
First Turkish Patient Diagnosed with BCL11A-Related Intellectual Disability with A De Novo Pathogenic Variant
*Corresponding Author(s):
Nejmiye AkkusDepartment Of Medical Genetics, Tokat Gaziosmanpasa University Faculty Of Medicine, Tokat, Turkey
Tel:+90 05308792685,
Email:drnejmiyeakkus@gmail.com
Abstract
Background: BCL11A-associated intellectual disability (BCL11A-ID) is a syndrome that can cause developmental delay or intellectual disability, neonatal hypotonia, microcephaly, behavioral problems, and the persistence of fetal hemoglobin without any symptoms.
Case presentation: Here, we report a 9-year-old patient with microcephaly, developmental delay/intellectual disability, behavior problems, and asymptomatic persistence of Fetal Hemoglobin (HbF). We detected a previously unreported heterozygous c.142T>C (p.Cys48Arg) variant in the BCL11A gene through exome sequencing, which leads to BCL11A-related intellectual disability. We used Sanger sequencing to confirm this genetic variant and performed a pathogenicity assessment according to ACMG.
Conclusion: We present the first Turkish patient with the rare syndrome BCL11A-ID, identified with this variant.
Introduction
BCL11A-related intellectual disability (BCL11A-ID) is known as Intellectual Developmental Disorder with the Persistence of Fetal Hemoglobin or Dias-Logan Syndrome. BCL11A-ID (OMIM: 617101) is a syndrome characterized by autosomal dominant inheritance, particularly delayed psychomotor development and intellectual disability. Other clinical features include behavioral problems, language delays, microcephaly, downward-curving palpebral fissures, strabismus, external ear abnormalities, and hereditary persistence of fetal hemoglobin (HPFH) [1,2]. BCL11A-ID was identified by Dias et al., [1] revealing BCL11A gene heterozygous mutations in chromosome 2p16 region.
The BCL11A (B-cell lymphoma/leukemia 11A) gene (OMIM: 606557) plays a role in silencing fetal hemoglobin after birth by providing C2H2 zinc-finger transcription factor expression [3-5]. It is thought that BCL11A variants are the cause of neurological deficits seen in the structural and functional infrastructure of the brain as a result of their effect on C2H2 zinc-finger transcription factor expression [6]. Identification of patients with neurodevelopmental disorders diagnosed with heterozygous mutation or copy number loss of BCL11A proves the vital role of BCL11A gene function in human brain development [7-12].
Peron et al., [7] in their review of patients to date, reported 60 unique variants including 30 frameshifts, 7 missenses, 6 splicing sites, 17 stop-gains, and 8 unique CNVs (microdeletions containing only BCL11A), in 75 patients diagnosed with BCL11A-IDD [7]. Exome sequencing revealed a previously unreported heterozygous c.142T>C (p.Cys48Arg) variant in the BCL11A gene, which led to BCL11A-related intellectual disability. We present the first clinical report of these variants with the BCL11A gene mutation in the literature.
Case Presentation
A 9-year-old male patient was referred to our clinic with a language delay and ID. He was born by normal vaginal delivery with a birth weight of 3000 g at term as the third child of nonconsanguineous parents. The 9-year-old patient had microcephaly, developmental delay/intellectual disability, hypermetropia, strabismus, scoliosis, and behavioral problems. The patient's first intelligible word pronunciation was at the age of 2. The patient, who has been receiving speech therapy for five years, can pronounce simple 3-word sentences. Moreover, he had aggressive behavior, complaints of difficulty walking, and frequent falls.
The hemoglobin electrophoresis showed that the patient’s HbF value was high; however, the results of the parents were normal (Figure 1). The patient's medical history includes an operation for an inguinal hernia. Additionally, cranial MRI imaging of the patient revealed hypoplasia in the lower cerebellar vermis (Figure 2).
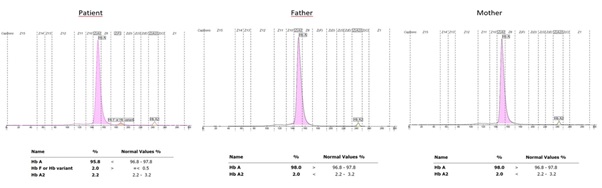 Figure 1: The hemoglobin electrophoresis showed that the patient’s HbF value was high; however, the results of the parents were normal.
Figure 1: The hemoglobin electrophoresis showed that the patient’s HbF value was high; however, the results of the parents were normal.
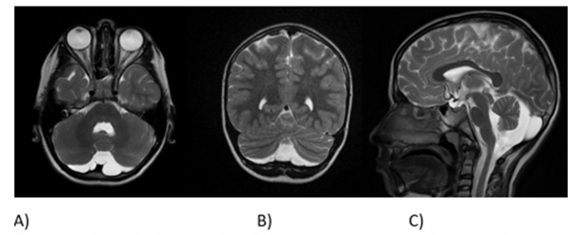 Figure 2: The patient's medical history includes an operation for an inguinal hernia. Additionally, cranial MRI imaging of the patient revealed hypoplasia in the lower cerebellar vermis
Figure 2: The patient's medical history includes an operation for an inguinal hernia. Additionally, cranial MRI imaging of the patient revealed hypoplasia in the lower cerebellar vermis
The patient was consulted by a child psychiatrist. As a result of the psychiatric examination and psychometric examination, Kent EGY, and Porteus Mazes intelligence tests results, there was thought to be a mild delay in cognitive development.
Whole-exome sequencing (WES) revealed a previously unreported heterozygous c.142T>C (p.Cys48Arg) variant detected in exon 2 of the BCL11A gene which, led to BCL11A-related intellectual disability. Exome sequencing was performed using the Roche Kapa HyperExom Kit and the Illumina Novaseq 6000 platform. The variants were evaluated according to the American College of Medical Genetics and Genomics (ACMG) [13]. In silico tools, the REVEL score of the variant was determined as 0.943 (PP3) and the ExAC value was determined as 3.62 (PP2). No variant was detected in either parent in the segregation analysis(PM6) (Figure 3). As a result of all these evaluations; heterozygous variant c.142T>C (p.Cys48Arg) in the BCL11A gene was reported a likely pathogenic (PP3+PM6+PP2+PM2) heterozygous variant. Sanger sequencing was conducted for the patient and his parents to confirm this genetic variant and a pathogenicity assessment was performed according to ACMG.
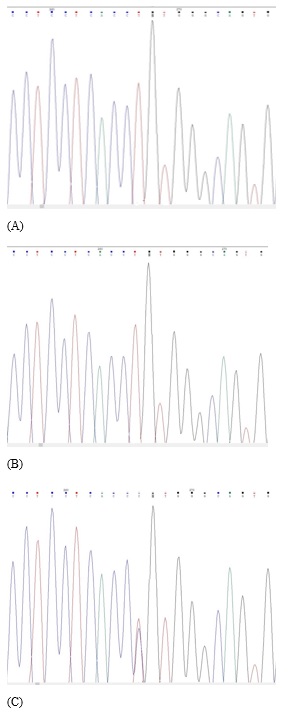 Figure 3: No variant was detected in either parent in the segregation analysis(PM6).
Figure 3: No variant was detected in either parent in the segregation analysis(PM6).
The patient underwent karyotype analysis, microarray, and fragile X examinations, all of which returned normal. Our patient's family history was unremarkable.
Informed consent for publication was obtained from the parents of our patient to participate in this study.
Discussion
BCL11A-ID is inherited as an autosomal dominant and occurs as a result of heterozygous variants in the BCL11A gene. BCL11A plays an important role in neurodevelopmental processes in the human brain. Moreover, BCL11A internal zinc finger domains selectively bind to g-globin promoter motifs, thus acting as a transcriptional repressor of fetal hemoglobin. Most patients may also develop ID, global delay in developmental milestones, Childhood Apraxia Of Speech (CAS), microcephaly, strabismus, flat midface, joint laxity, and persistence of HbF (OMIM: 617101) [14]. Our case involves delayed psychomotor development, ID, behavioral issues, CAS, microcephaly, strabismus, and the Hereditary Persistence of Fetal Hemoglobin (HPFH).
Of all previously reported individuals with BCL11A-ID, 73 of the 75 previous patients had a diagnosis of developmental delay and/or ID; this is the most common manifestation of the disease. The degree of ID in patients is usually moderate but varies from mild to severe/profound. The most common craniofacial findings in patients are external ear anomalies, malar flattening, thick or everted vermillion of the lower lip and thin vermillion of the upper lip, wide nose, full cheeks, and epicanthus [15-17]. Congenital anomalies such as polydactyly, cleft palate, stenosis of the pulmonary artery branches, craniosynostosis, and umbilical hernia are rarely seen. However, our patient had a congenital inguinal hernia.
BCL11A again reduces the HbF value after birth with its transcriptional repressive effect. In all patients diagnosed with BCL-11A-ID, HbF values were determined to be above the maximum reference for age in hemoglobin electrophoresis or high-performance liquid chromatography (HPLC) results [4,7]. In light of this information, hemoglobin electrophoresis showed that our patient’s HbF was high (Figure 2).
Findings such as a thin upper lip and/or an everted lower lip, microcephaly, intellectual disability, speech impairment, and behavior abnormalities have been reported in patients diagnosed with the BCL-11A-ID [1,2]. Our patient has been diagnosed with microcephaly, developmental delay/intellectual disability, hypermetropia, strabismus, and behavioral problems.
So far, Loss-of-Function (LoF) mutations in the BCL11A gene have mostly been reported, although fewer missense variants have been reported in the patients [14,18].
In conclusion, we detected a de novo heterozygous missense mutation c.142T>C (p.Cys48Arg) in the BCL11A gene in a male patient affected by ID and language delay. This case is the first Turkish patient diagnosed with BCL11A-ID with a previously unreported heterozygous variant.
Acknowledgement
All authors have contributed significantly. All authors have revised and approved the final manuscript.
Conflict of Interest Statement
There is no conflict of interest.
Funding
There is no funding.
Consent
Informed patient consent was obtained.
Ethics Approval and Consent to Participate
Not required.
References
- Dias C, Estruch SB, Graham SA, McRae J, Sawiak SJ, et al. (2016) BCL11A Haploinsufficiency Causes an Intellectual Disability Syndrome and Dysregulates Transcription. Am J of Hum Genet 99: 253-274.
- Wessels MW, Cnossen MH, Dijk TB, Gillemans N, Schmidt KLJ, et al. (2021) Molecular analysis of the erythroid phenotype of a patient with BCL11A haploinsufficiency. Blood Adv 11: 2339-2349.
- Uda M, Galanello R, Sanna S, Lettre G, Sankaran VG, et al. (2008) Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc Natl Acad Sci USA 105: 1620-1625.
- Liu H, Ippolito GC, Wall JK, Niu T, Probst L, et al. (2006) Functional studies of BCL11A: characterization of the conserved BCL11A-XL splice variant and its interaction with BCL6 in nuclear paraspeckles of germinal center B cells. Mol Cancer 5: 18.
- Smith EC, Luc S, Croney DM, Woodworth MB, Greig LC, et al. (2016) Strict in vivo specificity of the Bcl11a erythroid enhancer. Blood 128: 2338-2342.
- Shen Y, Li R, Teichert K, Montbleau KE, Verboon JM, et al. (2021) Pathogenic BCL11A variants provide insights into the mechanisms of human fetal hemoglobin silencing. PLoS Genet 17: 1009835.
- Peron A, D'Arco F, Aldinger KA, Smith-Hicks C, Zweier C, et al. (2021) BCL11A intellectual developmental disorder: Defining the clinical spectrum and genotype-phenotype correlations. medRxiv 2006: 21262776.
- Woodworth MB, Greig LC, Liu KX, Ippolito GC, Tucker HO, et al. (2016) C tip1 Regulates the Balance between Specification of Distinct Projection Neuron Subtypes in Deep Cortical Layers. Cell Rep 15: 999-1012.
- Greig LC, Woodworth MB, Greppi C, Macklis JD (2016) C tip1 Controls Acquisition of Sensory Area Identity and Establishment of Sensory Input Fields in the Developing Neocortex. Neuron 90: 261-77.
- Wiegreffe C, Simon R, Peschkes K, Kling C, Strehle M, et al. (2015) Bcl11a (Ctip1) Controls Migration of Cortical Projection Neurons through Regulation of Sema3c. Neuron 87: 311-325.
- Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, et al. (2014) Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515: 209-215.
- Basak A, Hancarova M, Ulirsch JC, Balci TB, Trkova M, et al. (2015) BCL11A deletions result in fetal hemoglobin persistence and neurodevelopmental alterations. J Clin Invest 125: 2363-2368.
- Richards S, Aziz N, Bale S, Bick D, Das S, et al. (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17: 405-424.
- Peter B, Matsushita M, Oda K, Raskind W (2014) De novo microdeletion of BCL11A is associated with severe speech sound disorder. Am J Med Genet A 164: 2091-2096.
- Cai T, Chen X, Li J, Xiang B, Yang L, et al. (2017) Identification of novel mutations in the HbF repressor gene BCL11A in patients with autism and intelligence disabilities. Am J Hematol 92: 653-656.
- Yoshida M, Nakashima M, Okanishi T, Kanai S, Fujimoto A, et al. (2018) Identification of novel BCL11A variants in patients with epileptic encephalopathy: expanding the phenotypic spectrum. Clin Genet 93: 368-373.
- Soblet J, Dimov I, Kalckreuth CG, Cano-Chervel J, Baijot S, et al. (2018) BCL11A frameshift mutation associated with dyspraxia and hypotonia affecting the fine, gross, oral, and speech motor systems. Am J Med Genet A 176: 201-208.
- Korenke GC, Schulte B, Biskup S, Neidhardt J, Owczarek-Lipska M (2020) A Novel de novo Frameshift Mutation in the BCL11A Gene in a Patient with Intellectual Disability Syndrome and Epilepsy. Mol Syndromol 11: 135-140.
Citation: Akkus N, Gokce E (2025) First Turkish Patient Diagnosed with BCL11A-Related Intellectual Disability with A De Novo Pathogenic Variant. J Genet Genomic Sci 10: 050.
Copyright: © 2025 Nejmiye Akkus, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

