroenterologists range from 3/7 (42.9%), 4/28 (14.3%), 7/51 (13.7%) to 9/74 (12.2%) are largely proportional to the severities of active gastritis. However, the difference in infection rates is not significant (p=0.177), and might be related to small sample sizes. The infection rates fluctuate among hospitals, pathologists and gastroenterologists, who have different tendency to do a gastric biopsy according to the endoscopic findings. If we wish to maintain the histological detection of H. pylori infection as a gold standard with higher specificity, the provision of a semiquantitative report of H. pylori infection should be essential.
Keywords
INTRODUCTION
Helicobacter pylori (H. pylori, HP) infection rate is different among nations [1,2], different age levels or social classes [1,3]. There are several methods developed according to various physical situations, feasibilities, techniques, costs, sensitivity, specificity and facility since the discovery of H. pylori [4-6]. Endoscopy biopsy with histological examination is recognized as one of the gold standard for detection of H. pylori [7-10]. It has been observed that specificity is less excellent than sensitivity for the detection of H. pylori by histology in a few literatures [4,8,11]. We compared the infection rates of H. pylori among 3 hospitals of different districts via histological detection on gastric endoscopic biopsy specimens. Some factors influencing the infection rate of the routine gastric tissue coming to the department of pathology are noticed.
MATERIALS AND METHODS
We would like to declare that this article has no conflict of interests. All the enrolled slides were delinked with their original general data. H. pylori infection rate of a hospital #1 of suburban region and two hospitals #2 and #3 of urban region are compared. 170, 90 and 110 cases of gastric biopsy specimens are collected during a 3-month period, respectively. Routine Giemsa stain for the first and third hospitals and routine immunohistochemical stain of HP bacteria for the second Hospital are performed. The HP density in gastric pits and on mucosal surface are detected through Giemsa stain (Giemsa’s stain solution of Muto Pure Chemicals Co., Ltd) or Immnohistochemical (IHC) stain (primary antibody: Zytomed Rabbit anti-Helicobacter pylori polyclonal antibody, and Bond Polymer Refine Detection kit of LeicaCo. The usage of 400X high power view under microscopy is used to confirm HP density. The HP densities can be categorized into high, medium, low and trace levels. The mucosal erosion and infiltration of neutrophils in nearby lamina propria are all recorded. Intergroup difference was calculated using chi square test; p<0.05 as significance level.
RESULTS
The infection rates among 3 hospitals #1, #2 and #3 are 13.5% (23/170), 21.1% (19/90) and 24.6% (27/110), respectively. We note that the third (urban) hospital has significantly higher HP infection rate than the suburban one (24.6% vs. 13.5%), p=0.01(Figure 1). The hospital #2 of urban area, detecting H. pylori with the method of IHC stain that is of high accuracy yet high cost had 21.1% HP infection rate. The HP infection rate of urban hospital of 110 cases had reached 41.8% (46/110) from previous 22.6% (7/31) after physician’s promotion. But the infection rate was corrected by reviewing the slides with the improved concepts mentioned in related article [11], and reducing the rate to 24.6% (27/110) by removing the false positive cases mainly of trace to low densities of H. pylori (Figure 1). Different doctors may have variable tendency or preference for gastric biopsy procedures during gastric endoscopic examination. The more biopsies on stomach with mild gastritis, the lower HP infection rate of the biopsy specimens is imaginable. However, the different infection rates of gastric tissue specimens 3/7 (42.9%), 4/28 (14.3), 7/51 (13.7) to 9/74 (12.2%) among four gastroenterologists make no statistical significance, p=0.177 (Figure 2). Finally, we take the suburban hospital (170 cases) as the example, the HP infection of all HP densities didn’t correlate with active gastritis (presence of neutrophils) or mucosal erosion significantly [30.4% (7/23) for HP infection cases vs. 20.4% (30/147) for non-HP infection cases], p=0.28 (Figures 3A and 3B). Only after neglecting the trace or low HP densities and appreciating the medium to high HP densities, it could lead to a significant correlation between H. pylori infection and active gastritis or gastric erosion [42.9% (6/14) of moderate to marked HP infection cases vs. 20.4% (30/147) of non-HPinfection cases], p=0.03 (Figures 3C and 3D).
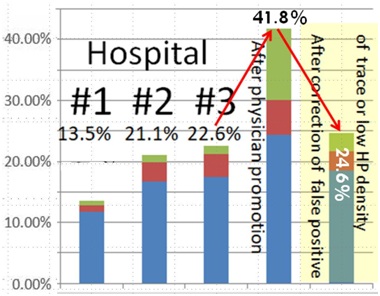 Figure 1: H. pylori infection rates by histology among 3 Hospitals #1, #2 and #3 (right 3rd to 5th bars) are 13.5% (23/170), 21.1% (19/90) and 24.6% (27/110). The 3rd bar of HP infection rate 22.6% (7/31) is before forceful hint of high HP infection rate by gastroenterologist, 41.8% (46/110) being after forceful hint and being corrected to 24.6% by reviewing the slides influenced by forceful hint. The intergroup difference between the infection rates of #1:13.5% vs. #3: 24.6% is significant; p=0.01.
Figure 1: H. pylori infection rates by histology among 3 Hospitals #1, #2 and #3 (right 3rd to 5th bars) are 13.5% (23/170), 21.1% (19/90) and 24.6% (27/110). The 3rd bar of HP infection rate 22.6% (7/31) is before forceful hint of high HP infection rate by gastroenterologist, 41.8% (46/110) being after forceful hint and being corrected to 24.6% by reviewing the slides influenced by forceful hint. The intergroup difference between the infection rates of #1:13.5% vs. #3: 24.6% is significant; p=0.01.
Blue+Brown+Light green: Total height of each bar at the percentage level of Y axis is the H. pyloriinfection rate of the given gastric specimens of each hospital
Brown: The percentage of brown portion in the total bar is the percentage of medium to high HP densities of given gastric specimens
Light green: The percentage of light green portion in the total bar is the percentage of trace to low HPdensities of given specimens
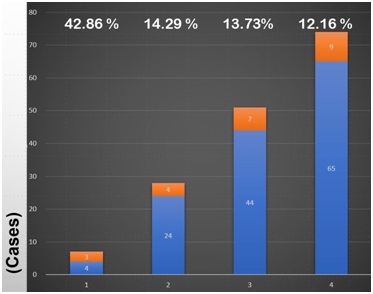 Figure 2: Different doctors might have different tendency of doing gastric endoscopic biopsy during endoscopic examination. It seems that the more active gastritis of the gastric specimens, the higher H. pylori infection rate, but no significant difference (p=0.177) among the HP infection rates of different doctor’s gastric specimens.
Figure 2: Different doctors might have different tendency of doing gastric endoscopic biopsy during endoscopic examination. It seems that the more active gastritis of the gastric specimens, the higher H. pylori infection rate, but no significant difference (p=0.177) among the HP infection rates of different doctor’s gastric specimens.
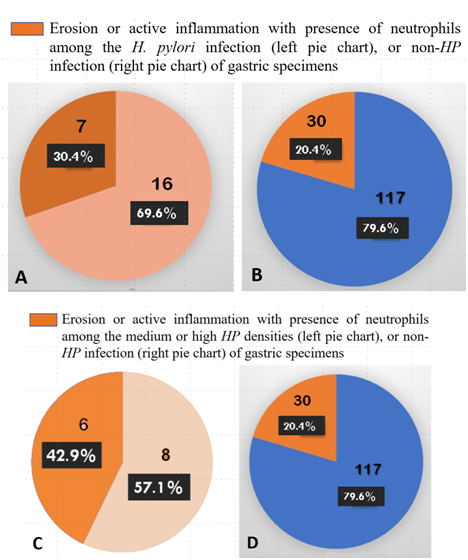 Figures 3 (A-D): A,B) 30.4% of all densities of H. pylori infection accompanied with gastric erosion or active inflammation (the remaining percentage is without gastric erosion or active inflammation) is not significantly different from non-HP infection with gastric erosion or active inflammation (20.4%); p=0.28. C,D) 42.9% of medium or high densities of HP infection specimens with gastric erosion or active inflammation is significantly higher than the rate of non-HP infection with gastric erosion or active inflammation (20.4%); p=0.03.
Figures 3 (A-D): A,B) 30.4% of all densities of H. pylori infection accompanied with gastric erosion or active inflammation (the remaining percentage is without gastric erosion or active inflammation) is not significantly different from non-HP infection with gastric erosion or active inflammation (20.4%); p=0.28. C,D) 42.9% of medium or high densities of HP infection specimens with gastric erosion or active inflammation is significantly higher than the rate of non-HP infection with gastric erosion or active inflammation (20.4%); p=0.03.
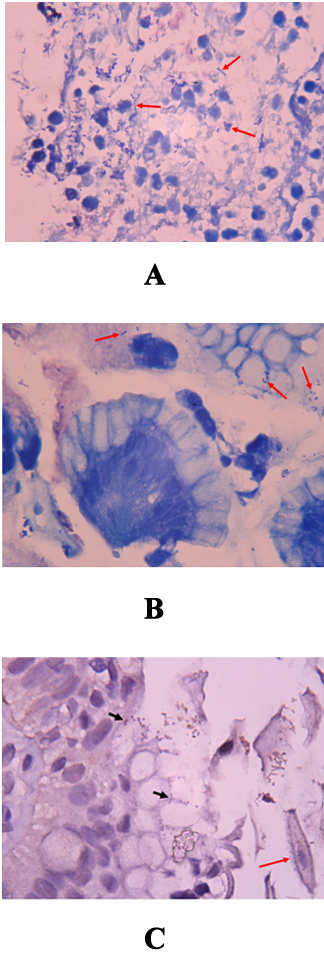 Figures 4 (A-C): Some bacilli (arrows) in necrotizing tissue shows too long and too straight to interpret as H. pylori (Giemsa stain, x1000) (A). Some coccoid bacteria (arrows) adjacent the surface glandular cells might be diagnosed as the coccoid form of HP (Giemsa stain, x1000) (B). However, IHC stains for HPantigen prove to be negative finding with no immunostaining (short arrows) on the bacteria. Few contaminated squamous epithelial cells (long arrow) of oropharyngeal origin are found nearby (IHC stain for H. pylori, x1000) (C).
Figures 4 (A-C): Some bacilli (arrows) in necrotizing tissue shows too long and too straight to interpret as H. pylori (Giemsa stain, x1000) (A). Some coccoid bacteria (arrows) adjacent the surface glandular cells might be diagnosed as the coccoid form of HP (Giemsa stain, x1000) (B). However, IHC stains for HPantigen prove to be negative finding with no immunostaining (short arrows) on the bacteria. Few contaminated squamous epithelial cells (long arrow) of oropharyngeal origin are found nearby (IHC stain for H. pylori, x1000) (C).
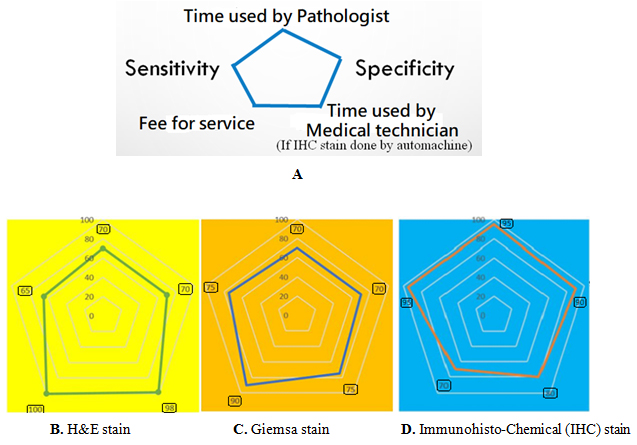 Figure 5: Five main parameters are considered for comparison with radar chart (A). The benefits among three staining methods are compared by the area of each pentagon (B, C and D), the larger area the more cost-effective (100 means no increase of cost, and high effectiveness). We can see the high effectiveness and higher cost of the IHC method (D).
Figure 5: Five main parameters are considered for comparison with radar chart (A). The benefits among three staining methods are compared by the area of each pentagon (B, C and D), the larger area the more cost-effective (100 means no increase of cost, and high effectiveness). We can see the high effectiveness and higher cost of the IHC method (D).
DISCUSSION
In our series, only adult patients receiving the procedure of gastric endoscopic biopsy are enrolled. The children seldom have endoscopic examination for detection of gastric lesion or peptic erosion, thus they are detected by non-invasive methods for H. pylori prevalence [5]. The fact that hospitals of urban regions tend to have a higher prevalence of H. pylori infection than that of countryside regions (noted on a previous study by Huang Y, et al., [12] displayed similarly in our study. However, it is reported that more than half of the population (53.9%) is infected with H. pylori in Taiwan in the past [13], unlike the lower infection rate in this study. While this infection rate may have decreased because of more affordable eradication treatments [14,15] and a reduction of false positive rate via recognition of interference factors that may lead to a overdiagnosis of H. pylori infection.
It has been reported that the more severe the active gastritis (or acute inflammation of gastric mucosa), the more likely the presence of H. pylori infection [12,16,17], as noted in our study (active gastritis and gastric erosion correlated significantly among medium to high H. pylori densities). Furthermore, the infection rate is lower among patients of a particular doctor who is tend to perform endoscopic biopsies on grossly near-normal gastric mucosa, although the statistical significance was not evident, probably because of small sample size (Figure 2).
Promotion of infection rate through hint or pressure by clinician is observed in this study. In the current articles, higher infection rates are found among some countries, especially in some of the developing countries [1,2]. It means they will have the hint of high H. pylori infection rate and it will be easier to have a false positive infection report by pathologists [11], though it may not happen in developed countries [18]. If it happens in developed countries, then possible negligence of other causes of active gastritis or its ulcer, a higher antimicrobial resistance rate, and a falsely lower sensitivity of the non-invasive detection methods might be induced by the falsely high positive histological detection results (currently so-called gold standard method). More first-line or second-line antibiotics may be prescribed due to false H. pylori infection or false drug-resistance, respectively. Therefore, drug resistance of H. pylori infection might increase relatively [19,20]. Here in, we attempt to exercise caution in the application of two-edged sword-like histological detection, and increase its necessary accuracy by the usage of a semi quantitative report of H. pylori density (infection intensity). Report of low HP intensity, coccoid form of H. pylori(Figure 4A) or bacterial colonization on necrotic debris (Figure 4B) should be cautious, because it might be simply contaminated bacteria (especially when it accompanied by minute oral debris or superficial squamous epithelial cells). When we are aware that the percentage of low H. pylori density should not be over one-third to one-fourth of all H. pylori infection densities (see 2nd and 3rd bars in figure 1), this will reduce the false positive H. pylori infection rate and thus increase its specificity. The report of ‘trace’ H. pylori density, which conflicts with the grading classification of Sydney system [21], and may represent an uncertain situation of H. pylori like contaminated bacteria or mucus precipitates, should be abandoned.
Reporting the presence of acute or active gastritis (gastric inflammation with presence of neutrophils or presence of mucosal erosion) could also improve the sensitivity and specificity of H. pylori infection (Figure 3). When we give more detailed information on infection density/intensity and its effects on mucosa, it will compensate not only the shortcoming of lower specificity influenced by interpretation pitfalls but also lower sensitivity caused by inadequate screening of tissue section or sampling of representative tissue [22]. By the way, an infiltration of eosinophils, a subtype of polymorphonuclear leukocyte, with chronic inflammatory cells does not belong to acute or active gastritis.
Employment of advanced non-invasive methods for follow-up, (such as fecal or urine H. pylori antigen detection), or histology detection method done by well-educated and well-trained medical technician (who is of more available manpower and of lower cost) could be used interchangeably with pathologists in the detection of H. pylori infection, and are indeed two other methods to be considered. With regards to clinical treatment, the commencement of anti H. pylori treatment with medium or high H. pylori intensity, versus merely regular follow-ups for those who has low, particularly trace H. pylori intensity should be recommended, when low/trace intensity seems misused or over-proportional, to prevent from unnecessary antibiotic treatment and eventual influence of antibiotic resistance rate. If the finding of low H. pylori density is solid and definite, the neutrophils would also present in adjacent gastric mucosa by our recent observation, antibiotic treatment for low intensity of HP infection is reasonable.
Finally, we compare additional Giemsa stain or H. pylori immunostaining, which will increase the cost to 1.3 folds or nearly double the cost of original H&E stain, respectively. However, both will save interpretation time, and increase specificity and sensitivity (Figure 5).
CONCLUSION
To correlate active or acute gastritis with ‘graded’ H. pylori infection report not only aids in the histopathologic detection of H. pylori, but also provides fair comparison of newly introduced non-invasive detection methods. The tendency of clinicians to perform gastric endoscopy biopsy on gastric mucosa without obvious mucosal changes, or inadequate sampling of representative sites of stomach may contribute to lower H. pylori infection/detection rate. An experienced and careful pathologist or medical technician could be an excellent H. pylori detector or screener, respectively, with adequate sensitivity and specificity.
REFERENCES
- Salih BA (2009) Helicobacter pylori Infection in Developing Countries: The Burden for How Long? Saudi J Gastroenterol 15: 201-207.
- Joshi YK (2004) Helicobacter pylori Infection : Current Status. J Indian Acad of Clin Medic 5: 148-155.
- Sitas F, Forman D, Yarnell JW, Burr ML, Elwood PC, et al. (1991) Helicobacter pylori infection rates in relation to age and social class in a population of Welsh men. Gut 32: 25-28.
- Khalifehgholi M, Shamsipour F, Ajhdarkosh H, Daryani NE, Pourmand MR, et al. (2013) Comparison of five diagnostic methods for Helicobacter pylori. Iran J Microbiol 5: 396-401.
- Kakiuchi T, Matsuo M, Endo H, Nakayama A, Sato K, et al. (2019) A Helicobacter pylori screening and treatment program to eliminate gastric cancer among junior high school students in Saga Prefecture: A preliminary report. J Gastroenterol Pg no: 1-9.
- Uotani T, Graham DY (2015) Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med 3: 9.
- Danciu M, Simion L, Poroch V, Padureanu SS, Constantinescu RN, et al. (2016) The role of histological evaluation of Helicobacter pylori infection in obese patients referred to laparoscopic sleeve gastrectomy. Rom J Morphol Embryol 57: 1303-1311.
- Tongtawee T, Dechsukhum C, Leeanansaksiri W, Kaewpitoon S, Kaewpitoon N, et al. (2015) Improved Detection of Helicobacter pylori Infection and Premalignant Gastric Mucosa Using “Site Specific Biopsy”: A Randomized Control Clinical Trial. Asian Pac J Cancer Prev 16: 8487-8490.
- Centers for Disease control and prevention (CDC) (1998) Helicobacter pylori: Fact Sheet for Health Care Provider. Depart of Health & Human servi, Washington, USA.
- Lee JY, Kim N (2015) Diagnosis of Helicobacter pylori by invasive test: histology. Ann Transl Med 3: 10.
- Shih CM, Chueh WY, Chung TT, Liu CK , Liu CC, et al. (2017) Histological Interpretation of Helicobacter pylori Density Relevant to Gastric Pathology and its Bias. J Cytol Tissue Biol 4: 1-5.
- Huang Y, Wang S, Zhao XH (2016) Helicobacter pylori infection and its risk factors in subjects receiving physical examination. Int J Clin Exp Med 9: 16802-16806.
- Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, et al. (2017) Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 153: 420-429.
- Rupnow MFT, Shachter RD, Owens DK, Parsonnet J (2000) A Dynamic Transmission Model for Predicting Trends in Helicobacter pylori and Associated Diseases in the United States. Emerg Infect Dis 6: 228-237.
- Hiroi S, Sugano K, Tanaka S, Kawakami K (2017) Impact of health insurance coverage for Helicobacter pylori gastritis on the trends in eradication therapy in Japan: Retrospective observational study and simulation study based on real-world data. BMJ Open 7: 015855.
- Tarkhashvili N, Beriashvili R, Chakvetadze N, Moistsrapishvili M, Chokheli M, et al. (2009) Helicobacter pylori Infection in Patients Undergoing Upper Endoscopy, Republic of Georgia. Emerg Infect Dis 15: 504-505.
- Ghasemi Basir HR, Ghobakhlou M, Akbari P, Dehghan A, Seif Rabiei MA (2017) Correlation between the Intensity of Helicobacter pylori Colonization and Severity of Gastritis. Gastroenterol Res Pract 2017: 8320496.
- TEl-Zimaity HM, Graham DY, Al-Assi MT, Malaty H, Karttunen TJ, et al. (1996) Interobserver variation in the histopathological assessment of Helicobacter pylori gastritis. Human Pathol 27: 35-41.
- Hu Y, Zhu Y and Lu NH (2017) Novel and Effective Therapeutic Regimens for Helicobacter pylori in an Era of Increasing Antibiotic Resistance. Front Cell Infect Microbiol 7: 168.
- Duck WM, Sobel J, Pruckler JM, Song Q, Swerdlow D, et al. (2004) Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis 10: 1088-1094.
- Dixon MF, Genta RM, Yardley JH, Correa P (1996) Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 20: 1161-1181.
- Abdel Latif ME, Shahin R, Abdrabou RM, Shawqy A, El-Ghadban HM, et al. (2016) Value of Additional Corpus Biopsy for Diagnosis of Helicobacter pylori in Atrophic Gastritis. J Gastro Hepato Dis 2: 108.
Citation: Shih CM, Chung TT, Tsai TY, Shu HF, Liu CK (2019) Experience about Improving the Histological Detection of Gastric Helicobacter pylori. J Cytol Tissue Biol 6: 020.
Copyright: © 2019 Chi-Min Shih, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

