Expression of KCNH2 (hERG1) and KCNE2 Correlates With Expression of Key Myometrial Genes in Term Pregnant Human Myometrium
*Corresponding Author(s):
Jonathan W PaulSchool Of Medicine And Public Health, Hunter Medical Research Institute, University Of Newcastle, 1 Kookaburra Circuit, New Lambton Heights 2305, NSW, Australia
Tel:+6140420348,
Fax:+61 4042 0045
Email:Jonathan.Paul@newcastle.edu.au
Abstract
Background
Loss of activity of Kv11.1 potassium channels (encoded by KCNH2) facilitates labor, and is associated with expression of an inhibitory subunit encoded by KCNE2.
Objective
To determine whether KCNH2 and KCNE2 expression was linked to expression of key genes involved in myometrial contractility, including the oxytocin receptor (OXTR), estrogen receptor 1 (ESR1), progesterone receptor (PGR) and Prostaglandin-Endoperoxidase Synthase 2 (PTGS2). We further aimed to examine KCNH2 and KCNE2 expression in preterm samples.
Study Design
Biopsies of term or preterm, non-laboring human myometrium were analysed by qPCR to determine KCNH2 and KCNE2 mRNA abundance, as well as abundance of OXTR, ESR1, PGR and PTGS2 mRNAs.
Results
KCNH2 and KCNE2 expression were significantly correlated at term, but not correlated with BMI. KCNH2 expression significantly correlated with OXTR, ESR1, PGR and PTGS2 expression, while KCNE2 expression correlated with the expression of ESR1, PGR and PTGS2. Preterm samples revealed no difference in KCNH2 and KCNE2 expression compared to term.
Conclusion
KCNH2 and KCNE2 expression correlate with the expression of key myometrial genes implicated in parturition, further strengthening a role for Kv11.1 channels in human pregnancy. Neither gene was correlated with BMI, suggesting that previously reported effects of obesity likely affect protein levels rather than gene expression.
Keywords
INTRODUCTION
A number of studies have demonstrated that ion channels encoded by the ether-à-go-go-related gene1 (ERG1) play a role in regulating the contractile activity of cardiac myocytes (reviewed by Vandenberg et al. [1]). Moreover, there is increasing evidence demonstrating a role for ERG1in regulating smooth muscle cell contractility. ERG1encodes the pore-forming α-subunit of the delayed rectifier voltage-gated potassium channel, Kv11.1. Herein we refer to the ERG gene and the cognate mRNA as ‘KCNH2’, and ‘KCNH2 mRNA’, respectively, to the channel protein as ERG and to the functional channel as Kv11.1. In addition to ERG, Kv11.1 channels contain regulatory β-subunits [2,3], such as the single transmembrane domain protein potassium voltage-gated channel subfamily E members 2, encoded by the gene KCNE2 (mRNA referred to as ‘KCNE2 mRNA’, and channel protein referred to as KCNE2).
Kv11.1 channels are activated following action potentials and function to repolarize the cell membrane by conducting potassium ions (K+) out of the cell, which constitutes the rapid component of the delayed rectifier current IKr [4,5]. Repolarization terminates the action potential and the associated contraction.
Kv11.1 channels have been shown in rat stomach and murine portal vein [6-8], as well as in opossum oesophagus [9]. In addition, selective Kv11.1 channel blockers have been shown to increase contractility in rat stomach [6], mouse portal vein [8], opossum oesophagus [9], mouse and guinea pig gall bladder [10], bovine epididymis [11] and human and equine jejunum [12,13].These smooth muscles all exhibit spontaneous contractile activity, which is also a property of uterine smooth muscle [14].
Kv11.1 channels were initially linked to myometrial contractility by Aaronson et al., who demonstrated that tetraethyl ammonium (TEA)- and 4-aminopyridine (4-AP)-sensitive voltage-dependent K+ (Kv) channels played a role in regulating action potential duration in rat myometrium [15].Greenwood et al. later examined mouse ERG (mERG) expression and function, and confirmed the presence of both the ERG1a and ERG1b splice variants, with ERG1a expression being more abundant than that of ERG1b [16]. They found that KCNH2 mRNA abundance did not change throughout gestation or with the onset of labor, however, mRNAs encoding auxiliary subunits, namely KCNE2, were significantly up-regulated approaching term [16].
More recently, our group reported that human ERG1 (hERG1) and KCNE2 were present in pregnant human myometrium during late gestation and labor [17]. We found that Kv11.1 activity supressed the amplitude and duration of contractions prior to labor, thereby supporting a role for Kv11.1 activity in helping to maintain uterine quiescence [17]. Previous reports have indicated that KCNE2 co-expression with hERG1 was not necessarily inhibitory of Kv11.1 activity [18], however, we found that the onset of labor was associated with the increased expression of KCNE2 and the decreased responsiveness of myocytes to the Kv11.1 inhibitor, dofetilide [17]. Together these findings support an inhibitory role for KCNE2 in the context of regulating hERG1 in the myometrium. The evidence also suggests that Kv11.1 activity contributes to the electrophysiological mechanisms that regulate uterine contractions and that inhibition of the α-subunit, ERG1, by the β-subunit, KCNE2, may facilitate labor [17]. Interestingly, the mechanism for reducing Kv11.1 activity to facilitate labor appears to be dysregulated in obese women. Maternal obesity is associated with increased rates of labor induction, dysfunctional labor requiring Caesarean section (CS) delivery, longer pregnancies, as well as postpartum haemorrhage [19-21]. We reported that high body mass index (BMI) was associated with increased levels of hERG1 and reduced levels of KCNE2 in the myometrium [17]. Furthermore, high BMI was associated with heightened Kv11.1 activity in vitro, suggesting that the delayed and protracted labor often observed in obese women is linked to elevated Kv11.1 activity in the myometrium [17].
Genes encoding the progesterone receptor (PGR) [22-24], estrogen receptor 1 (ESR1) [23,25], oxytocin receptor (OXTR) [24,26,27] and prostaglandin-endoperoxide synthase 2 (PTGS2) [24,28] have been identified as key genes involved human parturition. There is now extensive published literature linking the myometrial regulation of these genes, among others, to the maintenance of uterine quiescence and the transition to a contractile phenotype at labor [29-31]. Uncovering an association between expression of these key genes and genes encoding the Kv11.1 channel would ascertain the involvement of this channel in the increased contractility of the myometrium at term and as part of the myometrial transformation leading to labor.
The aim of this study therefore was to determine whether expression of KCNH2 and KCNE2 in term non-laboring human myometrium correlate with the expression of PGR, ESR1, OXTR and PTGS2. We report that in term pregnancy, KCNH2 and KCNE2 are expressed coordinatedly with these key parturition-associated genes, thus strengthening the link between uterine contractility and K+ channel abundance in myometrial cells. Furthermore, this study examined the relationship of myometrial KCNH2 and KCNE2 mRNA abundance with BMI, and explored the expression of both genes in myometrial biopsies from preterm deliveries.
MATERIALS AND METHODS
Consumables and reagents
Myometrial Tissue acquisition
RNA extraction, Reverse transcription and Real-time quantitative PCR
| Primer | Primer sequence (5’-3’) | Amplicon size | GeneBank # |
| KCNH2 | F: ACCTCATCGTGGACATCAR: CTCCTCGTTGGCATTGAC | 77 | NM_000238.3 |
| KCNE2 | F: CACGAGGCAAATCCAAATR: CTCCAACAAGCAAGCATAA | 141 | NM_172201.1 |
| OXTR | F: CTGGACGCCTTTCTTCTTCGTR: GAAGGCCGAGGCTTCCTT | 101 | NM_000963 |
| ESR1 | F: TGAAAGGTGGGATACGAAAAGACR: CATCTCTCTGGCGCTTGTGTT | 66 | NM_000125.3 |
| PGR | F: GTGGGAGCTGTAAGGTCTTCTTTAAR: AACGATGCAGTCATTTCTTCCA | 83 | NM000926.4 |
| PTGS2 | F: ATGTTCCACCCGCAGTACAGAR: CAGCATAAAGCGTTTGCGGTA | 73 | NM_000916.3 |
Table 1: cDNA primer sequences for KCNH2, KCNE2, OXTR, ESR1, PGR and PTGS2.
Data and statistical analysis
RESULTS
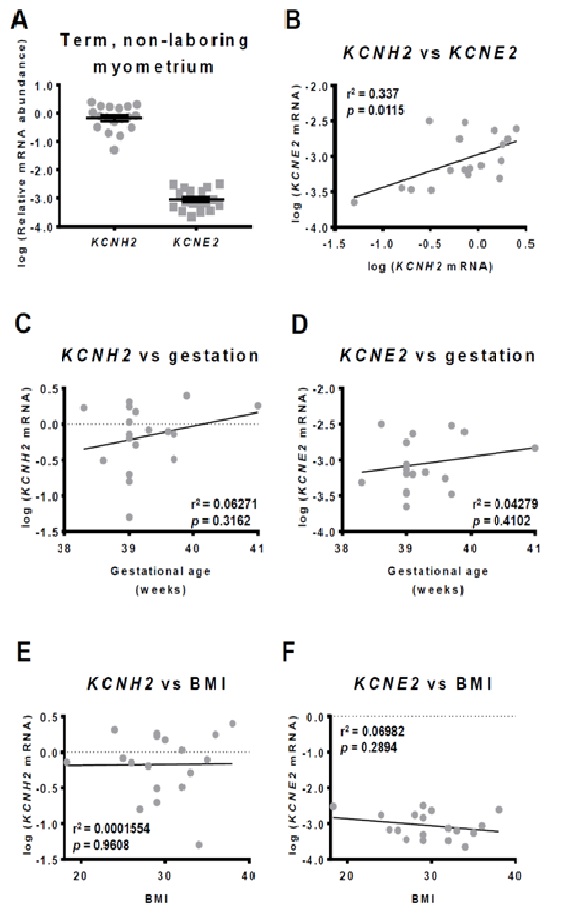
KCNH2and KCNE2 expression in human myometrium at term pregnancy
There was no statistically significant relationship between KCNH2 mRNA abundance and gestational age (r2=0.06, p=0.31) (Figure 1C), or KCNE2 mRNA abundance and gestational age (r2=0.04, p=0.41) (Figure 1D) within the term gestation range of 38.2-41.0 weeks.
Since we have found previously that hERG1levels increase whilst KCNE2 levels decrease with increasing BMI of term pregnant women, we have correlated KCNH2 mRNA as well as KCNE2 mRNA abundance to BMI. The BMI in our patients group ranged from 18.3 to 38.0. There was no statistically significant correlation between KCNH2 mRNA abundance (r2=0.0002, p=0.96) (Figure 1E) or KCNE2 mRNA abundance and BMI (r2=0.07, p=0.29) (Figure 1F).
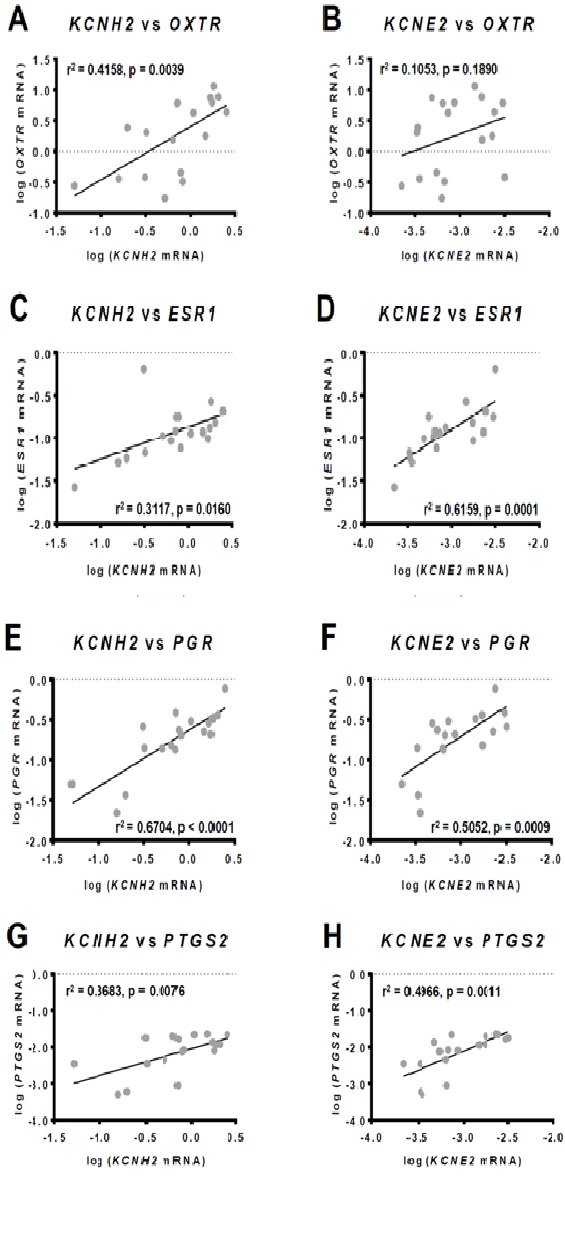
Correlations between the expression of KCNH2, KCNE2 and contraction-associated myometrial genes
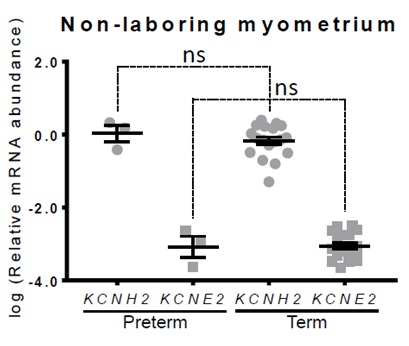
KCNH2 and KCNE2 expression in preterm human myometrium
DISCUSSION
We previously reported the presence of hERG1 and KCNE2 in pregnant human myometrium in late gestation, and demonstrated that labor onset is associated with diminished Kv11.1 activity in association with enhanced expression of the inhibitory subunit, KCNE2 [17]. Furthermore, we reported a significant positive correlation between Kv11.1 activity and BMI, which was attributable to increased hERG1 levels and decreased KCNE2 levels [17].In follow up to that functional study, we examined KCNH2 and KCNE2 gene expression in term non-laboring myometrium and found that KCNE2 expression was relatively low, and correlated with KCNH2 expression. Moreover, the levels of both mRNAs correlated positively with the expression of myometrial genes that are key regulators of parturition, but showed no relationship with the BMI of the participants. Patients comprising our term non-laboring cohort ranged from underweight (BMI 18.3) to Class II obese (BMI 38.0) (World Health Organisation guidelines). The lack of correlation suggests that within the examined BMI range, the effect of obesity is not on KCNH2 or KCNE2 gene expression, but rather on protein levels. This finding refines the link between Kv11.1 activity and BMI, and suggests that future studies should focus on the effect of obesity on KCNH2 and KCNE2 mRNA translation as well as protein turnover.
Another potassium channel that plays a crucial role in pregnancy is the ATP-sensitive K+ (KATP) channel. KATP-mediated K+ efflux plays a role in maintaining the resting membrane potential of myocytes [36]. Several studies have found that in late pregnancy, the number of myometrial KATP channelsis reduced, which increases uterine excitability, thereby promoting the establishment of labor [37-39]. Du et al. [40] found that all KATP channel subunits, apart from the SUr2A subunit, were down-regulated in the late pregnant uterus compared to the non-pregnant uterus. More importantly, expression of SUr2B/Kir6.1 in term pregnant human myometrium was found to be increased in women older than 35 years [40]. Advanced maternal age is associated with increased obstetric risks in general, as well as increased risk of elective and emergency CS [41,42]. The study therefore suggests that increased risk of birth complications in women aged over 35 years may be linked to reduced myocyte excitability attributable to increased numbers of KATP channels in the myometrium [40]. This is consistent with our previous study on hERG1, which similarly links poor labor outcomes for obese women with the elevated activity of a potassium channel [17]. Both studies illustrate the importance of understanding how different potassium channels regulate uterine contractility, as dysregulation can lead to obstetric complications.
In pregnant human myometrium, we observed a significant positive correlation between KCNH2 and KCNE2 mRNA abundance at term (Figure 1B). This correlation, if it translates to protein abundance, could suggest an underlying subunit stoichiometry in myometrial Kv11.1 channels. Given that all term samples analysed were non-laboring, preservation of the high KCNH2:KCNE2 ratio may play a role in maintaining quiescence in non-laboring tissue.
The uterine quiescence, which persists for the majority of pregnancy, is maintained by inhibitors of uterine contraction, such as progesterone [24,43-46]. As term approaches there is a shift from progesterone to estrogen dominance and the uterus undergoes a phenotypic transition. This transition is characterised by up-regulated expression of a series of contraction-associated genes (reviewed by Smith [47]), including receptors for oxytocin and prostaglandins, increased expression of genes encoding myometrial gap junctions, such as connexin 43, which facilitates synchronous contractions, and alterations in resting membrane potential of myocytes, which renders myocytes more prone to excitation [24,43-46,48]. Collectively these changes increase the likelihood that sporadic contractions of the uterus will propagate in synchrony and lead to established labor [49]. In fulfilling a role as a regulator of myometrial contractility, it would therefore be reasonable to expect Kv11.1 activity, and thus KCNH2 and KCNE2 expression, to correlate with the expression of key genes that are known to play a role in this phenotypic transition.
OXTR expression in the myometrium is reportedly constant between 24 - 36 weeks gestation, but rises significantly in term samples (>37 weeks) prior to the onset of labor [48,50]. Within this latter time frame (term), we found that KCNH2 expression correlated positively with OXTR expression, whereas KCNE2 did not. Given that KCNH2 mRNA abundance is significantly correlated with both KCNE2 and OXTR mRNA abundance, a correlation should be expected between KCNE2 and OXTR mRNA levels. It is possible that with greater sample numbers a positive correlation would be demonstrated between KCNE2 and OXTR expression. Given that the role of Kv11.1 within the myometrium is to rapidly terminate contractions in order to prevent propagation of uterine contractility and the establishment of labor, it is reasonable to conjecture that KCNH2 expression is co-regulated with OXTR expression as a counter-measure to offset increased sensitivity to oxytocin.
Similar considerations may apply to the observed positive correlation of KCNH2 and KCNE2 with ESR1 and PTGS2. ESR1 encodes estrogen receptor α (ERα), which drives the estrogen-dependent expression of contraction-associated proteins, such as connexin 43 [51-54]. PTGS2 is responsible for the biosynthesis of prostaglandins (PGs). Prostaglandin F2α(PGF2α) is produced mainly by the maternal decidua and is involved in the up-regulation of OXTR levels and gap junctions in the myometrium, thus promoting uterine contractions [55]. Prostaglandin E2(PGE2) is produced by the fetus and placenta and is involved in collagen degradation and dilation of small blood vessels in the cervix, thus promoting cervical ripening as well as spontaneous rupture of the fetal membranes [56]. It is important to remain mindful, however, that non-laboring tissues were analysed in our study suggesting the possibility that women with high expression of ESR1 and PTGS2 may require higher levels of KCNH2 in order to maintain uterine quiescence than women with lower ESR1 and PTGS2 expression. Moreover, in the heart KCNE2 expression is up-regulated by estrogen [57]. Taking into consideration that in humans circulating levels of estrogen are high for most of pregnancy, and remain elevated during parturition [46,58], it is likely that estrogen regulation of KCNE2 expression, and thus Kv11.1 activity, plays an important role in modulating the transition from quiescence to contractility.
KCNH2 and KCNE2 mRNA levels were both positively correlated with PGR expression. Consistent with previous studies we report PGR total expression [30] as within our term non-laboring cohort PR-A expression was barely detectable. The strong positive correlations between KCNH2 and KCNE2 expression with PGR expression are therefore in relation to PR-B, which is consistent with the observed KCNH2:KCNE2 ratio potentially contributing to pregnancy maintenance. A follow up analyses determining whether KCNH2 and KCNE2 expression continue to correlate with PGR expression in laboring tissue would provide valuable insight into whether progesterone regulates hERG1 and KCNE2 levels as means of maintaining uterine quiescence.
In mice, KCNH2 expression remains constant across gestation and there is no change in ERG1 protein levels [16]. KCNE2, however, is gestationally regulated in mice in that mRNA abundance is significantly up-regulated by day 14 of a 20 day pregnancy, and KCNE2 protein levels are significantly up-regulated by day 17 [16]. Our analyses of preterm non-laboring samples showed no change in KCNH2 or KCNE2 gene expression earlier in gestation suggesting that, unlike mice, KCNE2 gene expression may not be gestationally regulated in humans. If confirmed by analysis of additional preterm samples, this could indicate that alternative auxiliary subunits need to be explored in the context of human myometrium. To date, studies exploring the role of Kv11.1 channels in human myometrium have focused on KCNE2 co-expression alongsidehERG1. However, a variety of auxiliary subunits are known to modulate Kv11.1 activity [1], and studies by Greenwood et al. [16] indicate that KCNE4 expression is gestationally regulated in mouse uterine tissue in addition to KCNE2. As such it is possible that KCNE4, or another regulatory subunit, is gestationally regulated in humans.
CONCLUSION
We have previously provided functional data examining the role of Kv11.1 in regulating myometrial contractility at term labor. Here we have followed up the functional study with an analysis of KCNH2 and KCNE2 gene expression in term non-laboring women. We have shown that KCNH2 and KCNE2 expression correlate with the expression of key myometrial genes implicated in parturition. Our data suggest that KCNH2 and KCNE2 are participants in the gene network controlling myometrial contractility at term. Uncovering this association advances our understanding of the mechanisms that underpin myometrial transformation, and reiterates the complexity of the parturition process. A complementary analysis using laboring myometrium would assist with the further interpretation of these results as alterations of the correlative network with the onset of labor would provide insight into which relationships are critical in maintaining uterine quiescence. We have also performed an initial investigation into KCNH2 and KCNE2 expression in preterm samples, which did not show any robust association between the preterm status and KCNH2 or KCNE2 expression. This investigation should be followed up with additional preterm samples to strengthen the study, as well as the analysis of preterm in-labor samples. Furthermore, future studies include examining whether these correlations translate into correlations at the protein level.
ACKNOWLEDGEMENT
The authors wish to thank the obstetricians from the John Hunter Hospital, NSW, our research midwife, Anne Wright, and the research participants who donated samples toward this study.
CONFLICT OF INTERESTS DISCLOSURE
Roger Smith holds a patent through the University of Newcastle in relation to the hERG potassium channel, titled “Compositions and methods for modulating uterine contractions”. The remaining authors declare that there is no conflict of interest regarding the publication of this paper.
REFERENCES
- Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, et al. (2012) hERG K(+) channels: structure, function, and clinical significance. Physiol Rev 3: 1393-1478.
- Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, et al. (1999) MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell 2: 175-187.
- McDonald TV, Yu Z, Ming Z, Palma E, Meyers MB, et al. (1997) A minK-HERG complex regulates the cardiac potassium current I(Kr). Nature 388: 289-292.
- Sanguinetti MC, Jiang C, Curran ME, Keating MT (1995) A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell 81: 299-307.
- Trudeau MC, Warmke JW, Ganetzky B, Robertson GA (1995) HERG, a human inward rectifier in the voltage-gated potassium channel family. Science 269: 92-95.
- Ohya S, Asakura K, Muraki K, Watanabe M, Imaizumi Y (2002) Molecular and functional characterization of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. Am J Physiol Gastrointest Liver Physiol 282: 277-287.
- Ohya S, Horowitz B, Greenwood IA (2002) Functional and molecular identification of ERG channels in murine portal vein myocytes. Am J Physiol Cell Physiol 283: 866-877.
- Yeung SY, Greenwood IA (2007) Pharmacological and biophysical isolation of K+ currents encoded by ether-a-go-go-related genes in murine hepatic portal vein smooth muscle cells. Am J Physiol Cell Physiol 292: 468-476.
- Akbarali HI, Thatte H, He XD, Giles WR, Goyal RK (1999) Role of HERG-like K(+) currents in opossum esophageal circular smooth muscle. Am J Physiol 277: 1284-1290.
- Parr E, Pozo MJ, Horowitz B, Nelson MT, Mawe GM (2003) ERG K+ channels modulate the electrical and contractile activities of gallbladder smooth muscle. Am J Physiol Gastrointest Liver Physiol 284: 392-398.
- Mewe M, Wulfsen I, Schuster AM, Middendorff R, Glassmeier G, et al. (2008) Erg K+ channels modulate contractile activity in the Bovine Epididymal Duct. Am J Physiol Regul Integr Comp Physiol 294: 895-904.
- Farrelly AM, Ro S, Callaghan BP, Khoyi MA, Fleming N, et al. (2003) Expression and function of KCNH2 (HERG) in the human jejunum. Am J Physiol Gastrointest Liver Physiol 284: 883-895.
- Lillich JD, Rakestraw PC, Roussel AJ, Finley MR, Ganta S, et al. (2003) Expression of the ether-a-go-go (ERG) potassium channel in smooth muscle of the equine gastrointestinal tract and influence on activity of jejunal smooth muscle. Am J Vet Res 64: 267-272.
- Parkington HC, Tonta MA, Brennecke SP, Coleman HA (1999) Contractile activity, membrane potential, and cytoplasmic calcium in human uterine smooth muscle in the third trimester of pregnancy and during labor. American Journal of Obstetrics and Gynecology 181: 1445-1451.
- Aaronson PI, Sarwar U, Gin S, Rockenbauch U, Connolly M, et al. (2006) A role for voltage-gated, but not Ca2+-activated, K+ channels in regulating spontaneous contractile activity in myometrium from virgin and pregnant rats. Br J Pharmacol 147: 815-824.
- Greenwood IA, Yeung SY, Tribe RM, Ohya S (2009) Loss of functional K+ channels encoded by ether-a-go-go-related genes in mouse myometrium prior to labour onset. J Physiol 587: 2313-2326.
- Parkington HC, Stevenson J, Tonta MA, Paul J, Butler T, et al. (2014) Diminished hERG K+ channel activity facilitates strong human labour contractions but is dysregulated in obese women. Nat Commun 5.
- Weerapura M, Nattel S, Chartier D, Caballero R, Hebert TE (2002) A comparison of currents carried by HERG, with and without coexpression of MiRP1, and the native rapid delayed rectifier current. Is MiRP1 the missing link? J Physiol 540: 15-27.
- Higgins CA, Martin W, Anderson L, Blanks AM, Norman JE, et al. (2010) Maternal obesity and its relationship with spontaneous and oxytocin-induced contractility of human myometrium in vitro. Reprod Sci 17: 177-185.
- Jie Z, Kendrick A, Quenby S, Wray S (2007) Contractility and calcium signaling of human myometrium are profoundly affected by cholesterol manipulation: implications for labor? Reprod Sci 14: 456-466.
- Fyfe EM, Anderson NH, North RA, Chan EH, Taylor RS, et al. (2011) Risk of first-stage and second-stage cesarean delivery by maternal body mass index among nulliparous women in labor at term. Obstet Gynecol 117: 1315-1322.
- Mesiano S (2007) Myometrial progesterone responsiveness. Semin reprod med 25: 5-13.
- Mesiano S, Welsh TN (2007) Steroid hormone control of myometrial contractility and parturition. Semin Cell Dev Biol 18: 321-331.
- Challis JRG, Matthews SG, Gibb W, Lye SJ (2000) Endocrine and paracrine regulation of birth at term and preterm. Endocrine reviews 21: 514-550.
- Welsh T, Johnson M, Yi L, Tan H, Rahman R, et al. (2012) Estrogen receptor (ER) expression and function in the pregnant human myometrium: estradiol via ERα activates ERK1/2 signaling in term myometrium. J Endocrinol 12: 227-238.
- Mitchell BF, Fang X, Wong S (1998) Oxytocin: a paracrine hormone in the regulation of parturition? Rev Reprod 3: 113-122.
- Blanks AM, Thornton S (2003) The role of oxytocin in parturition. BJOG 20: 46-51.
- Blesson CS, Sahlin L (2014) Prostaglandin E and F receptors in the uterus. Receptors & Clinical Investigation 1: 127-138.
- Bollapragada S, Youssef R, Jordan F, Greer I, Norman J, et al. (2009) Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol 200: 1-11.
- Chan YW, van den Berg HA, Moore JD, Quenby S, Blanks AM (2014) Assessment of myometrial transcriptome changes associated with spontaneous human labour by high-throughput RNA-seq. Exp Physiol 99: 510-524.
- Mittal P, Romero R, Tarca AL, Gonzalez J, Draghici S, et al. (2010) Characterization of the myometrial transcriptome and biological pathways of spontaneous human labor at term. J Perinat Med 38: 617-643.
- Cicinnati VR, Shen Q, Sotiropoulos GC, Radtke A, Gerken G, et al. (2008) Validation of putative reference genes for gene expression studies in human hepatocellular carcinoma using real-time quantitative RT-PCR. BMC Cancer 8: 350.
- Ilicic M, Zakar T, Paul JW (2017) Modulation of Progesterone Receptor Isoform Expression in Pregnant Human Myometrium. Biomed Res Int.
- Huggett J, Dheda K, Bustin S, Zumla A (2005) Real-time RT-PCR normalisation; strategies and considerations. Genes Immun 6: 279-284.
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402-408.
- Brainard AM, Korovkina VP, England SK (2007) Potassium channels and uterine function. Semin Cell Dev Biol 18: 332-339.
- Curley M, Cairns MT, Friel AM, McMeel OM, Morrison JJ, et al. (2002) Expression of mRNA transcripts for ATP-sensitive potassium channels in human myometrium. Mol Hum Reprod 8: 941-945.
- Longo M, Jain V, Vedernikov YP, Hankins GD, Garfield RE, et al. (2003) Effects of L-type Ca(2+)-channel blockade, K(+)(ATP)-channel opening and nitric oxide on human uterine contractility in relation to gestational age and labour. Mol Hum Reprod 9: 159-164.
- Xu C, You X, Gao L, Zhang L, Hu R, et al. (2011) Expression of ATP-sensitive potassium channels in human pregnant myometrium. Reprod Biol Endocrinol 9.
- Du Q, Jovanovic S, Tulic L, Sljivancanin D, Jack DW, et al. (2013) KATP channels are up-regulated with increasing age in human myometrium. Mech Ageing Dev 134: 98-102.
- Heffner LJ, Elkin E, Fretts RC (2003) Impact of labor induction, gestational age, and maternal age on cesarean delivery rates. Obstet Gynecol 102: 287-293.
- Zasloff E, Schytt E, Waldenstrom U (2007) First time mothers’ pregnancy and birth experiences varying by age. Acta Obstet Gynecol Scand 86: 1328-1336.
- Mesiano S (2004) Myometrial progesterone responsiveness and the control of human parturition. J Soc Gynecol Investig 11: 193-202.
- Norwitz ER, Robinson JN, Challis JR (1999) The control of labor. N Engl J Med 341: 660-666.
- Tulchinsky D, Hobel CJ, Yeager E, Marshall JR (1972) Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol 112: 1095-1100.
- Walsh SW, Stanczyk FZ, Novy MJ (1984) Daily hormonal changes in the maternal, fetal, and amniotic fluid compartments before parturition in a primate species. J Clin Endocrinol Metab 4: 629-39.
- Smith R (2007) Parturition. N Engl J Med 3: 271-283.
- Fuchs AR, F Fuchs, P Husslein, MS Soloff (1984) Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol 6: 734-741.
- Smith R, M Imtiaz, D Banney, JW Paul, RC Young (2015) Why the heart is like an orchestra and the uterus is like a soccer crowd. Am J Obstet Gynecol 2: 181-185.
- Wathes DC, Borwick SC, Timmons PM, Leung ST, Thornton S (1999) Oxytocin receptor expression in human term and preterm gestational tissues prior to and following the onset of labour. J Endocrinol 1: 143-151.
- Ambrus G, Rao CV (1994) Novel regulation of pregnant human myometrial smooth muscle cell gap junctions by human chorionic gonadotropin. Endocrinology 6: 2772-2779.
- Garfield RE, Kannan MS, Daniel EE (1980) Gap junction formation in myometrium: control by estrogens, progesterone, and prostaglandins. Am J Physiol 3: 81-89.
- Petrocelli T, Lye SJ (1993) Regulation of transcripts encoding the myometrial gap junction protein, connexin-43, by estrogen and progesterone. Endocrinology 1: 284-290.
- Skinner KA, Challis JR (1985) Changes in the synthesis and metabolism of prostaglandins by human fetal membranes and decidua at labor. Am J Obstet Gynecol 4: 519-523.
- Falcone TLA (1994) Placental synthesis of steroid hormones. In: Tulchinsky D, Little AB (eds.). Maternal-fetal endocrinology, Saunders, Philadelphia, USA.
- Kundu P, Ciobotaru A, Foroughi S, Toro L, Stefani E, et al. (2008) Hormonal regulation of cardiac KCNE2 gene expression. Mol Cell Endocrinol 292: 50-62.
- Yen S (1991) Endocrine-metabolic adaptations in pregnancy. In: Yen S, Jaffe R (eds.). Reproductive endocrinology. Saunders, Philadelphia, USA.
Citation: Paul JW, Ilicic M, Zakar T, Smith R (2017) Expression of KCNH2 (hERG1) and KCNE2 correlates with expression of key myometrial genes in term pregnant human myometrium. J Hum Endocrinol 2: 008.
Copyright: © 2017 Jonathan W Paul, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.