
Extract of Parkia Biglobosa ("Dawadawa") Seed is A Rich Antioxidant Source Which Enhances Glucose Tolerance and Inhibits the Growth of Cancer Cells
*Corresponding Author(s):
Perez Frimpong DansoDepartment Of Biochemistry And Biotechnology, KNUST, Kumasi-Ghana, Ghana
Email:Perezfrimpong@gmail.com
Abstract
Background: Diet is a major indicator in preventing and managing chronic diseases like cancer and diabetes. Parkia biglobosa seed, also known as "dawadawa" in most West African languages, is used in all parts of Ghana and West Africa to season traditional soups and stews and manage chronic diseases.
Objective: This study was aimed at evaluating the anticancer, antidiabetic, antioxidant, and phytochemical potential of fermented dawadawa extract (FDE) and unfermented dawadawa (UDE) aqueous seed extracts of Parkia biglobosa.
Methodology: Total antioxidant capacity was assessed using DPPH assay, and total phenolic was assessed using Folin Ciocalteu assay. The antidiabetic potential was investigated using the yeast cell glucose uptake assay. The anticancer screening was performed on the prostate (PC3 and LNCAP), breast (MCF7), liver (HEPG2) cancer cells and prostate normal cells (PNT-2). The Standard used was curcumin. The selectivity index was determined for each extract. Qualitative screening for terpenoids, saponins, tannins, and flavonoids was conducted. Gas chromatography-Mass Spectrometry analysis was performed on the methanolic extracts for the detection of major compounds.
Results: Unfermented Parkia biglobosa had the highest DPPH scavenging activity with an EC50= 0.54 mg/ml and fermented had an EC50 = 2.27 mg/ml. The FDE had the highest phenolic content (0.24 ± 0.01 mg/100gGAE) while the UDE had 0.18 ± 0.01 mg/100gGAE. The FDE had the highest total flavonoid content of 1178.91mg/100g QE per 10 mg of the extract while the UDE had 511.95mg/100g QE per 10 mg extract. The FDE showed the highest glucose uptake followed by UDE and metformin recorded the lowest uptake. All samples were cytotoxic against cancer cell lines except UDE on LNCAP. The highest activity in a FDE was recorded in MCF7 with an IC50 of 257.20 µg/ml and selectivity of 1.17. In the UDE sample, the highest activity was recorded in PC3 with an IC50 of 259.5µg/ml selectivity of 1.106. GC-MS analysis showed peaks of Cis-Vaccenic acid and 9-octadecenoic acid present in the methanolic extracts. Terpenoids and flavonoids were concentrated in both extracts.
Conclusion: The study showed that Parkia biglobosa seed possesses remarkable antioxidant, antidiabetic and anticancer properties.
Keywords
Antioxidant; Cancer; Diet; Diabetes; HEPG2; LNCAP; MCF7; Parkia biglobosa; PC3; PNT-2
Abbreviations
FDE- Fermented "Dawadawa" Extract
UDE- Unfermented "Dawadawa" Extract
DPPH - 2, 2- diphenyl-1-picrylhydrazyl
GAE- Gallic Acid Equivalent
QE- Quercetin Equivalent
GC-MS- Gas Chromatography- Mass Spectrometry
RPMI- Roswell Park Memorial Institute Medium
FBS- Foetal Bovine Serum
DMSO- Dimethyl sulfoxide
SI- Selectivity Index
Background
Most African countries are burdened with communicable diseases, however, non-communicable diseases are the major burden both globally and regionally as a result of changing shifts in diet and lifestyle [1]. Cancer is a group of diseases characterized by uncontrolled growth and spread of abnormal cells. The disease is caused by both internal factors (inherited genetic mutations, immune conditions and mutations that occur from metabolism) and external factors (nutritional deficiencies, infectious organisms, radiations and chemicals) that may act together or in sequence to initiate or promote its development [2]. Cancer is a major public health problem globally. It is the second leading cause of death in the United States with one in four deaths [3]. Research in this area has become paramount as understanding of the disease mechanism and susceptibility of individuals to it, will go a long way to aid in the search for a cure. In the search for a cure, diet is a major indicator in the prevention and management of chronic disease and cancer is no exception. Treatment options available for cancers are very expensive and these include chemotherapy, surgery, hormone therapy, radiotherapy, and cryotherapy, among others. The standard method of treating these people has been chemotherapy, which comes with numerous side effects – nausea, toxicity, and excessive hair loss, among others. Also, the non-selective nature of some chemotherapeutic agents makes them affect cancer and normal cells alike, often leading to undesirable side effects. These problems are also compounded by the high cost of Western anticancer drugs. Due to these, a large proportion of cancer patients in Ghana and other developing countries have resorted to the use of Complementary and Alternative Medicine either as an adjunct or to completely supplant the use of chemotherapy [4]. Particularly, herbal medicines are widely used, with about 80% of cancer patients in developing countries (particularly African and Asian populations) relying on this method for cancer treatment [5]. This has been the case as these medicinal plants such as Parkia biglobosa. They are considered to be cheaper, safer and with less side effects [4].
Parkia biglobosa is a perennial deciduous tree found in a wide range of environments in Africa. The fermented seeds of P. biglobosa are used in all parts of Ghana and West Africa to season traditional soups. Some tribes in Ghana use the seed and the fruit to treat stomach aches. However, the anticancer properties of the seed of P. biglobosa have not been scientifically validated although its traditional use is reported among these groups of people. P. biglobosa is less expensive and readily available than chemotherapeutic drugs hence the need to investigate the anticancer activities of the plant to serve as an alternative source of anticancer agents that will aid in the formulation of cheaper and safer anticancer drugs. Also, P. biglobosa is added as a spice in the preparation of food, hence knowledge about its, antioxidant, anticancer effect and antidiabetic effects will help in its promotion and consumption at the public health education level.
Materials And Methods
- Cell lines and reagents
Cell lines for the study were obtained from the Cell Bank of the Noguchi Memorial Institute for Medical Research (NMIMR), Legon. Cell lines used included HepG2 (Liver), MCF-7 (Breast), PC3 and LNCAP (Prostate), and PNT-2 (Prostate epithelial). Culture media used included Foetal Bovine Serum (FBS), supplemented with antibiotics (Glutamine, Streptomycin and Penicillin), Roswell Park Memorial Institute Medium (RPMI), Dulbecco’s Minimum Essential Medium (DMEM), Phosphate buffered saline (PBS), Resazurin (7-Hydroxy-3H-phenoxazin-3-one 10-oxide), trypan blue solution, Potassium Ferricyanide (KCN), Trichloroacetate (TCA), Iron (III) Chloride (FeCl3), Butylated hydroxytoluene (BHT), 2, 2- diphenyl-1-picrylhydrazyl (DPPH), Ascorbic acid, Sodium Carbonate (Na2CO3), Folin Ciocalteu, Pyro-Gallic Acid, Aluminium Chloride (AlCl3), Quercetin, Commercial Yeast, Glucose and metformin. All reagents were analytical grade and were obtained from standard suppliers.
- Plant material
The seed was collected from a farm in Bolgatanga in the Upper East Region of Ghana. They were identified and authenticated. They were identified as those of P. biglobosa at the Department of Herbal Medicine, KNUST, Kumasi, Ghana (with voucher no. KNUST/HM1/2018/S001). A portion of the seed was soaked in water and allowed to ferment. The fermented and unfermented samples were air-dried and milled.
- Aqueous Crude Extract Preparation
In the aqueous extract of P. biglobosa seed sample preparation, 500 g of milled seed sample, both fermented and unfermented, was suspended in 500 mL of distilled water for 48 hours. The samples were centrifuged (Homef LC-30 centrifuge, LH) for ten minutes at a speed of 1106 ×g at room temperature in 50 mL Eppendorf tubes. The supernatants were taken up and the pellets were subsequently re-suspended with the same volume of distilled water and extracted again. All the supernatants were frozen at -20°C and lyophilized using a vacuum freeze dryer (YK-118, Taiwan). The fermented extract was designated as FDE and unfermented as UDE.
- Phytochemical Assessment of Crude Extracts of Biglobosa
The procedure used by [6], was adopted for the in vitro qualitative phytochemical investigation of extracts.
- In Vitro Antioxidant Activity
DPPH (2,2-DIPHENYL-1-PICRYL HYDRAZYL) assay: The free radical scavenging activities of FDE and UDE were determined by DPPH assay with slight modification as described by [7].
Total Phenolic Content Determination: Total phenolic content was determined using the Folin-Ciocalteu assay as described by [8].
Total flavonoid content: In the total flavonoid assay used procedure described by [9]. In the total flavonoid content assay, quercetin was used as standard and a stock solution of quercetin was prepared by dissolving 1 mg of quercetin in 1 mL of absolute methanol into a 2 mL Eppendorf tube and then vortexed for 30 seconds to ensure dissolution. Sample concentration was obtained through ten-fold serial dilution in absolute methanol. A stock solution of Aluminium Chloride (AlCl3) was prepared by dissolving 2 g of AlCl3 in 100 mL of absolute methanol to make 2% AlCl3. A stock solution of FDE and UDE was prepared by dissolving 20 mg of each sample in 1 mL of distilled water in a 2 mL Eppendorf tube. The samples were vortexed for 1 minute and sonicated for 5 minutes to ensure complete dissolution. Sample concentration of 10 and 5, mg/mL was obtained through two-fold serial dilution in absolute ethanol. Furthermore, 100 μL of the various concentration of sample and standard was pipetted in triplicates into the 96 well plate which was followed by the addition of 100 μL of 2% AlCl3 into the well and sample incubated for 20 mins and absorbance read at 412 nm.
- Glucose Uptake Assay- In Vitro Antidiabetic Assay
In the yeast preparation, 1 g of yeast was weighed into a falcon tube and 10 mL of distilled water was added and repeatedly washed via centrifugation at 3000 g for 5 mins. This was done repeatedly until the supernatant was clean. Furthermore, 10% w/v of the supernatant was prepared with distilled water. In the preparation of the glucose solution, 18.016 mg of glucose was dissolved in 10 mL of distilled water. Metformin was used as a standard. In the metformin preparation, 100 mg of metformin was weighed into an Eppendorf tube and dissolved in 1 mL of distilled water and afterwards, 20% of the metformin solution was picked and run through nine-fold serial dilution to obtain concentrations of 20, 10, 5, 2.5, 1.25, 0.625, 0.3125 mg/mL.
Stock solution of extracts of the sample was prepared by dissolving 20 mg of each sample in 1 mL of distilled water in a 2 mL Eppendorf tube. The sample was vortexed for 1 minute and sonicated for 5 minutes to ensure complete dissolution.
In the assay, 200 μL of the extract and metformin were added to 200 μL of the glucose solution in a 2 mL Eppendorf and incubated at 370C for 10 minutes. Furthermore, 20 μL of yeast suspension was added to each Eppendorf tube and vortexed for 30 seconds and afterwards incubated at 370C for 60 minutes. After incubation, the Eppendorf tube was centrifuged at 2500 x g for 5 minutes. The supernatant was collected after centrifugation and the absorbance was read at 720 nm to estimate glucose uptake.
- Antiproliferative Assay
Cell Culture: Cells were cultured as described by [10]. Jurkat, LNCap, and PNT2 cells were cultured in the RPMI 1640 medium. MCF7 cell line was cultured in D-MEM medium. All culture media were supplemented with 1% PSG and 10% FBS. The cells were maintained in an incubator with 5% CO2 concentration at 37 °C and passaged on reaching about 80% confluence.
Cell Viability Assay: The Alamar Blue assay or Resazurin-based Colorimetric Assay was used to determine the cytotoxicity of P. biglobosa on cancer and normal cell lines. The protocol used by [11] was used.
In the preparation of the stock solution, 100 mg of each extract was dissolved in 1 mL of distilled water. The solution was vortexed for 1 minute and afterwards, filter sterilized into cryotubes in a biosafety cabinet through 0.45 μm pore filters and subsequently, a twofold serial dilution was made of each extract to obtain four concentrations of 0.0625, 0.125, 0.25 and 0.5 mg/mL. Curcumin was used as a standard for the experiment and the concentration range used was 10-100 μg/mL in 1% DMSO.
A volume of 100 μL (1 × 10^5 cells/mL) of cells was seeded into 96-well plate. The cells were immediately treated with 10 μL of each extract dilutions in triplicates and incubated in an incubator with 5% CO2 concentration at 37°C for 72 hours. The untreated experiment which is the negative control was included in the plate. A blank of culture media on;ly was also included. And for each of the test extracts including the standard curcumin, a colour control plate was set up.
Resazurin solution was prepared by dissolving 0.15 mg of resazurin in 1 mL of PBS. The solution was vortexed for 1 minute and afterwards filter sterilized. After 72 hours of incubation at 37°C, under 5% CO2, in a humidified atmosphere, 10 µL of the prepared resazurin solution was added to each well. The excitation and emission were measured at 560 nm and 590 nm respectively on a microplate reader.
Dose response curves were plotted as percentages of cell viability against concentration.
% Cell Viability = [(ODT0-ODT1)/(ODU0-ODU1)] × 100
where ODT0 is the average absorbance of wells treated with test extracts for all cell lines; ODT1 is the average absorbance of wells with curcumin or test extract control; ODU0 is the average absorbance of wells with untreated cells (negative control) for all cell lines; ODU1 is the average absorbance of wells containing blank (culture media only).
Since the experiment was done in triplicates, the mean percentage of the cell viability was used to plot the dose-response curve and the inhibitory concentration at 50% was also determined using nonlinear regression. The inhibitory concentration at 50% IC50) is the concentration of P. biglobosa extracts or Curcumin (the test substance) that induces 50% inhibition of the cell lines.
The selectivity Index (SI) which is the ratio of the IC50 of each sample (including standard drug curcumin) on normal cell line PNT-2 to the IC50 of each sample (including standard drug curcumin) on the cancer cell lines. According to [12], samples were said to have high selectivity towards cancer cells if their SI values were greater than 2.
- Gas Chromatography-Mass Spectrometry (GC-MS)
The methanolic extracts were analysed using the GC-MS to identify the major compounds present using the PerkinElmer GC Clarus 580 Gas Chromatograph interfaced to a Mass Spectrometer (PerkinElmer Clarus SQ 8 S) equipped with ZB-5HTMS (5% diphenyl/95% dimethyl polysiloxane) fused capillary column (30 × 0.25 μm ID × 0.25 μm DF). The conditions of GC-MS were previously described [13]. The total GC-MS running time was 50 min respectively. Turbo-Mass (ver. 6.1.0) was used in this analysis and compounds were identified using the National Institute of Standard and Technology (NIST) database with over 62,000 patterns.
- Statistical Analysis
Microsoft Excel (2013) was used for the calculation and plotting of mean and SD estimates in the graph. Mean EC50 and IC50 values were compared by one-way ANOVA using GraphPad prism (8.0) and values with p < 0.05 were considered statistically significant.
Results
- Phytochemical constituents
Both the FDE and UDE were screened for phytochemicals namely: saponins, tannins, terpenoids and flavonoids (Table 1).
|
|
Saponins |
Tannins |
Terpenoids |
Flavonoids |
|
Standard |
+ |
+++ |
+++ |
+++ |
|
FDE |
- |
- |
++ |
+ |
|
UDE |
- |
- |
+ |
+ |
Table 1: Phytochemical constituent of aqueous extracts of fermented and unfermented Parkia biglobosa.
+++ means present in high concentration; ++Means present in moderate concentration; +Means present in low concentration; - means absent.
- In vitro antioxidant assay
(2,2-DIPHENYL-1-PICRYL HYDRAZYL) DPPH Assay: The antioxidant effect was evaluated by calculating the EC50. All the extracts reduced DPPH to diphenylpicrylhydrazine and diminished the absorbance at 517 nm. The UDE had the highest DPPH scavenging activity with an EC50= 0.54 and while the FDE had an EC50= 2.27 (Figure 1 & Table 2).
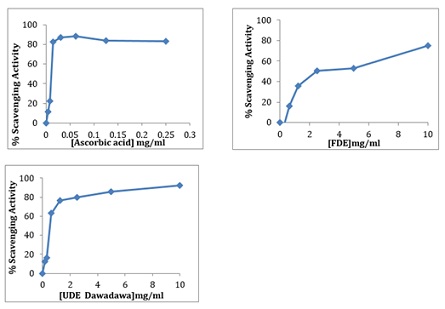 Figure 1: DPPH scavenging activity of aqueous extracts of fermented and unfermented Parkia biglobosa compared to ascorbic acid (standard compound).
Figure 1: DPPH scavenging activity of aqueous extracts of fermented and unfermented Parkia biglobosa compared to ascorbic acid (standard compound).
|
Sample/Standard |
EC50 value (mg/mL) |
P VALUE |
|
Ascorbic Acid (Standard) |
0.01141 |
≤0.001 |
|
UDE |
0.537177 |
≤0.001 |
|
FDE |
2.274214 |
≤0.001 |
Table 2: DPPH scavenging activity of the fermented and unfermented Parkia biglobosa with their various EC-50 values.
Values are means ± standard deviation of three replicates
- Total Phenolic Content
The results showed that the FDE had a higher concentration of phenols (0.24 ± 0.01) than the UDE (0.18 ± 0.01). Regarding the gallic acid equivalence, the total phenolic content of the FDE was 2408.96 GAE and the UDE was 1774.9 GAE. This was based on the Gallic acid standard curve (y=0.3927x + 0.0501, R2 =0.9934) (Figure 2).
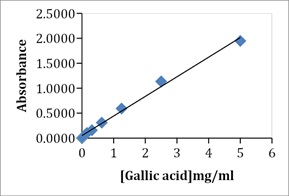 Figure 2: Standard concentration curve of absorbance against Gallic acid.
Figure 2: Standard concentration curve of absorbance against Gallic acid.
- Total Flavonoids Content
The fermented P. biglobosa had the highest total flavonoid content of 812.36 mg/100g QE per 10 mg of the extract while the unfermented recorded 576.19 mg/100g QE per 10 mg extract. This was based on a standard Quercetin curve (y= 16.077x +0.082, R2 = 0.999) (Figure 3).
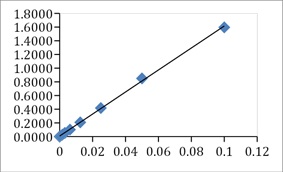 Figure 3: Standard concentration curve of absorbance against the concentration of Quercetin.
Figure 3: Standard concentration curve of absorbance against the concentration of Quercetin.
- In-vitro antidiabetic assay (Yeast Cells Glucose Uptake Assay)
The glucose uptake at an initial concentration of 20mg/mL aqueous extracts of P. biglobosa which was reduced through a twofold serial dilution to obtain 6 different concentrations was comparable to standard drug metformin. However, the effect of unfermented seed and fermented seed aqueous extracts on the uptake of glucose by yeast cells was higher at all concentrations as compared to metformin. Fermented seed aqueous extracts showed the highest glucose uptake followed by unfermented and metformin recording the lowest uptake (Figure 4 & Table 3).
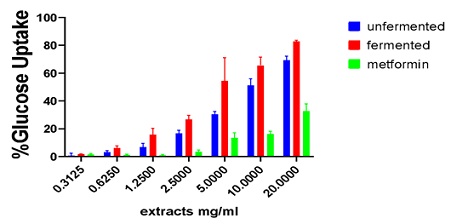 Figure 4: Glucose uptake by yeast cells on aqueous extracts of fermented and unfermented Parkia biglobosa as compared to metformin.
Figure 4: Glucose uptake by yeast cells on aqueous extracts of fermented and unfermented Parkia biglobosa as compared to metformin.
|
Concentration (mg/mL ) |
%Glucose uptake of sample/drug |
|
|
|
|
Metformin |
Fermented |
Unfermented |
|
20 |
32.7 ± 5.256 |
82.9 ± 0.969 |
69.3 ± 2.943 |
|
10 |
16.2 ± 2.189 |
65.6 ± 5.959 |
51.3 ± 4.742 |
|
5 |
13.6 ± 3.554 |
54.6 ± 16.543 |
30.4 ± 2.247 |
|
2.5 |
3.5 ± 1.251 |
26.7 ± 3.090 |
16.7 ± 2.433 |
|
1.225 |
0.9 ± 0.722 |
15.8 ± 4.599 |
6.9 ± 2.856 |
|
0.625 |
1.1 ± 0865 |
6.2 ± 1.705 |
3.2 ± 1.247 |
|
0.3125 |
1.7 ± 0.583 |
1.9 ± 0.217 |
0.8 ± 1.667 |
Table 3: Summary of percentage uptake of glucose by yeast cells on aqueous extracts of fermented and unfermented Parkia biglobosa as compared to metformin.
Values are means ± standard deviation of three replicates
- In vitro cytotoxic assay
The Fermented and unfermented P. biglobosa seed had a cytotoxic effect on liver cancer cell lines (HepG2), breast (MCF-7), prostate (PC3) and normal human prostate cells (PNT2). IC50 values were not obtained for the prostate cancer cell (LNCAP). UDE was most cytotoxic to PC3 and MCF 7. There was no IC50 value for the UDE on LNCAP. For the FDE, breast cancer cells MCF-7 had the highest activity (Figures 5-9 & Tables 4 & 5).
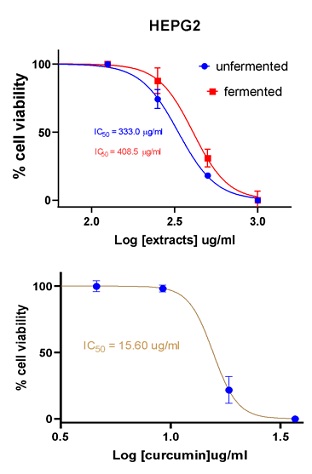 Figure 5: Cell viability curves showing cytotoxicity effect of FDE and UDE and the standard curcumin against liver cancer cell line (HepG2).
Figure 5: Cell viability curves showing cytotoxicity effect of FDE and UDE and the standard curcumin against liver cancer cell line (HepG2).
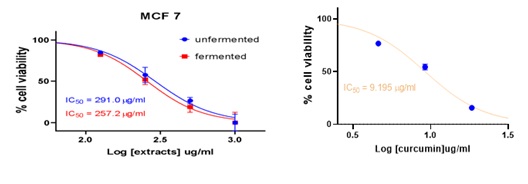 Figure 6: Cell viability curves showing cytotoxicity effect of FDE and UDE and the standard curcumin against breast cancer cell line.
Figure 6: Cell viability curves showing cytotoxicity effect of FDE and UDE and the standard curcumin against breast cancer cell line.
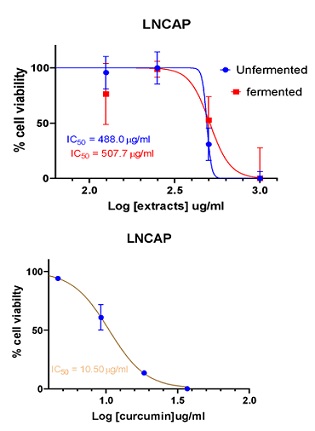 Figure 7: Cell viability curves showing cytotoxicity effect of of FDE and UDE and the standard curcumin against prostate cancer cell line (LNCAP).
Figure 7: Cell viability curves showing cytotoxicity effect of of FDE and UDE and the standard curcumin against prostate cancer cell line (LNCAP).
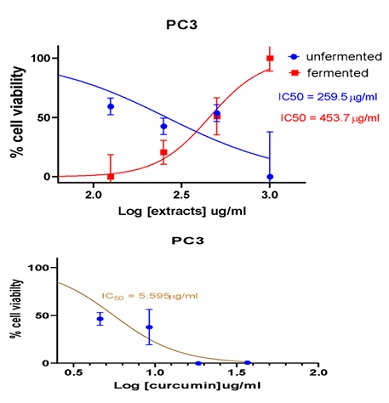 Figure 8: Cell viability curves showing cytotoxicity effect of FDE and UDE and the standard curcumin against prostate cancer cell line.
Figure 8: Cell viability curves showing cytotoxicity effect of FDE and UDE and the standard curcumin against prostate cancer cell line.
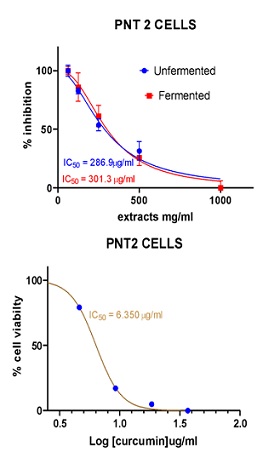 Figure 9: Cell viability curves showing cytotoxicity effect of FDE and UDE and the standard curcumin against Human normal prostate cell line (PNT-2).
Figure 9: Cell viability curves showing cytotoxicity effect of FDE and UDE and the standard curcumin against Human normal prostate cell line (PNT-2).
|
Cell Lines |
FDE |
UDE |
Curcumin |
P-value |
|
HepG2 |
408.5 |
333.0 |
15.60 |
≤0.001 |
|
MCF-7 |
257.2 |
291.0 |
9.195 |
≤0.001 |
|
LNCAP |
507.7 |
>1000 |
10.50 |
≤0.001 |
|
PC3 |
453.7 |
259.5` |
5.595 |
≤0.001 |
|
PNT-2 |
301.3 |
286.9 |
6.350 |
≤0.001 |
Table 4: Comparison of IC50 values of samples tested and curcumin standard on the various cell lines.
Values are means ± standard deviation of three replicates
|
Cell Lines |
FDE |
UDE |
Curcumin |
|
HepG2 |
0.738 |
0.862 |
0.407 |
|
MCF-7 |
1.171 |
0.986 |
0.691 |
|
LNCAP |
0..593 |
0.287 |
0.605 |
|
PC3 |
0.664 |
1.106 |
1.135 |
Table 5: Selectivity indices of the aqueous extract of fermented and unfermented Parkia biglobosa.
- Selectivity Index
The SI of various extracts which is fermented, unfermented and curcumin was calculated by dividing the IC50 of the normal prostate cell line (PNT-2) by the IC50 of the cancer cell lines (HepG2, MCF-7, LNCAP, PC3).
- GC-MS Analyses
Figure 10 shows the total ion chromatogram for the fraction which sums up the intensities of all mass spectral peaks. Also, some active compounds with their Retention Times (RT) in the fermented sample only are shown in table 6.
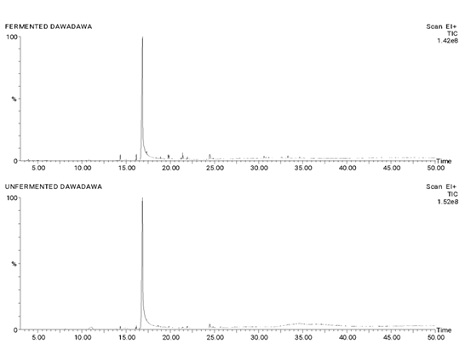 Figure 10: Total Ion Chromatogram (TIC) for the fraction which sums up the intensities of all mass spectral peaks.
Figure 10: Total Ion Chromatogram (TIC) for the fraction which sums up the intensities of all mass spectral peaks.
|
RF |
Scan |
Height |
Area |
Area % |
Norm % |
SI |
COMPOUND |
|
16.163 |
718 |
6,945,628 |
232,439.00 |
0.627 |
0.95 |
99.77
99.88
100.00 |
9-Octadecanoic acid (Z)-, methyl ester Trans-13-Octadecanoic, methyl ester 11-Octadecenoic acid, methyl ester |
|
16.860 |
756 |
141,783,424 |
24,365,656.00 |
65.732 |
100 |
100.00 99.78 100.00 |
Cis-Vaccenic acid 9-Octadecenoic acid, (E)- cis- 13- octadecenoic acid |
|
21.407 |
1004 |
7,871,788 |
509,356.80 |
1.374 |
2.09 |
97.03
96.87
|
Cyclopropaneoctanoic acid,2- [[2-[(2-ethylcyclopropyl) methylcyclopropyl] methyl]-, methyl ester 8, 11, 14 – Eicosatrienoic acid, (Z, Z, Z)- E, E- 1, 9, 17- Docasatriene |
Table 6: Compounds Present in Extracts of Fermented seed of P. biglobosa.
Discussion
The DPPH test is the oldest indirect method for determining the antioxidant activity based on the ability of the stable free radical 2, 2-diphenyl-1-picrylhydrazyl to react with hydrogen donors including phenols. DPPH, an N centered radical, has a characteristic absorbance at 517 nm, which decreases with the scavenging of the proton radical [14]. Phenolic compounds are also known to be potent antioxidants; flavonoids, tannins, etc. Total phenol content is measured by the Folin-Ciocalteu method at an absorbance of 750 nm [15]. Phenolic compounds have been suggested to induce the cellular antioxidant system by approximately 50% cellular glutathione concentration increment. Flavonoids are significant in the modulation of γ-glutamylcysteine synthase in both cellular antioxidant defences and detoxification of xenobiotics [16]. Among the extracts, the aqueous unfermented extract showed the strongest DPPH scavenging activity with an EC50 value of 0.54 followed by the aqueous fermented extract with an EC50 value of 2.27. The presence of phenolic compounds was also observed in all extracts, with the aqueous fermented extract recording the highest total phenolic content, 2408.964 mg/g plant extract (GAE) in crude extracts of P. biglobosa while the aqueous unfermented extract recorded a lower total phenolic content, 1774.892 mg/g plant extract (GAE). This suggests that the antioxidant activity of the P. biglobosa could be partly attributed to the presence of the various phenolic compounds in the extracts which may act through their ability to adsorb, neutralize, and quench free radicals. The result of the present investigation has shown that Parkia biglobosa possesses antioxidant activity and this property is predominantly found in the aqueous unfermented extract. In the total flavonoid assay, the fermented P. biglobosa had the highest total flavonoid content of 812.3613mg/100g QE per 10 mg of the extract and the unfermented showed a total flavonoid content of 576.1854mg/100g QE per 10mg extract. Therefore flavonoids were present in both aqueous fermented and unfermented extracts of Parkia biglobosa despite the disparity in the amounts. This suggests that the antioxidant activity of the Parkia biglobosa could be partly attributed to the presence of various phenolic compounds, particularly flavonoids. The extracts may act through their ability to adsorb, neutralize, and quench free radicals. The result of the present investigation has shown that P. biglobosa possesses antioxidant activity and this property is predominantly found in the aqueous fermented extract.
This research was also aimed at evaluating the antiproliferative effect of this Ghanaian food spice P. biglobosa, using the aqueous extract of fermented and unfermented seeds. To date, no known study has been conducted or published investigating the in vitro anticancer activity of P. biglobosa seed extract. The In Vitro Cytotoxic assay was conducted for both the fermented and the unfermented Parkia biglobosa on four cancer cell lines which are LNCAP (prostate), MCF-7 (breast), HepG2 (liver), PC3 (prostate) and a normal human prostate cell line (PNT2). Curcumin was used as a positive control. The result from this study indicates that fermented and unfermented aqueous extracts of P. biglobosa had cytotoxic activity on Liver Cancer Cells (HepG2), breast cancer cells (MCF-7) and Prostate Cancer Cells (PC3). Aqueous extracts of fermented P. biglobosa seed had cytotoxic activity on Prostate Cancer Cells (LNCAP) but at the same time, no significant cytotoxic activity was recorded with aqueous extracts of unfermented on prostate cancer cells (LNCAP). The high amount of flavonoids and phenolics observed for the extracts might account for the promising antiproliferative activity observed in the various cancer cell lines as these secondary metabolites have proved to be good anticancer agents [17].
An IC50 was obtained for HepG2, MCF-7 and PC3 cells with respect to both fermented and unfermented seed extracts in a concentration-response curve. When sample extracts were tested against LNCAP cells, IC50 value was obtained only in the fermented sample as the unfermented sample showed IC50 greater than 1000. When the cytotoxic effects of various samples on cancer cells were compared, it was observed that in HepG2 and LNCAP cells, unfermented samples showed more pronounced effects than fermented samples. It was also observed that in MCF-7 and LNCAP cells the fermented sample showed a more pronounced cytotoxic effect than the unfermented sample. Also, it was observed that the most pronounced cytotoxic effect was observed in fermented samples against MCF-7 cells and the least cytotoxic activity was observed in unfermented samples against LNCAP cells in which case IC50 was greater than 1000. Furthermore, in the positive control test experiment in which Curcumin was used as the positive control, the most profound cytotoxic effect was observed in prostate cancer cell (PC3) followed by breast cancer cells (MCF-7), and then prostate cancer cells (LNCAP) with liver cancer cells (HepG2) showing the least profound cytotoxic effect. It is important to note that the half maximal inhibitory concentration (IC50) as observed in the positive control sample that is curcumin against all the cancer cells had a relatively lower IC50, that is to say that curcumin had a more profound cytotoxic activity than both fermented and unfermented Parkia biglobosa seed extracts. Potential anticancer agents should exhibit cytotoxic specificity for cancer cells hence a factor called selectivity index is used to determine if a candidate is a good anticancer agent. Selectivity indexes greater than 2 make it a good candidate for further anticancer studies. It should be noted that none of the test samples had an SI of 2 including the positive control curcumin which is a very good anticancer agent showing distinctive antiproliferative activity like inhibiting proliferation, induction of apoptosis and invasion of tumours [18]. The highest selectivity index for all the test samples including the positive control curcumin obtained for breast cancer cells (MCF-7) for aqueous crude fermented sample followed by prostate cancer cell (PC3) for the positive control curcumin and then followed by prostate cancer cell (PC3) for aqueous crude unfermented sample. It can be inferred from this study that liver cancer cell (HepG2), breast cancer cell (MCF-7), Prostate Cancer Cell (PC3) are all susceptible to Parkia biglobosa seed while prostate cancer cell (LNCAP) was most resistant to the seed. This study has also shown that obtaining fractions of both the unfermented and the fermented sample should be obtained and this can increase the selectivity index which will help us make very good judgement.
In the GC-MS analysis prominent High peaks on the total ion Chromatogram showed the presence of Cis Vaccenic Acid and 9-octadecenoic acid present in both the fermented and the unfermented extracts. Preliminary Studies conducted by [19], showed that Cis-Vaccenic acid lowers risk of breast cancer. According to [20], Cis-Vaccenic acid present in the both extracts is associated with lower risk of coronary heart disease which subsequently leads to heart failure. 9-octadecenoic acid significantly decreased Reactive oxygen species (ROS) and Lipid Peroxidation (LPO) by raising the activity of antioxidant enzymes [21]. Hence the presence of 9-octadecenoic acid may account for the high antioxidant scavenging activity of the extracts. The presence of Cis-Vaccenic acid may account for the anti-cancer activity of the extracts. Fermented extracts showed prominent peaks of eicosatrienoic acid, docasatriene, cyclopropaneoctanoic acid which were absent in the total ion chromatogram of unfermented extracts. Fermentation plays a major role in the changes of the components in the secondary protein structure. Furthermore, a study showed that fermentation increased the total phenolic content of an extract [22]. The compound eicosatrienoic acid which was formed during fermentation as it was present only in the fermented extracts of Parkia biglobosa has proven to be a very promising compound which according to a study conducted by [23], has proven to lower insulin resistivity. The formation of eicosatrienoic acid during fermentation may account for the increased antidiabetic activity in the fermented extracts. The study also proved that eicosatrienoic acid has anti-inflammatory properties hence the high total phenolics, total flavonoids and anticancer levels on breast cancer cells may be accounted for by the presence of eicosatrienoic acid in the fermented extracts [23].
In this study, both fermented and unfermented aqueous extracts of Parkia biglobosa were screened for the presence of phytochemicals. These included tannins, saponins, flavonoids, and terpenoids. In both extracts, only the presence of flavonoids and terpenoids were detected.Terpenoids, which plays an important role in wound and scar healing [24], treatment of skin disease [25]. Terpenoids are known to fight malaria and cancer [26], very active in wound healing [25]. From clinical studies, it is shown that terpenoids strengthen the skin, increase the concentration of antioxidants in wounds, and restore inflamed tissues by increasing blood supply. The observed antioxidant and anticancer activities in this study could partly be attributed to the presence of flavonoids, terpenoids as well as phenolic compounds.
Investigations revealed that both test samples, the aqueous fermented and unfermented Parkia biglobosa seed extracts, helped yeast cells to uptake glucose. The glucose uptake at a final concentration of 0.3125 mg/mL and an initial concentration of 20mg/mL of fermented and unfermented seed was comparable to standard drug metformin. However, the effect metformin on glucose uptake by yeast cells was lower compared to the two test samples. Of the two test samples, aqueous fermented Parkia biglobosa extract readily helped yeast cells to uptake glucose better than the unfermented. The antidiabetic properties present of P. biglobosa seed extracts can be attributed to bioactive compounds that were revealed during the phytochemistry and the antioxidant assays. Therefore, the presence of one of these compounds in the extract might help in the uptake of glucose by yeast cells in the present study.
Conclusion
The present investigation suggests that Parkia biglobosa seed possesses remarkable antioxidant, antidiabetic and anticancer properties and the chemical compounds detected by the phytochemical analysis and gas chromatography mass spectrometry analysis could be responsible for these properties.
Author’s Contribution
Perez Frimpong Danso and Christopher Larbie conceived and designed the study. Perez Frimpong Danso and Adwoa Frema Amanfo, wrote the manuscript. Afua Ampem Gyenfi, Rosemond Serwaa Opoku helped in editing the manuscript. Regina Appiah-Oppong, Christopher Larbie Reviewed the manuscript and did the required editing.
Declarations
- Competing interests
The Authors declare that they have no competing interest.
- Funding
This work was funded by the authors.
- Acknowledgement
We Thank the Department of Biochemistry and Biotechnology of Kwame Nkrumah University of Science and Technology, Kumasi and Department of Clinical Pathology, Noguchi Memorial Institute of Medical Research, Universiy of Ghana, Accra for providing us a space to conduct this study.
References
- GBD 2017 Causes of Death Collaborators (2018) Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. The lancet 392: 1736-1788.
- Sakarkar D, Deshmukh V (2011) Ethnopharmacological review of traditional medicinal plants for anticancer activity. Int J Pharm Tech Res 3: 298-308.
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA: a cancer journal for clinicians 61: 69-90.
- Olaku O, White JD (2011) Herbal therapy use by cancer patients: a literature review on case reports. European Journal of Cancer 47: 508-514.
- Gurib-Fakim A (2006) Medicinal plants: traditions of yesterday and drugs of tomorrow. Molecular aspects of Medicine 27: 1-93.
- Ayoola G, Coker H, Adesegun S, Adepoju-Bello A, Obaweya K, et al. (2008) Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Tropical Journal of Pharmaceutical Research 7: 1019-1024.
- Gyamfi E, Anim M, Zakaria N, Kpattah L, Enti-Brown S, et al. (2016) Evaluation of metal ions concentrations, total flavonoid content and dpph free radicals scavenging in the fruits of Tetrapleura tetraptera (Prekese) from Ghana. Annals Food Science and Technology 17: 124-131.
- Marinova D, Ribarova F, Atanassova M (2005) Total phenolics and total flavonoids in Bulgarian fruits and vegetables. Journal of the university of chemical technology and metallurgy 40: 255-260.
- Ordonez A, Gomez J, Vattuone M (2006) Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food chemistry 97: 452-458.
- Ham YM, Yoon WJ, Park SY, Jung YH, Kim D, et al. (2012) Investigation of the component of Lycopodium serratum extract that inhibits proliferation and mediates apoptosis of human HL-60 leukemia cells. Food and chemical toxicology 50: 2629-2634.
- Ayisi NK, Appiah-Opong R, Gyan B, Bugyei K, Ekuban F (2011) Plasmodium falciparum: assessment of selectivity of action of chloroquine, Alchornea cordifolia, Ficus polita, and other drugs by a tetrazolium-based colorimetric assay. Malaria research and treatment 2011: 816250.
- Badisa RB, Darling-Reed SF, Joseph P, Cooperwood JS, Latinwo LM (2009) Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer research 29: 2993-2996.
- Larbie C, Mills-Robertson FC, Quaicoe EB, Opoku R, Kabiri NC, et al. (2019) Tetrapleura tetraptera of Ghanaian Origin: Phytochemistry, Antioxidant and Antimicrobial Activity of Extracts of Plant Parts. J Pharm Res Int 32: 78-96.
- Jao C-L, Ko W-C (2002) 1, 1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging by protein hydrolyzates from tuna cooking juice. Fisheries Science 68: 430-435.
- Ghasemi K., Ghasemi Y, Ebrahimzadeh MA (2009) Antioxidant activity, phenol and flavonoid contents of 13 citrus species peels and tissues. Pak J Pharm Sci 22: 277-281.
- Muchuweti M, Kativu E, Mupure C, Chidewe C, Ndhlala A, et al. (2007) Phenolic composition and antioxidant properties of some spices. American Journal of Food Technology 2: 414-420.
- Han HJ, Tan NH, Zeng GZ, Fan JT, Huang HQ, et al. (2008) Natural inhibitors of DNA topoisomerase I with cytotoxicities. Chemistry & biodiversity 5: 1364-1368.
- Kunnumakkara AB, Bordoloi D, Padmavathi G, Monisha J, Roy NK, et al. (2017) Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. British journal of pharmacology 174: 1325-1348.
- Simonsen NR, Navajas JF-C, Martin-Moreno JM, Strain JJ, Huttunen JK, et al. (1998) Tissue stores of individual monounsaturated fatty acids and breast cancer: the EURAMIC study. European Community Multicenter Study on Antioxidants, Myocardial Infarction, and Breast Cancer. The American journal of clinical nutrition 68: 134-141.
- Djoussé L, Matsumoto C, Hanson NQ, Weir NL, Tsai MY, et al. (2014) Plasma cis-vaccenic acid and risk of heart failure with antecedent coronary heart disease in male physicians. Clinical nutrition 33: 478-482.
- Shukla R, Banerjee S, Tripathi YB (2018) Antioxidant and Antiapoptotic effect of aqueous extract of Pueraria tuberosa (Roxb. Ex Willd.) DC. On streptozotocin-induced diabetic nephropathy in rats. BMC complementary and alternative medicine 18: 1-11.
- Alrosan M, Tan T-C, Easa AM, Gammoh S, Alu’datt MH (2021) Effects of Fermentation on the Quality, Structure, and Nonnutritive Contents of Lentil (Lens culinaris) Proteins. Journal of Food Quality 2021: 5556450.
- Baker EJ, Miles EA, Calder PC (2021) A review of the functional effects of pine nut oil, pinolenic acid and its derivative eicosatrienoic acid and their potential health benefits. Progress in Lipid Research 82: 101097.
- Hawkins E Ehrlich S (2006) Gotu Kola. University of Maryland Medical Center. Baltimore. USA.
- Sotheeswaran S, Doyle M, Aalbersberg WG (1998) Medicinal plants in the South Pacific: World Health Organisation, Regional office for the Western Pacific, USA.
- Salah N, Miller NJ, Paganga G, Tijburg L, Bolwell GP, et al. (1995) Polyphenolic flavonoids as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Archives of biochemistry and biophysics 322: 339-346.
Citation: Danso PF, Larbie C, Appiah-Opong R, Opoku RS, Gemfi AA, et al. (2023) Extract of Parkia Biglobosa ("Dawadawa") Seed is A Rich Antioxidant Source Which Enhances Glucose Tolerance and Inhibits the Growth of Cancer Cells. J Altern Complement Integr Med 9: 435.
Copyright: © 2023 Perez Frimpong Danso, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

