
Explore the Mechanism of LiYin Decoction in Treating Nonalcoholic Steatohepatitis and Atherosclerosis with Homotherapy for Heteropathy Based on Network Pharmacology and Molecular Docking
*Corresponding Author(s):
Chai XinlouCollege Of Chinese Medicine, Beijing University Of Chinese Medicine, Beijing, China
Tel:+86 15811484091,
Email:mmxin3@126.com
Abstract
Background: LiYin Decoction (LYD) is a classic Chinese herbal formula most commonly used for the treatment of Nonalcoholic Steatohepatitis (NASH) and Atherosclerosis (AS) with homotherapy for heteropathy. In order to understand its mechanisms action, this study based on the theory of homotherapy for heteropathy used network pharmacology and molecular docking approaches to illustrate the molecular mechanism and substance basis of LYD for the treatment of NASH and AS.
Methods: The active ingredients and their potential targets of LYD were obtained and screened from TCMSP database, and the names of targets were standardized by the UniProt database. DisGeNET, CTD and TTD databases were searched to obtain two disease-related targets. A Venn diagram was drawn by online software “Bioinformatics” and acquired common targets, software Cytoscape3.7.2 was used to construct and visualize the “LYD ingredients-common targets” network. The common targets were imported into STRING11.5 database to construct Protein-Protein Interaction (PPI) network, software Cytoscape3.7.2 was used to visualize the results and screen out the core network. Metascape database was used to analyze the GO and KEGG pathway enrichment results. Finally, the main active ingredients were docked with the core targets by using software AutoDockTools1.5.7.
Results: The core active components of LYD are quercetin, kaempferol, naringenin, isorhamnetin and formononetin, and the core targets are ESR1, PPARG, ESR2, MAPK14, PIK3CG, MAPK8, MAPK3, PRKCA etc. The key targets are mainly enriched in significant pathways such as lipid and atherosclerosis, IL-17 signaling pathway and so on. Molecular docking verification results indicated that most of the core components have strong binding activity to the core targets.
Conclusion: This study preliminarily revealed the potential mechanism of multiple ingredients in LYD to treat NASH and AS with homotherapy for heteropathy through multiple targets and ways, provided theoretical basis for further research on the mechanism of action and clinical application of LYD.
Keywords
Atherosclerosis; Homotherapy for heteropathy; LiYin Decoction; Molecular docking; Network
Introduction
Nonalcoholic Steatohepatitis (NASH) is a severe form of Nonalcoholic Fatty Liver Disease (NAFLD) that results from lipid accumulation leading to hepatitis and hepatocellular damage (ballooning) [1], it is considered to be an asymptotic form of NAFLD and characterized by hepatic steatosis, inflammation, hepatocellular injury and fibrosis of varying degrees. The mechanism of inflammation can help us to prevent and reverse the further development of NASH [2]. Atherosclerosis (AS) is a chronic inflammatory cardiovascular disease that is harmful to human health, its main pathological feature is that the lipid deposition in the coronary artery and accompanied by the proliferation of smooth muscle cells and fibrous matrix proliferation, which gradually forms atherosclerotic plaques. At the same time, it is generally believed that various inflammatory cells and inflammatory factors also play an important role in its pathogenesis [3]. Existing research shows that pathological changes in NASH are not only associated with the liver, but also with an increased risk of cardiovascular events; patients with NAFLD have a higher risk of cardiovascular events compared with patients without NAFLD, which is the main cause of death [4]. The pathogenesis and pathological changes of NASH and AS are related to lipid and inflammation. According to the theory of “homotherapy for heteropathy” in traditional Chinese medicine, the same treatment method is used for different diseases with the same pathogenesis, and the same etiology or pathogenesis in different diseases is the key to treatment [5]. In TCM syndrome differentiation and treatment, NASH belongs to “hypochondriac pain” and “abdominal mass”, the main pathogenesis of NASH is the endogenous turbid dampness, which runs through the whole process of the disease [6]. AS can be attributed to “chest discomfort” and “angina pectoris” in TCM and its pathogenesis should also be classified as the endogenous turbid dampness, which is considered to be main pathogenesis of AS [7]. The classic famous prescription LiYin Decoction originated from the chapter “Prescription for Treating Phlegm and Fluid” in Integrating Chinese and Western Medicine written by Zhang Xichun [8], and it is composed of eight herbs including Rhizoma Zingiberis(GJ),Cassia Twig(GZ),Atractylodes macrocephala(BZ),Hoelen(FL), Radix Paeoniae Alba (BS), Pericarpium Citri Reticulatae (CP), Cortex Magnoliae Officinalis (HP) and Glycyrrhiza uralensis Fisch(GC),which is used to treat phlegm and fluid retention and is widely used in clinic [9]. Therefore, the use of LYD to treat NASH and AS, which are mainly characterized by “endogenous turbid dampness”, conforms to the theoretical basis of “homotherapy for heteropathy” in TCM.Meanwhile, modern pharmacological and clinical studies show that the drugs contained in LYD have definite therapeutic effects on interfering with NASH and AS [10-13], providing a theoretical basis for treating different diseases with the same treatment (Figure 1).
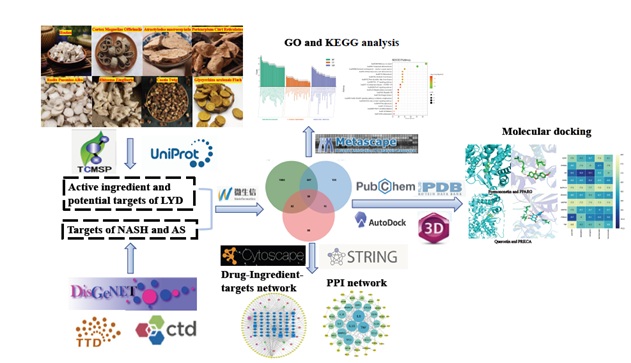 Figure 1: Workflow of the study.
Figure 1: Workflow of the study.
With the rapid development of biomedical big data and artificial intelligence era, network pharmacology has formed a new generation of research mode characterized by “network” and “system”. It explores the relationship between drugs and diseases by building a network of “drug components-targets-pathways-diseases”, which is in line with the characteristics of “whole” and “treating different diseases with the same treatment” of TCM in treating diseases [14]. In this study, the network pharmacology and molecular docking methods were used to explore the mechanism of LYD in treating NASH and AS with homotherapy for heteropathy, and provide reference for further clinical research and application.
Materials and Methods
Screening and Target Prediction of Active Components in LYD
The Traditional Chinese Medicine Systems Pharmacology Database and Analysis Platform (TCMSP) was used to obtain the active ingredients of GJ, GZ, BZ, FL, BS, CP, HP and GC based on the oral bioavailability (OB ≥ 30%) and drug-like (DL ≥ 0.18). The targets of the active ingredient were predicted by TCMSP, and then the gene symbols were obtained after standardized by UniProt database.
Screening NASH and AS related targets
NASH and AS related targets were acquired from the DisGeNET, Comparative Toxicogenomics Database (CTD) and Therapeutic Target Database (TTD), and then get disease-related targets after sorting. By using online software “Bioinformatics” (https://www.bioinformatics.com.cn) to intersect the targets of LYD and the targets of the two diseases and draw the VENN diagram.
Building the PPI network of the intersection target of NASH and AS
Import the obtained LYD-NASH and AS intersection targets into Search Tool for the Retrieval of Interacting Genes (String11.5) database, select the species as “Homo sapiens”, and set the “minimum required interaction score” as 0.900, so as to obtain PPI network relationship. The results were imported into the Cytoscape 3.7.2 software for visualization, and the plug-in Network Analyzer is used to analyze the relationship between protein interactions, then the core proteins are screened and analyzed.
Construction of “LYD active-ingredients -intersection targets of NASH and AS” network Cytoscape 3.7.2 software was used to build the “drug-active ingredient-disease targets” network
by combining the target of LYD active-ingredient with the intersection targets of NASH-AS, where node represents the drug ingredient or disease target, and edge represents the relationship between the drug ingredient and disease target. Network Analyzer plug-in in Cytoscape 3.7.2 was used to analyze network characteristics, including topological parameters such as Degree, Betweenness and Closeness, and study the interaction relationship between drug components and targets.
Enrichment analysis of the target Function and pathway of LYD intervening NASH and AS
The intersection targets obtained from 2.4 were analyzed by using the Metascape (http://metascape.org) database for biological information enrichment. The species selection is Homo sapie, including KEGG pathway analysis, Biological Process (BP), Molecular Function (CC) and Molecular Function (MF) of GO analysis. After the analysis results were sorted according to the P value and Count value, respectively P< 0.01, the top 20 KEGG pathways and GO analysis results were selected.
Construction of “LYD active-ingredients -intersection targets -pathways” network
Combining the information of drug active component-disease target in 1.4 and pathway interaction obtained by KEGG enrichment analysis in 1.5, using Cytocape3.7.2 to construct “drug component-target-pathway” network diagram for visualization processing and analyzing the topological parameters of the network, and screening the main active components and core targets of LYD for interfering NASH and AS according to the topological parameters in the network. Combining the drug active component-disease targets in 2.4 and pathways interaction obtained by KEGG enrichment analysis in 2.5, using Cytocape3.7.2 to construct “drug component-target-pathway” network diagram for visualization processing and analyzing the topological parameters of the network. Then screening the main active components and core targets of LYD for interfering NASH and AS according to the topological parameters in the network.
Molecular docking verification
The main active ingredients of LYD and the core targets of interfering NASH and AS screened in 2.6 were obtained through analysis. The names of the active ingredients were searched through PubChem database(https://pubchem.ncbi.nlm.nih.gov) and the 3D structure files of compounds were downloaded in pdb format. At the same time, the core targets were searched in Uniprot database to obtain Entry names, which were imported into PROTEIN DATA BANK(https://www.rcsb.org) database to search and download the 3D structure files of target proteins, which were saved in pdb format. PyMOL was used to remove water molecules and small molecular ligands from the target protein. AutoDockTools-1.5.7 was used to add hydrogen bonds to the receptor protein, and the size of the active pocket was determined and stored in pdbqt format. After the minimum binding energy of the active ingredient ligands were calculated by Chem3D software, the format was converted into pdbqt format by AutoDockTools-1.5.7 and stored. By using AutoDock Vina for molecular docking operation, the active components and targets with better binding activity are selected based on the minimum binding energy (Affinity value of ligand-receptor). The Affinity score is less than - 5 kcal • mol-1, which means that the binding activity is good, and less than - 7kcal • mol-1, which means that the ligand-receptor has strong docking activity.
Results
Active components and targets of LYD
In the TCMSP database, 8 components of BZ,5 components of GJ,7 components of GZ, 92 components of GC, 15 components of FL,13 components of BS, 9 components of JH and 2 components of HP were obtained, including kaempferol, naringenin, beta-sitosterol, formononetin, Hedysarimcoumestan B, etc. A total of 236 targets of LYD active ingredients were obtained after sorting and deleting duplicate values.
Disease-related targets of NASH and AS
Through searching DisGeNET, CTD and TTD databases, a total of 529 NASH-related disease targets were obtained. After sorting out the potential targets, 448 targets were finally obtained. Similarly, 2069 AS-related disease targets were finally obtained. The potential targets of these two diseases are intersected to obtain 303 targets. The targets of LYD and the disease-related targets of NASH and AS were intersected to obtain 56 targets, and the VENN diagram was shown in figure 2.
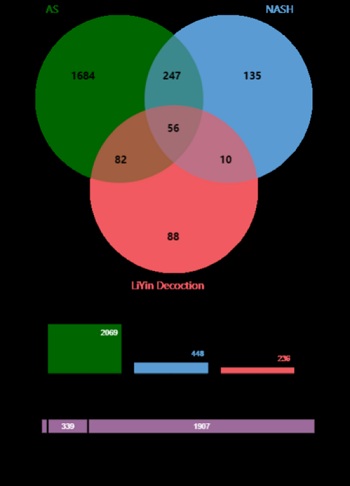 Figure 2: Venn diagram of the targets of the AS, NASH and LiYin Decoction.
Figure 2: Venn diagram of the targets of the AS, NASH and LiYin Decoction.
“LYD- the intersection targets of NASH and AS” PPI network
The 56 targets obtained from 3.2 are used to build PPI network through STRING 11.5 database, the species was selected as “Homo sapiens”, and the “minimum required interaction score” was set as 0.900, so as to acquired PPI network relationship. The results are imported into Cytoscape 3.7.2 software for visualization, as shown in figure 3. There are 53 nodes and 131 edges in the network, in which the nodes represent protein-coding genes, and the lines represent the interaction relationship between two genes. The larger the area of the nodes is in the network, the degree of connection of the nodes is higher. The results showed that IL6, TNF, IL10, TP53, MAPK3, IL4, IL8, MAPK14 and ESR1 were the main targets.
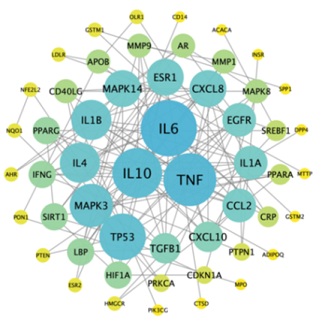 Figure 3: The common targets PPI network.
Figure 3: The common targets PPI network.
“LYD drugs-active ingredients-intersection targets” network
The “drugs - active ingredients - intersection targets” network was built through Cytoscape 3.7.2 software, as shown in figure 4. There are 157 nodes, 579 edges, 94 active ingredients (Table 1) and 56 intersection targets in the network. The blue round rectangle node represents the disease target, the purple octagon node represents the drug, the pink octagon and the yellow and green diamond nodes represent the active ingredient. After the network was analyzed through the plug-in Network Analyzer, the higher the degree value was, the correlation of the node was greater. The larger the area of all nodes was in the network, their degree value was higher. The results showed that kaempferol (MOL000422), naringin(MOL004328), β-sitosterol(MOL000358), Hedysarimcoumestan B(MOL004913), formononetin (MOL000392) and kanzonols W (MOL004820) had higher degree value, it suggested that these active ingredients might be the key ingredient of LYD and played an important role in the intervention of NASH and AS. ESR1, AR, PPARG, ESR2, MAPK14, DPP4, PIK3CG and other targets have high degree value in the network, which may be the core genes of LYD interfering with NASH and AS.
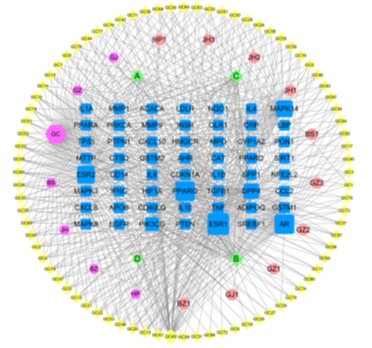 Figure 4: “Drugs-active ingredients-intersection targets” network.
Figure 4: “Drugs-active ingredients-intersection targets” network.
(Blue round rectangle node - disease target, purple octagon node - drug, pink octagon and the yellow and green diamond nodes - active ingredient.)
|
Main active ingredients of LYD for intervention of NASH and AS |
|||||
|
NAME |
MOLID |
INGREDIENT |
OB(%) |
DL |
SOURCE |
|
BZ1 |
MOL000049 |
3β-acetoxyatractylone |
54.07 |
0.22 |
BZ |
|
GJ1 |
MOL002514 |
Sexangularetin |
62.86 |
0.3 |
GJ |
|
GZ1 |
MOL001736 |
(-)-taxifolin |
60.51 |
0.27 |
GZ |
|
GZ2 |
MOL000073 |
ent-Epicatechin |
48.96 |
0.24 |
GZ |
|
GZ3 |
MOL004576 |
taxifolin |
57.84 |
0.27 |
GZ |
|
BS1 |
MOL001924 |
paeoniflorin |
53.87 |
0.79 |
BS |
|
JH1 |
MOL001040 |
(2R)-5,7-dihydroxy-2-(4-hydroxyphenyl)chroman-4-one |
42.36 |
0.21 |
JH |
|
JH2 |
MOL005849 |
didymin |
38.55 |
0.24 |
JH |
|
JH3 |
MOL001798 |
neohesperidin_qt |
71.17 |
0.27 |
JH |
|
HP1 |
MOL005970 |
Eucalyptol |
60.62 |
0.32 |
HP |
|
GC1 |
MOL002311 |
Glycyrol |
90.78 |
0.67 |
GC |
|
GC2 |
MOL004990 |
7,2',4'-trihydroxy-5-methoxy-3-arylcoumarin |
83.71 |
0.27 |
GC |
|
GC3 |
MOL004904 |
licopyranocoumarin |
80.36 |
0.65 |
GC |
|
GC4 |
MOL004891 |
shinpterocarpin |
80.3 |
0.73 |
GC |
|
GC5 |
MOL005017 |
Phaseol |
78.77 |
0.58 |
GC |
|
GC6 |
MOL004841 |
Licochalcone B |
76.76 |
0.19 |
GC |
|
GC7 |
MOL004810 |
glyasperin F |
75.84 |
0.54 |
GC |
|
GC8 |
MOL001484 |
Inermine |
75.18 |
0.54 |
GC |
|
GC9 |
MOL000500 |
Vestitol |
74.66 |
0.21 |
GC |
|
GC10 |
MOL005007 |
Glyasperins M |
72.67 |
0.59 |
GC |
|
GC11 |
MOL004941 |
(2R)-7-hydroxy-2-(4-hydroxyphenyl)chroman-4-one |
71.12 |
0.18 |
GC |
|
GC12 |
MOL004959 |
1-Methoxyphaseollidin |
69.98 |
0.64 |
GC |
|
GC13 |
MOL000392 |
formononetin |
69.67 |
0.21 |
GC |
|
GC14 |
MOL004863 |
3-(3,4-dihydroxyphenyl)-5,7-dihydroxy-8-(3-methylbut-2- enyl)chromone |
66.37 |
0.41 |
GC |
|
GC15 |
MOL004808 |
glyasperin B |
65.22 |
0.44 |
GC |
|
GC16 |
MOL004829 |
Glepidotin B |
64.46 |
0.34 |
GC |
|
GC17 |
MOL004855 |
Licoricone |
63.58 |
0.47 |
GC |
|
GC18 |
MOL004914 |
1,3-dihydroxy-8,9-dimethoxy-6-benzofurano[3,2-c]chromenone |
62.9 |
0.53 |
GC |
|
GC19 |
MOL004835 |
Glypallichalcone |
61.6 |
0.19 |
GC |
|
GC20 |
MOL004907 |
Glyzaglabrin |
61.07 |
0.35 |
GC |
|
GC21 |
MOL005000 |
Gancaonin G |
60.44 |
0.39 |
GC |
|
GC22 |
MOL004824 |
(2S)-6-(2,4-dihydroxyphenyl)-2-(2-hydroxypropan-2-yl)-4- methoxy-2,3-dihydrofuro[3,2-g]chromen-7-one |
60.25 |
0.63 |
GC |
|
GC23 |
MOL004849 |
3-(2,4-dihydroxyphenyl)-8-(1,1-dimethylprop-2-enyl)-7-hydroxy-5-methoxy-coumarin |
59.62 |
0.43 |
GC |
|
GC24 |
MOL005003 |
Licoagrocarpin |
58.81 |
0.58 |
GC |
|
GC25 |
MOL004838 |
8-(6-hydroxy-2-benzofuranyl)-2,2-dimethyl-5-chromenol |
58.44 |
0.38 |
GC |
|
GC26 |
MOL005012 |
Licoagroisoflavone |
57.28 |
0.49 |
GC |
|
GC27 |
MOL005018 |
Xambioona |
54.85 |
0.87 |
GC |
|
GC28 |
MOL005020 |
dehydroglyasperins C |
53.82 |
0.37 |
GC |
|
GC29 |
MOL004993 |
8-prenylated eriodictyol |
53.79 |
0.4 |
GC |
|
GC30 |
MOL004908 |
Glabridin |
53.25 |
0.47 |
GC |
|
GC31 |
MOL004910 |
Glabranin |
52.9 |
0.31 |
GC |
|
GC32 |
MOL004879 |
Glycyrin |
52.61 |
0.47 |
GC |
|
GC33 |
MOL004912 |
Glabrone |
52.51 |
0.5 |
GC |
|
GC34 |
MOL004885 |
licoisoflavanone |
52.47 |
0.54 |
GC |
|
GC35 |
MOL003656 |
Lupiwighteone |
51.64 |
0.37 |
GC |
|
GC36 |
MOL004856 |
Gancaonin A |
51.08 |
0.4 |
GC |
|
GC37 |
MOL000239 |
Jaranol |
50.83 |
0.29 |
GC |
|
GC38 |
MOL004820 |
kanzonols W |
50.48 |
0.52 |
GC |
|
GC39 |
MOL005001 |
Gancaonin H |
50.1 |
0.78 |
GC |
|
GC40 |
MOL005016 |
Odoratin |
49.95 |
0.3 |
GC |
|
GC41 |
MOL000354 |
isorhamnetin |
49.6 |
0.31 |
GC |
|
GC42 |
MOL004848 |
licochalcone G |
49.25 |
0.32 |
GC |
|
GC43 |
MOL002565 |
Medicarpin |
49.22 |
0.34 |
GC |
|
GC44 |
MOL004857 |
Gancaonin B |
48.79 |
0.45 |
GC |
|
GC45 |
MOL004827 |
Semilicoisoflavone B |
48.78 |
0.55 |
GC |
|
GC46 |
MOL004913 |
1,3-dihydroxy-9-methoxy-6-benzofurano[3,2-c]chromenone |
48.14 |
0.43 |
GC |
|
GC47 |
MOL000417 |
Calycosin |
47.75 |
0.24 |
GC |
|
GC48 |
MOL004961 |
Quercetin der. |
46.45 |
0.33 |
GC |
|
GC49 |
MOL000098 |
quercetin |
46.43 |
0.28 |
GC |
|
GC50 |
MOL004898 |
(E)-3-[3,4-dihydroxy-5-(3-methylbut-2-enyl)phenyl]-1-(2,4- dihydroxyphenyl)prop-2-en-1-one |
46.27 |
0.31 |
GC |
|
GC51 |
MOL004911 |
Glabrene |
46.27 |
0.44 |
GC |
|
GC52 |
MOL004974 |
3'-Methoxyglabridin |
46.16 |
0.57 |
GC |
|
GC53 |
MOL004811 |
Glyasperin C |
45.56 |
0.4 |
GC |
|
GC54 |
MOL004949 |
Isolicoflavonol |
45.17 |
0.42 |
GC |
|
GC55 |
MOL004828 |
Glepidotin A |
44.72 |
0.35 |
GC |
|
GC56 |
MOL004948 |
Isoglycyrol |
44.7 |
0.84 |
GC |
|
GC57 |
MOL004866 |
2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-6-(3-methylbut-2- enyl)chromone |
44.15 |
0.41 |
GC |
|
GC58 |
MOL004966 |
3'-Hydroxy-4'-O-Methylglabridin |
43.71 |
0.57 |
GC |
|
GC59 |
MOL004915 |
Eurycarpin A |
43.28 |
0.37 |
GC |
|
GC60 |
MOL003896 |
7-Methoxy-2-methyl isoflavone |
42.56 |
0.2 |
GC |
|
GC61 |
MOL004883 |
Licoisoflavone |
41.61 |
0.42 |
GC |
|
GC62 |
MOL005008 |
Glycyrrhiza flavonol A |
41.28 |
0.6 |
GC |
|
GC63 |
MOL000497 |
licochalcone a |
40.79 |
0.29 |
GC |
|
GC64 |
MOL004980 |
Inflacoumarin A |
39.71 |
0.33 |
GC |
|
GC65 |
MOL004815 |
(E)-1-(2,4-dihydroxyphenyl)-3-(2,2-dimethylchromen-6-yl)prop-2- en-1-one |
39.62 |
0.35 |
GC |
|
GC66 |
MOL004989 |
6-prenylated eriodictyol |
39.22 |
0.41 |
GC |
|
GC67 |
MOL004884 |
Licoisoflavone B |
38.93 |
0.55 |
GC |
|
GC68 |
MOL004991 |
7-Acetoxy-2-methylisoflavone |
38.92 |
0.26 |
GC |
|
GC69 |
MOL004957 |
HMO |
38.37 |
0.21 |
GC |
|
GC70 |
MOL004945 |
(2S)-7-hydroxy-2-(4-hydroxyphenyl)-8-(3-methylbut-2- enyl)chroman-4-one |
36.57 |
0.32 |
GC |
|
GC71 |
MOL004978 |
2-[(3R)-8,8-dimethyl-3,4-dihydro-2H-pyrano[6,5-f]chromen-3-yl]- 5-methoxyphenol |
36.21 |
0.52 |
GC |
|
GC72 |
MOL004935 |
Sigmoidin-B |
34.88 |
0.41 |
GC |
|
GC73 |
MOL004882 |
Licocoumarone |
33.21 |
0.36 |
GC |
|
GC74 |
MOL001792 |
DFV |
32.76 |
0.18 |
GC |
|
GC75 |
MOL004988 |
Kanzonol F |
32.47 |
0.89 |
GC |
|
GC76 |
MOL004833 |
Phaseolinisoflavan |
32.01 |
0.45 |
GC |
|
GC77 |
MOL004814 |
Isotrifoliol |
31.94 |
0.42 |
GC |
|
GC78 |
MOL004805 |
(2S)-2-[4-hydroxy-3-(3-methylbut-2-enyl)phenyl]-8,8-dimethyl-2,3-dihydropyrano[2,3-f]chromen-4-one |
31.79 |
0.72 |
GC |
|
GC79 |
MOL004864 |
5,7-dihydroxy-3-(4-methoxyphenyl)-8-(3-methylbut-2- enyl)chromone |
30.49 |
0.41 |
GC |
|
GC80 |
MOL004806 |
euchrenone |
30.29 |
0.57 |
GC |
|
A |
MOL000358 |
beta-sitosterol |
36.91 |
0.75 |
GJ,GZ,BS,JH |
|
B |
MOL000422 |
kaempferol |
41.88 |
0.24 |
BS,GC |
|
C |
MOL004328 |
naringenin |
59.29 |
0.21 |
JH,GC |
|
D |
MOL000492 |
(+)-catechin |
54.83 |
0.24 |
GZ,BS |
Table 1: Main active ingredients of LYD for intervention of NASH and AS.
Gene ontology and Kyoto Encyclopedia of Genes and Genomes enrichment analyses
By using the Metascape platform to conduct bio-information enrichment analysis on 56 intersection targets, a total of 157 KEGG enrichment and analysis pathways (P < 0.01) were obtained, including Lipid and atherosclerosis, Pathways in cancer, IL-17 signaling pathway, Fluid shear stress and atherosclerosis, Alcoholic liver disease, Non-alcoholic fatty liver disease and Chemical carcinogenesis - reactive oxygen species. These are the main signal pathways of LYD in treating NASH and AS with homotherapy for heteropathy. According to the P value of each enrichment pathway, the top 20 pathways are visualized, as shown in figure 5 and table 2.
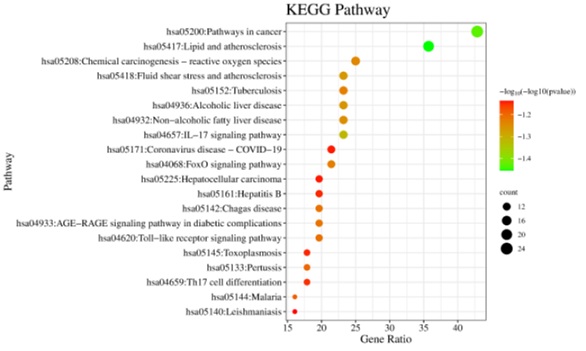 Figure 5: The results of the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment.
Figure 5: The results of the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment.
|
GO |
Pathway |
LogP |
Count |
Hits |
|
hsa05417 |
Lipid and atherosclerosis |
28.54 |
20 |
APOB,CD14,CD40LG,MAPK14,IL1B,IL6,CXCL8,LBP,LDLR,MMP1,MMP9,NFE2L2,OLR1, PPARG,PRKCA,MAPK3,MAPK8,CCL2,TNF,TP53 |
|
hsa05200 |
Pathways in cancer |
26.92 |
24 |
AR,CDKN1A,NQO1,EGFR,ESR1,ESR2,GSTM1,GSTM2,HIF1A,IFNG,IL4,IL6,CXCL8,MMP1, MMP9,NFE2L2,PPARD,PPARG,PRKCA,MAPK3,MAPK8,PTEN,TGFB1,TP53 |
|
hsa04657 |
IL-17 signaling pathway |
20.73 |
13 |
MAPK14,IFNG,IL1B,IL4,IL6,CXCL8,CXCL10,MMP1,MMP9,MAPK3,MAPK8,CCL2,TNF |
|
hsa05418 |
Fluid shear stress and atherosclerosis |
18.42 |
13 |
MAPK14,NQO1,GSTM1,GSTM2,IFNG,IL1A,IL1B,MMP9,NFE2L2,MAPK8,CCL2,TNF,TP53 |
|
hsa04936 |
Alcoholic liver disease |
18.3 |
13 |
ACACA,CD14,MAPK14,IL1B,IL6,CXCL8,LBP,PPARA,MAPK8,SREBF1,TNF,ADIPOQ, SIRT1 |
|
hsa04932 |
Non-alcoholic fatty liver disease |
17.79 |
13 |
MAPK14,IL1A,IL1B,IL6,CXCL8,INSR,PPARA,PPARG,MAPK8,SREBF1,TGFB1,TNF,ADIPOQ |
|
hsa05208 |
Chemical carcinogenesis - reactive oxygen species |
17.37 |
14 |
AHR,CAT,MAPK14,CYP1A2,NQO1,EGFR,GSTM1,GSTM2,HIF1A,NFE2L2, MAPK3,MAPK8,PTEN,PTPN1 |
|
hsa05152 |
Tuberculosis |
16.93 |
13 |
CD14,MAPK14,CTSD,IFNG,IL1A,IL1B,IL6,IL10,LBP,MAPK3,MAPK8,TGFB1,TNF |
|
hsa04068 |
FoxO signaling pathway |
16.89 |
12 |
CAT,CDKN1A,MAPK14,EGFR,IL6,IL10,INSR,MAPK3,MAPK8,PTEN,TGFB1,SIRT1 |
|
hsa04933 |
AGE-RAGE signaling pathway indiabetic complications |
16.4 |
11 |
MAPK14,IL1A,IL1B,IL6,CXCL8,PRKCA,MAPK3,MAPK8,CCL2,TGFB1,TNF |
|
hsa05142 |
Chagas disease |
16.3 |
11 |
MAPK14,IFNG,IL1B,IL6,CXCL8,IL10,MAPK3,MAPK8,CCL2,TGFB1,TNF |
|
hsa04620 |
Toll-like receptor signaling pathway |
16.21 |
11 |
CD14,MAPK14,IL1B,IL6,CXCL8,CXCL10,LBP,MAPK3,MAPK8,SPP1,TNF |
|
hsa05133 |
Pertussis |
15.74 |
10 |
CD14,MAPK14,IL1A,IL1B,IL6,CXCL8,IL10,MAPK3,MAPK8,TNF |
|
hsa05144 |
Malaria |
15.5 |
9 |
CD40LG,IFNG,IL1B,IL6,CXCL8,IL10,CCL2,TGFB1,TNF |
|
hsa04659 |
Th17 cell differentiation |
14.15 |
10 |
AHR,MAPK14,HIF1A,IFNG,IL1B,IL4,IL6,MAPK3,MAPK8,TGFB1 |
|
hsa05161 |
Hepatitis B |
14.04 |
11 |
CDKN1A,MAPK14,IL6,CXCL8,MMP9,PRKCA,MAPK3,MAPK8,TGFB1,TNF,TP53 |
|
hsa05145 |
Toxoplasmosis |
13.99 |
10 |
CD40LG,MAPK14,IFNG,IL10,LDLR,PIK3CG,MAPK3,MAPK8,TGFB1,TNF |
|
hsa05171 |
Coronavirus disease - COVID-19 |
13.88 |
12 |
MAPK14,EGFR,IL1B,IL6,CXCL8,CXCL10,MMP1,PRKCA,MAPK3,MAPK8,CCL2,TNF |
|
hsa05225 |
Hepatocellular carcinoma |
13.86 |
11 |
CDKN1A,NQO1,EGFR,GSTM1,GSTM2,NFE2L2,PRKCA,MAPK3,PTEN,TGFB1,TP53 |
|
hsa05140 |
Leishmaniasis |
13.71 |
9 |
MAPK14,IFNG,IL1A,IL1B,IL4,IL10,MAPK3,TGFB1,TNF |
Table 2: Top 20 pathways of KEGG enrichment.
In the results of GO analysis, 1082 biological processes (BP) were obtained after screening according to the set P < 0.01, including cellular response to lipid, response to hormone, inflammatory response, etc; 76 Molecular Function (MF) including signaling receptor regulator activity, receptor ligand activity, cytokine activity, etc; 41 Cellular Component (CC) including receptor complex, lysosome, lytic vacuole, etc. According to the Count value, the top 20 enrichment results are taken for visualization, as shown in figure 6.
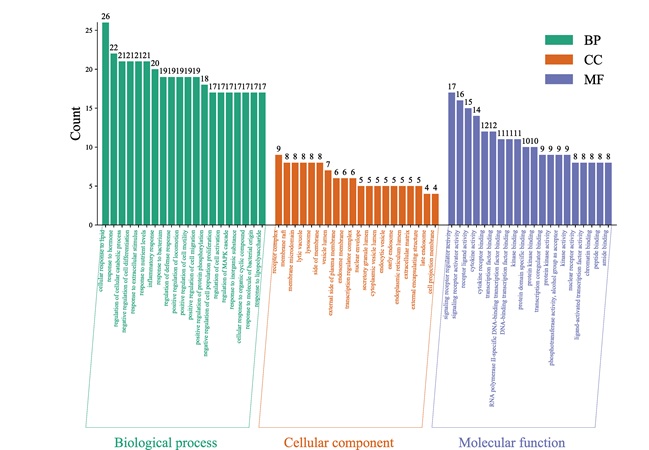 Figure 6: The results of Gene Oncology (GO) analysis.
Figure 6: The results of Gene Oncology (GO) analysis.
“LYD active-ingredients- intersection targets -pathways” network
By using Cytoscape 3.7.2 to build a network of “drug active-ingredients - disease targets – pathways”, and analyze the topological parameters of the disease targets and related pathways of LYD acting on NASH and AS, so as to obtain the main active ingredients and core targets and pathways. As shown in figure 7, the network consists of 168 nodes and 667 edges. Blue nodes represent disease-related targets, yellow nodes represent active ingredients of drugs, pink nodes represent common active ingredients of drugs, green nodes represent signal pathways, and the connecting lines between nodes represent the interaction relationship between nodes. The larger the area of nodes was in the network, the effect of drugs on disease intervention was stronger.
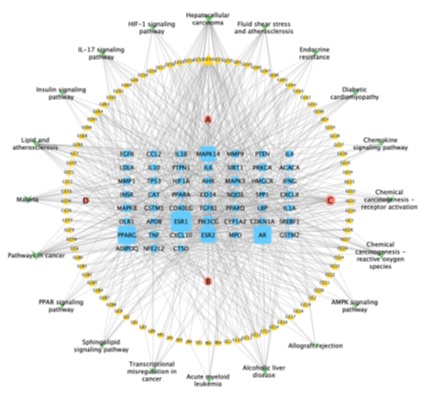 Figure 7: “LYD active-ingredients- intersection targets -pathways” network.
Figure 7: “LYD active-ingredients- intersection targets -pathways” network.
(Blue nodes - disease-related targets, yellow nodes - active ingredients of drugs, pink nodes - common active ingredients of drugs, green nodes - signal pathways).
Cytoscape network analysis showed that the highest degree of the active component GC52 (MOL000098: quercetin) was 36,it was predicted that quercetin was the main component of LYD in the treatment of NASH and AS. And the second active component B [the common component of BZ and GC (MOL000422: kaempferol)], its degree was 24; The active component C (common component of JH and GC) named naringenin (MOL004328: naringenin), its degree is 22; The degree of active component GC44(MOL000354: isorhamnetin) and GC13(MOL000392: formononetin) was 9 and 8 respectively. Specific network topology parameters were shown in table 3. The connectivity of ESR1 in the network was 87, which predicted that ESR1 was the main target of LYD in treating NASH and AS. In addition, PPARG, AR, ESR2, MAPK14, PIK3CG, MAPK8, MAPK3, PRKCA and TNF were also relatively important targets table 4.
|
MOLID |
INGREDIENT |
Degree |
BetweennessCentrality |
ClosenessCentrality |
|
MOL000098 |
Quercetin |
36 |
0.1171346 |
0.49137931 |
|
MOL000422 |
Kaempferol |
12 |
0.0147753 |
0.42964824 |
|
MOL004328 |
Naringenin |
11 |
0.02720369 |
0.4453125 |
|
MOL000354 |
Isorhamnetin |
9 |
0.02030442 |
0.4453125 |
|
MOL000392 |
Formononetin |
8 |
0.00856406 |
0.41911765 |
Table 3: Network node parameters of core active ingredients of LYD.
|
Target |
Degree |
Betweenness Centrality |
Closeness Centrality |
Target |
Degree |
Betweenness Centrality |
Closeness Centrality |
|
ESR1 |
87 |
0.2667568 |
0.55519481 |
PIK3CG |
26 |
0.10323653 |
0.40909091 |
|
PPARG |
74 |
0.16942978 |
0.5310559 |
MAPK8 |
14 |
0.01176744 |
0.39041096 |
|
AR |
72 |
0.14659936 |
0.51197605 |
MAPK3 |
14 |
0.02025408 |
0.39041096 |
|
ESR2 |
58 |
0.0699745 |
0.45478723 |
PRKCA |
13 |
0.01914337 |
0.38340807 |
|
MAPK14 |
56 |
0.10365811 |
0.48033708 |
TNF |
12 |
0.01527333 |
0.38513514 |
Table 4: Network node parameters of the core target of LYD.
Molecular docking results
The five potential core components were respectively docked with 10 core targets (ESR1, PPARG, AR, ESR2, MAPK14, PIK3CG, MAPK8, MAPK3 and PRKCA), and 50 receptor-ligand docking results were obtained. Among 50 sets of receptor-ligand docking results, 49 sets of docking results had Affinity <-7kcal·mol-1, accounting for 98%. This indicated that the screened core components had strong binding activity with the core targets. The highest docking score was naringenin-AR, with Affinity=-10.1kcal·mol -1; The lowest docking score was quercetin-TNF, with Affinity=-6.8kcal·mol - 1. The heatmap was drawn according to the score of docking results, as shown in Figure8, and the docking conformation of some compounds were shown in figures 8 & 9.
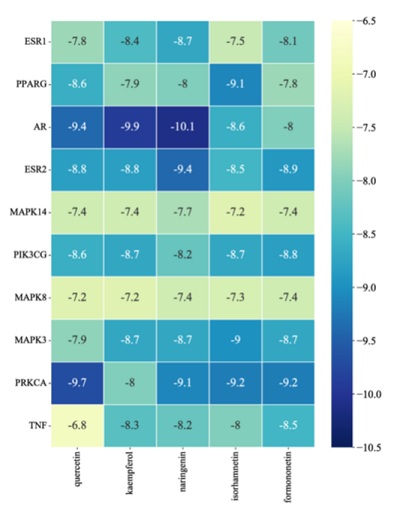 Figure 8: The heatmap of molecular docking results.
Figure 8: The heatmap of molecular docking results.
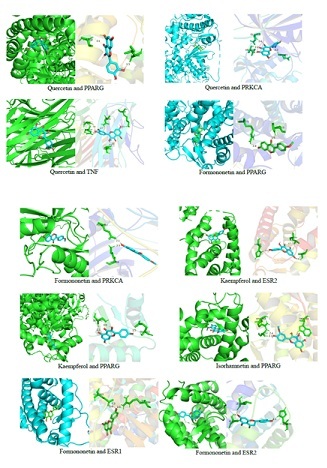 Figure 9: The results of docking conformation of some compounds.
Figure 9: The results of docking conformation of some compounds.
Discussion
In recent years, the prevalence of nonalcoholic steatohepatitis (NASH) has been rising and linked to cardiovascular disease, which is also the leading cause of death in NASH. NASH shares common risk factors with AS, and studies have demonstrated that the key molecular processes of NASH that drive AS is lipid metabolism (SC5D, LCAT, and HMGCR) and inflammation (IL1A) - related specific regulators and the development of AS is driven by NASH through vascular inflammation (TNFA) - and atherosclerosis signaling (CCL2 and FDFT1) [15]. It is well known that macrophage infiltration plays a central role in atherosclerotic lesions, and similarly activation and infiltration of macrophage are the main causes of steatosis during the pathogenesis of NASH. Thus, it is possible to consider macrophage activation as a common cause of Nash and AS, and some scholars have described both diseases as two aspects of a common disease [16]. The theory of TCM holds that the main pathogenesis of NASH and AS both result from phlegm and fluid, and LYD was created by famous physician Zhang Xichun for treating phlegm and fluid, which is widely used in clinical practice. According to the common pathogenesis of TCM, using LYD to treat NASH and AS can give full play to characteristics and advantages of Chinese medicine based on “syndrome differentiation and treatment” and “homotherapy for heteropathy”. It may achieve more significant therapeutic effects in the clinical treatment process.
In this study, we used network pharmacology method to find that quercetin, kaempferol, naringenin, isorhamnetin and formononetin in LYD can treat NASH and AS by acting on core targets such as ESR1, PPARG, AR, ESR2, MAPK14, PIK3CG, MAPK8, MAPK3 and PRKCA. Quercetin, a typical flavonol, has the protective effect on atherosclerosis. It has been shown that quercetin attenuates the progression of atherosclerosis by regulating dendritic cell (DC) activation via expression of Dab2 protein [17]. Quercetin can significantly reduce serum triglycerides, cholesterol, TNF-α and IL-6 levels, reduce the formation of atherosclerotic plaques and protect damaged coronary arteries caused by HFD, and has good anti-atherosclerotic properties [18,19]. Liver VLDL assembly and fat phagocytosis are the main pathways of quercetin against NAFLD through IRE1a/XBP1s pathway [20], Hu Y et al. found that quercetin could reduce the excessive cholesterol accumulation in vivo and in vitro by inhibiting the mRNA and protein expression levels of SREBP2 and HMGCR, and play a protective role in the treatment of NAFLD [21]. Kaempferol's anti-atherosclerosis effect is mainly through regulating gene and protein expression of inflammatory molecules [22], the potential molecular basis of kaempferol's anti-atherosclerosis activity has been reviewed as antioxidant, anti-inflammatory and cardioprotective activity [23]. The result of relevant cellular experimental study showed that kaempferol could alleviate OA induced lipid accumulation and oxidative stress in HepG2 cells and inhibit expression of peroxisome proliferator activated receptors gamma (PPAR γ) is beneficial for the treatment of NAFLD [24]. Naringenin can decrease the levels of serum Low Density Lipoprotein (LDL) and Triglyceride (TG) and increase High-Density Lipoprotein (HDL). It also can inhibit macrophage inflammation, monocyte adhesion and foam cell formation, reduce plaque macrophage production while modestly increasing smooth muscle cells to enhance arteriosclerosis repair with better anti-atherosclerotic effect [25,26]. Naringenin can inhibit Mitogen-Activated Protein Kinase (MAPK), block the anti-inflammatory activity of NF-κB, change the pathway of growth factor (TGF-β), prevent the differentiation of Hepatic Stellate Cells (HSC) and lead to the decrease of collagen synthesis. And it also can play a beneficial role in alleviating inflammation and protecting liver in the treatment of NAFLD by reducing liver lipid deposition, inducing apoptosis and inhibiting oxidative stress reaction [27,28]. Isorhamnetin protects against ox-LDL induced endothelial cell injury by inhibiting lectin-like ox-LDL receptor-1 and interfering with ox-LDL mediated intracellular signaling pathways (p38MAPK activation, NF kappaB nuclear translocation, eNOS expression), and it has the functions of anti-atherosclerosis, reducing serum fat, resisting inflammation, resisting oxidation and protecting endothelium. In vitro experiments have also shown that isorhamnetin also inhibited CYP activity in liver microsomes to restore liver injury [29]. Relevant pharmacological studies have shown that isorhamnetin, as a metabolite of quercetin, is a novel antagonist of peroxisome proliferator-activated receptor gamma (PPAR gamma), which can inhibit adipocyte differentiation and improve hepatic steatosis [30]. Isorhamnetin reduces lipid accumulation and Triglyceride (TG) content in liver by inhibiting de novo adipose-derived pathway in mice, and shows reduction of liver injury markers and collagen deposition in the process of treating NASH mice, as well as reduction of expression of related fibrin genes, significantly relieving pathological characteristics of NASH [31]. Formononetin may significantly attenuate the development of atherosclerosis by modulating the interaction between KLF4 and SRA [32], it activates peroxisome proliferator activated receptors γ (PPAR gamma) signaling to alleviate endothelial injury induced by bovine LDL in HUVECs, which exhibited good anti-inflammatory or antioxidant properties in their process, thus providing a rationale for further development of potential drugs against atherosclerosis [33]. Wang Y et al., found that with the intervention of formononetin, the blockage of fat autophagy flux was alleviated through mechanisms such as TFEB mediated lysosome biogenesis and autophagosome lysosome fusion, which further induced fat phagocytosis, prevented hepatocyte lipid accumulation and improved liver steatosis, providing new evidence for the treatment of NAFLD [34].
LYD mainly intervenes NASH and AS through these core targets such as ESR1, PPARG, AR, ESR2, MAPK14, PIK3CG, MAPK8, MAPK3, PRKCA and TNF, and most of them participate in the regulation pathways related to anti-oxidative stress, lipid metabolism and inflammation. Estrogen receptors (ESR1,ESR2) may be involved in the control of osteoblast-like cell differentiation to regulate the calcification of atherosclerotic lesions [35], they are involved in inducing foam cell formation related signal transduction to produce hyperkinetic effect and regulate cardiovascular function to protect the heart [36,37]. Peroxisome Proliferator-Activated Receptor Gamma (PPARG) plays an important role in adipocyte differentiation and lipid deposition, potent PPARG antagonists can significantly reduce lipid accumulation, and fatty acid-derived molecules can also modulate inflammatory responses via PPARG [38,39]. PPARG is also indispensable in fatty acid metabolism and fatty acid storage of liver and adipose tissue, and in addition its expression in atherosclerotic lesions has been shown to affect gene transcription in vascular endothelial cells, smooth muscle cells, monocytes and macrophages [40], PPARG agonists suppress inflammatory responses within the vessel wall, inhibit migration and proliferation of vascular smooth muscle cells, and affect foam cell formation by altering scavenger receptor expression [41]. PPARG has a protective effect on hepatic inflammation and fibrosis, and rosiglitazone, a PPARG agonist, inhibits TGF-β1/ Smad signaling pathway to suppress Hepatic Stellate Cell (HSC) activation and alleviate liver fibrosis and inflammation [42] MAPK (MAPK3, MAPK8, MAPK14)-related signal transduction reduces the levels of TG, TC, LDL, IL-6, IL-1β, TNF-α, increases the level of HDL and promotes macrophage apoptosis to improve the stability of atherosclerotic plaque [43,44]. Related experimental studies have shown that inhibiting the expression of MAPK signaling pathway can not only improve the oxidative stress response, lipid accumulation and degeneration of NASH mouse liver, but also improve the fibrosis of steatosis liver and reduce hepatocyte death [45,46]. According to the related signal pathways involved in PIK3CG research, it is known that the related pathways mediated by PIK3CG can improve hepatic oxidative stress and further inhibit NF- κB-mediated expression of associated inflammatory, it can also reduce the level of serum lipid, improve intimal hyperplasia, reduce the accumulation of carotid artery lipid, regulate cell proliferation and apoptosis to improve atherosclerosis [47,48]. Androgen Receptor (AR) in monocytes/macrophages upregulates tumor necrosis factor alpha (TNF-α), which is involved in a major inflammatory process of atherosclerosis. AR mediated AMP activated protein kinase (AMPK) - acetyl coenzyme (ACC) signaling slows the progression of NAFLD by regulating lipogenic processes in the liver and hepatocytes [49,50]. Protein kinase C alpha type (PRKCA) plays an important role in regulating platelet function and arterial thrombotic pathways, and inhibiting PRKCA expression can further reduce interleukin 6(IL-6) release and improve inflammatory conditions [51,52]. Tumor necrosis factor alpha (TNF-α) can induce oxidative stress and inflammatory response in endothelial cells, and its related pathway of inducing PRKCA has significant protective effect in endothelial cells [53].
Through KEGG and GO analysis, it was found that the treatment of LYD for NASH and AS mainly through lipid metabolism and inflammatory response related pathways, in which interleukin 17(IL-17) signaling pathway could drive cytokines of autoimmune and inflammatory diseases, and played a decisive role in promoting inflammatory response [54]. Inflammatory cytokines produced by IL-17 are key factors in the development of atherosclerosis, and can induce vascular endothelial cell senescence by mediating NF-κB/p53/Rb signaling pathway [55]. In addition, as a proinflammatory cytokine, IL-17 can promote hepatocyte apoptosis by mediating the ERK1/2/p65 signaling pathway, which exacerbates the progression of NASH [56]. FoxO insulin signaling can mediate the inhibitory effects of insulin or Insulin-like Growth Factor (IGF) on key functions of cell metabolism, growth, differentiation, oxidative stress, aging, autophagy, and senescence, especially in mechanisms combining hepatic glucose and lipid metabolism [57]. Zhang L et al., found that the increase of cytoplasmic calcium signaling in hepatocyte steatosis inhibits autophagy through FoxO signaling pathway, leading to lipid accumulation and degeneration of hepatocytes and exacerbating the development of NAFLD [58]. In addition, other researchers have found that FoxO signaling pathway plays a key role in maintaining liver metabolism and cell homeostasis, including maintaining glucose, triglyceride and cholesterol homeostasis, and regulating inflammation and fibrosis. FoxO may help prevent liver fibrosis in NAFLD by inhibiting proliferation and differentiation of hepatic stellate cells [59]. FoxO can intervene cardiovascular disease by promoting cell activation, apoptosis and proliferation, participating in inflammation and anti-oxidative stress response, regulating glucose production and lipid consumption and other biological processes [60]. Both in vivo and in vitro studies demonstrated that knockout of FoxO in myeloid cells increased the production of reactive oxygen species, further increased the oxidative stress response and accelerated the development of atherosclerosis in mice [61]. The activation of AGE-RAGE signaling pathway can cause sustained oxidative stress in vascular tissues and promote calcification of vascular smooth muscle cells (VSMC) by activating Nox-1 and reducing SOD-1 expression [62]. Activation of Toll-like receptor signaling promotes hepatic steatosis [63], it may also provide a signal for the activation of inflammasomes in the relevant organs [64]. Related experimental studies have demonstrated that the use of Corilagin can improve the development of atherosclerotic plaques and reduce the release of pro-inflammatory cytokines by inhibiting Toll-like receptor signaling pathways in monocytes/macrophages [65]. Pterostilbene can inhibit the secretion of proinflammatory cytokines by regulating NF-κB signaling pathway through Toll-like receptor signaling, thus playing a role in preventing high-fat-induced atherosclerosis [66].
The molecular docking results showed that the 5 active components in LYD had good binding activity with 10 core disease targets. According to the binding energy value, the groups with Affinity<-7 kcal·mol-1 accounted for 98% of the 50 receptor-ligand docking groups, and the binding sites were all formed into relatively stable conformation through hydrogen bonding. This also preliminarily verified that LYD in treating NASH and AS with homotherapy for heteropathy mainly through these core targets. Through searching the relevant literature and combining the analysis of this study, we found that combination: quercetin with TNF; kaempferol, isorhamnetin and formononetin with PPARG respectively may be the “star combination” of LYD in intervening NASH and AS. Therefore, the combination with higher docking score and the main active ingredients and core targets in the above combination may play a major role in the process of Chinese herbal compound intervention in diseases.
In conclusion, this study predicts that multiple active ingredients in LYD exert effects such as antioxidant stress response, regulating lipid metabolism and inflammatory response to achieve treatment of NASH and AS with homotherapy for heteropathy through a multi-target and multi pathway manner, which provides a theoretical basis and research ideas for subsequent clinical and animal experiments. Because of the limitation of network pharmacology and molecular docking, the prediction results need to be further verified by experimental studies. The results of this study can provide theoretical basis for the following experimental studies.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for publication was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Funding
This study was supported by National Science and Technology Major Project of CHINA. NO.2019ZX09201004-001-021.
Author’s Contribution
Chai Xinlou proposed and designed this study. Cong Shibo contributed to writing the manuscript and performed the analyses. Both Chai Xinlou and Cong Shibo revised the manuscript. All authors read and approved the final manuscript.
Acknowledgement
Throughout the writing of this article I have received a great deal of support and assistance. I would particularly like to acknowledge my teammate for their wonderful collaboration and patient support.
Availability of Data and Material
All data generated or analyzed during this study are included in this published article.
References
- Fraile JM, Palliyil S, Barelle C, Porter AJ, Kovaleva M (2021) Non-Alcoholic Steatohepatitis (NASH) - A Review of a Crowded Clinical Landscape, Driven by a Complex Disease. Drug Des Devel Ther 15: 3997-4009.
- Schuster S, Cabrera D, Arrese M, Feldstein AE (2018) Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol 15: 349-364.
- Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, et al. (2018) Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 8: 80.
- Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C (2016) Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J Hepatol 65: 589-600.
- Lijie H (2020) Research on the theory of the same treatment of different diseases in traditional Chinese medicine [J]. Chinese Community Physicians 36: 93-94.
- Cheng C, Yongping M, Qin F (2016) Discussion on the pathogenesis of nonalcoholic steatohepatitis in traditional Chinese medicine. World Science and Technology - Modernization of Traditional Chinese Medicine 18: 1483-1487.
- Bo N, Chao P, Mingjun Z (2022) Research progress in pathogenesis of coronary heart disease based on different syndrome differentiation systems. Journal of Liaoning University of Traditional Chinese Medicine 8: 11-24.
- Xichun Z (2009) Integrating Chinese and Western Medicine. Taiyuan: Shanxi Science and Technology Press, China.
- Wei G (2021) Thinking and clinical experience of treating difficult and complicated diseases in internal medicine with Zhang Xichun Liyin Decoction. Global Chinese Medicine 14: 1500-1503.
- Xiaoxuan X, Chune X (2020) Clinical effect and mechanism of Yinchen Linggui Zhugan Decoction in the treatment of nonalcoholic steatohepatitis. Chinese Medicine 15: 907-911.
- Tangyou M, Tsunami H, Weihan Z, Yunliang W, Kangli G, et al. (2016) Decoction on liver tissue DGAT2, PKC of NASH rats ε Study on the role of. Chinese Journal of Integrated Traditional and Western Medicine Digestion 24: 87-91.
- Na L, Chenhao G, Cunan H, Jianhua W (2022) Exploring the mechanism of action of the main active ingredients of Linggui Zhugan Decoction based on network pharmacology-molecular docking technology. Guangdong Chemical Industry 49: 73-76.
- Lilong Z (2020) Effect of Linggui Zhugan Decoction on serum inflammatory factors, blood lipids and oxidation levels in patients with coronary heart disease. Chinese Community Physician 36: 83-84.
- Ziyi W, Xin W, Daiyan Z, Yuanjia H, Shao L (2022) Network pharmacology of traditional Chinese medicine: the development of a new era led by the Guide. Chinese Journal of Traditional Chinese Medicine 47: 7-17.
- Hoek AM, Özsezen S, Caspers MPM, Koppen A, Hanemaaijer R, et al. (2022) Unraveling the Transcriptional Dynamics of NASH Pathogenesis Affecting Atherosclerosis. Int J Mol Sci 23: 8229.
- Bieghs V, Rensen PC, Hofker MH, Sverdlov RS (2012) NASH and atherosclerosis are two aspects of a shared disease: central role for macrophages. Atherosclerosis 220: 287-293.
- Lin W, Wang W, Wang D, Ling W (2017) Quercetin protects against atherosclerosis by inhibiting dendritic cell activation. Mol Nutr Food Res 61: 31.
- Wu DN, Guan L, Jiang YX, Ma SH, Sun YN, et al. (2019)Microbiome and metabonomics study of quercetin for the treatment of atherosclerosis. Cardiovasc Diagn Ther 9: 545-560.
- Juzwiak S, Wójcicki J, Mokrzycki K, Marchlewicz M, Bialecka M, et al. (2005) Effect of quercetin on experimental hyperlipidemia and atherosclerosis in rabbits. Pharmacol Rep 57: 604-609.
- Zhu X, Xiong T, Liu P, Guo X, Xiao L, et al. (2018) Quercetin ameliorates HFD-induced NAFLD by promoting hepatic VLDL assembly and lipophagy via the IRE1a/XBP1s pathway. Food Chem Toxicol 114: 52-60.
- Hu Y, Xu J, Chen Q, Liu M, Wang S, et al. (2020) Regulation effects of total flavonoids in Morus alba L. on hepatic cholesterol disorders in orotic acid induced NAFLD rats. BMC Complement Med Ther 20: 257.
- Kong L, Luo C, Li X, Zhou Y, He H (2013) The anti-inflammatory effect of kaempferol on early atherosclerosis in high cholesterol fed rabbits. Lipids Health Dis 12: 115.
- Chen M, Xiao J, Seedi HR, Wozniak KS, Daglia M, et al. (2022) Kaempferol and atherosclerosis: From mechanism to medicine. Crit Rev Food Sci Nutr 13: 1-19.
- Tie F, Ding J, Hu N, Dong Q, Chen Z, et al. (2021) Kaempferol and Kaempferide Attenuate Oleic Acid-Induced Lipid Accumulation and Oxidative Stress in HepG2 Cells. Int J Mol Sci 22: 8847.
- Orhan IE, Nabavi SF, Daglia M, Tenore GC, Mansouri K, et al. (2015) Naringenin and atherosclerosis: a review of literature. Curr Pharm Biotechnol 16: 245-251.
- Burke AC, Sutherland BG, Telford DE, Morrow MR, Sawyez CG, et al. (2019) Naringenin enhances the regression of atherosclerosis induced by a chow diet in Ldlr-/- Atherosclerosis 286: 60-70.
- Aquino EH, Muriel P (2018) Beneficial effects of naringenin in liver diseases: Molecular mechanisms. World J Gastroenterol 24: 1679-1707.
- Hu R, Liu S, Shen W, Chen C, Cao Y, et al. (2021) Study on the Inhibitory Effects of Naringenin-Loaded Nanostructured Lipid Carriers Against Nonalcoholic Fatty Liver Disease. J Biomed Nanotechnol 17: 942-951.
- Li WQ, Li J, Liu WX, Wu LJ, Qin JY, et al. (2022) Isorhamnetin: A Novel Natural Product Beneficial for Cardiovascular Disease. Curr Pharm Des 28: 2569-2582.
- Zhang Y, Gu M, Cai W, Yu L, Feng L, et al. (2016) Dietary component isorhamnetin is a PPARγ antagonist and ameliorates metabolic disorders induced by diet or leptin deficiency. Sci Rep 6: 19288.
- Ganbold M, Owada Y, Ozawa Y, Shimamoto Y, Ferdousi F, et al. (2019) Isorhamnetin Alleviates Steatosis and Fibrosis in Mice with Nonalcoholic Steatohepatitis. Sci Rep 9: 16210.
- Ma C, Xia R, Yang S, Liu L, Zhang J, et al. (2020) Formononetin attenuates atherosclerosis via regulating interaction between KLF4 and SRA in apoE-/- Theranostics 10: 1090-1106.
- Zhang B, Hao Z, Zhou W, Zhang S, Sun M, et al. (2021) Formononetin protects against ox-LDL-induced endothelial dysfunction by activating PPAR-γ signaling based on network pharmacology and experimental validation. Bioengineered 12: 4887-4898.
- Wang Y, Zhao H, Li X, Wang Q, Yan M, et al. (2019) Formononetin alleviates hepatic steatosis by facilitating TFEB-mediated lysosome biogenesis and lipophagy. J Nutr Biochem 73: 108214.
- McRobb LS, McGrath KCY, Tsatralis T, Liong EC, Tan JTM, et al. (2017) Estrogen Receptor Control of Atherosclerotic Calcification and Smooth Muscle Cell Osteogenic Differentiation. Arterioscler Thromb Vasc Biol 37: 1127-1137.
- Xiang J, Wang Y, Su K, Liu M, Hu PC, et al. (2014) Ritonavir binds to and downregulates estrogen receptors: molecular mechanism of promoting early atherosclerosis. Exp Cell Res 327: 318-330.
- Aryan L, Younessi D, Zargari M, Banerjee S, Agopian J, et al. (2020) The Role of Estrogen Receptors in Cardiovascular Disease. Int J Mol Sci 21: 4314.
- Imai T, Takakuwa R, Marchand S, Dentz E, Bornert JM, et al. (2004) Peroxisome proliferator-activated receptor gamma is required in mature white and brown adipocytes for their survival in the mouse. Proc Natl Acad Sci USA 101: 4543-4547.
- Varga T, Czimmerer Z, Nagy L (2011) PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta 1812: 1007-1022.
- Neve BP, Fruchart JC, Staels B (2000) Role of the peroxisome proliferator-activated receptors (PPAR) in atherosclerosis. Biochem Pharmacol 60: 1245-1250.
- Beltowski J, Wójcicka G, Jamroz A (2003) Peroxisome proliferator-activated receptors (PPAR) in pathophysiology of the circulatory system and prospective use of agonists of these receptors in therapy. Postepy Hig Med Dosw 57: 199-217.
- Ni XX, Li XY, Wang Q, Hua J (2021) Regulation of peroxisome proliferator-activated receptor-gamma activity affects the hepatic stellate cell activation and the progression of NASH via TGF-β1/Smad signaling pathway. J Physiol Biochem 77: 35-45.
- Fang S, Sun S, Cai H, Zou X, Wang S, et al. (2021) IRGM/Irgm1 facilitates macrophage apoptosis through ROS generation and MAPK signal transduction: Irgm1+/- mice display increases atherosclerotic plaque stability. Theranostics 11: 9358-9375.
- Du H, Zhang H, Yang R, Qiao L, Shao H, et al. (2021) Small interfering RNA-induced silencing lncRNA PVT1 inhibits atherosclerosis via inactivating the MAPK/NF-κB pathway. Aging (Albany NY) 13: 24449-24463.
- Ding J, Wu L, Zhu G, Zhu J, Luo P, et al. (2022) HADHA alleviates hepatic steatosis and oxidative stress in NAFLD via inactivation of the MKK3/MAPK pathway. Mol Biol Rep 50: 961-970.
- Shen X, Guo H, Xu J, Wang J (2019) Inhibition of lncRNA HULC improves hepatic fibrosis and hepatocyte apoptosis by inhibiting the MAPK signaling pathway in rats with nonalcoholic fatty liver disease. J Cell Physiol 234: 18169-18179.
- Li J, Wang T, Liu P, Yang F, Wang X, et al. (2021) Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct 12: 3898-3918.
- Song T, Chen WD (2021) Berberine inhibited carotid atherosclerosis through PI3K/AKTmTOR signaling pathway. Bioengineered 12: 8135-8146.
- Huang CK, Pang H, Wang L, Niu Y, Luo J, et al. (2014) New therapy via targeting androgen receptor in monocytes/macrophages to battle atherosclerosis. Hypertension 63: 1345-1353.
- Heo JH, Lee SR, Jo SL, Ko JW, Kwon HJ, et al. (2021) Hepatic LKB1 Reduces the Progression of Non-Alcoholic Fatty Liver Disease via Genomic Androgen Receptor Signaling. Int J Mol Sci 22: 7904.
- Liu D, Cao Y, Zhang X, Peng C, Tian X, et al. (2018) Chemokine CC-motif ligand 2 participates in platelet function and arterial thrombosis by regulating PKCα-P38MAPK-HSP27 pathway. Biochim Biophys Acta Mol Basis Dis 1864: 2901-2912.
- Devaraj S, Venugopal SK, Singh U, Jialal I (2005) Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-{alpha} and -{beta}. Diabetes 54: 85-91.
- Shiraki A, Oyama J, Komoda H, Asaka M, Komatsu A, et al. (2012) The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis 221: 375-382.
- Li X, Bechara R, Zhao J, McGeachy MJ, Gaffen SL (2019) IL-17 receptor-based signaling and implications for disease. Nat Immunol 20: 1594-1602.
- Zhang L, Liu M, Liu W, Hu C, Li H, et al. (2021) Th17/IL-17 induces endothelial cell senescence via activation of NF-κB/p53/Rb signaling pathway. Lab Invest 101: 1418-1426.
- Harley IT, Stankiewicz TE, Giles DA, Softic S, Flick LM, et al. (2014) IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology 59: 1830-1839.
- Lee S, Dong HH (2017) FoxO integration of insulin signaling with glucose and lipid metabolism. J Endocrinol 233: 67-79.
- Zhang L, Zhang Y, Jiang Y, Dou X, Li S, et al. (2021) Upregulated SOCC and IP3R calcium channels and subsequent elevated cytoplasmic calcium signaling promote nonalcoholic fatty liver disease by inhibiting autophagy. Mol Cell Biochem 476: 3163-3175.
- Dong XC (2017) FOXO transcription factors in non-alcoholic fatty liver disease. Liver Res 1: 168-173.
- Menghini R, Casagrande V, Iuliani G, Rizza S, Mavilio M, et al. (2020) Metabolic aspects of cardiovascular diseases: Is FoxO1 a player or a target? Int J Biochem Cell Biol 118: 105659.
- Tsuchiya K, Westerterp M, Murphy AJ, Subramanian V, Ferrante AW, et al. (2013) Expanded granulocyte/monocyte compartment in myeloid-specific triple FoxO knockout increases oxidative stress and accelerates atherosclerosis in mice. Circ Res 112: 992-1003.
- Kay AM, Simpson CL, Stewart JA (2016) The Role of AGE/RAGE Signaling in Diabetes-Mediated Vascular Calcification. J Diabetes Res 2016: 6809703.
- Todoric J, Caro GD, Reibe S, Henstridge DC, Green CR, et al. (2020) Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat Metab 2: 1034-1045.
- Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ (2014) Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 146: 1763-1774.
- Li Y, Wang Y, Chen Y, Wang Y, Zhang S, et al. (2020) Corilagin Ameliorates Atherosclerosis in Peripheral Artery Disease via the Toll-Like Receptor-4 Signaling Pathway in vitro and in vivo. Front Immunol 11: 1611.
- Zhang Y, Zhang Y (2016) Pterostilbene, a novel natural plant conduct, inhibits high fat-induced atherosclerosis inflammation via NF-κB signaling pathway in Toll-like receptor 5 (TLR5) deficient mice. Biomed Pharmacother 81: 345-355.
Citation: Shibo C, Xinlou C (2023) Explore the Mechanism of LiYin Decoction in Treating Nonalcoholic Steatohepatitis and Atherosclerosis with Homotherapy for Heteropathy Based on Network Pharmacology and Molecular Docking. J Altern Complement Integr Med 9: 339.
Copyright: © 2023 Cong Shibo , et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

