
Fully matured F1 Seed Formed in the Cross between Cultivated Chickpea (Cicer arietinum L.) and the Tertiary Gene Pool Species, Cicer pinnatifidum without Embryo Rescue but Albinism Persists
*Corresponding Author(s):
Shivali SharmaGlobal Crop Diversity Trust, Bonn, Germany
Tel:+91 9440397294,
Email:shivalipbg@gmail.com
Abstract
Wild Cicer species, especially those in the tertiary gene pool, carry useful alleles for chickpea improvement. The aim of this study was to evaluate the crossability and geneflow between three chickpea cultivars (as female parents) and four cross-incompatible Cicer pinnatifidum accessions (as pollen parents) from the tertiary gene pool. Ten crosses were conducted. One fully developed healthy F1 seed was harvested in vivo from the ICC 4958 × ICC 17269 cross, but the seedling developed an albino phenotype at 4-5 days after germination. Unlike other crosses, those involving the cultivar ICCV 96030 generated a large number of pods with comparatively large ovules. One albino plantlet was obtained from the ICCV 96030 × ICC 17269 cross by embryo rescue. Crosses involving ICCV 10 resulted in flower drop and poor pod set. These variable genotype-specific responses of pod, ovule, and seed development indicate that genetic factors affect the formation of interspecific hybrids. Although pod and seed formation in these interspecific crosses can be improved by involving diverse genotypes in crossing, geneflow between these materials is hindered by a strong genetic factor conferring albinism in the F1 hybrids.
Keywords
Chickpea; Cicer arietinum; Cicer pinnatifidum; Cross-incompatibility; Geneflow; Tertiary gene pool
Introduction
Chickpea (Cicer arietinum L.), the second most important dietary legume after common bean, is a rich source of proteins, carbohydrates, micronutrients, and vitamins [1]. It is a potential staple food crop in about 55 countries. India is the largest producer of chickpea with an annual production of 11.1 million tonnes [2]. Chickpea production worldwide is affected by biotic and abiotic stresses. Because there is limited genetic variation in the cultivated chickpea germplasm, it is necessary to utilize wild Cicer species for its genetic improvement. Wild Cicer species are strongly resistant to major biotic stresses like Ascochyta blight [3-8], Botrytis gray mold [6,8,9] and Fusarium wilt [5, 6,10] and tolerant to abiotic stresses such as drought [5,11,12], cold [5,13,14] and combined drought and heat [15]. Wild Cicer species also have desirable nutrition-related traits such as high seed protein and mineral contents [6,16].
Various incompatibility barriers, linkage drag, and poor viability and sterility of F1 hybrids and progenies mean that potential wild Cicer species are underutilized in chickpea breeding programs. Two annual wild Cicer species, Cicer reticulatum and Cicer echinospermum, are crossable with cultivated chickpea. However, the sterility of F1 hybrids and progenies has limited the utilization of C. echinospermum in crossing programs. Little is known about the crossability of the other six annual wild Cicer species with cultivated chickpea. To utilize those species in chickpea improvement, specialized techniques such as the application of growth hormones, ovule culture, and embryo rescue are required [17-20].
Few attempts have been made to generate interspecific hybrids between Cicer arietinum from the primary gene pool and wild Cicer pinnatifidum from the tertiary gene pool [17,19]. Systematic crossing efforts involving diverse parental combinations are required to advance the production of viable interspecific hybrids involving tertiary gene pool species. The aim of this study, therefore, was to evaluate the crossability and geneflow between three cultivars of C. arietinum and four wild C. pinnatifidum accessions originating/collected from Turkey and Syria.
Material and Methods
Three chickpea cultivars (ICCV 10, ICC 4958, and ICCV 96030) and four wild accessions (ICC 17126, ICC 17276, ICC 17200, and ICC 17269) belonging to the tertiary gene pool species C. pinnatifidum were used (Table 1). The seeds of all wild accessions were scarified by incising the hard seed coat. Seeds were treated with fungicides (2 g thiram + 1 g carbendazim kg-1 seed) before sowing in pots in a 2:1:1 mixture of sterilized black soil, farmyard manure, and sand. Seed sowing was staggered to synchronize the flowering of cultivated genotypes and wild accessions. At 1 month after germination, C. pinnatifidum seedlings were exposed to an 18-h light/6-h dark photoperiod with light supplied by 60-W incandescent lights to induce early flowering [21]. Interspecific crosses were made using the three cultivars as female parents and the four wild accessions as pollen parents. ICCV 10 and ICC 4958 were each crossed with all four C. pinnatifidum accessions, and ICCV 96030 was crossed only with two accessions, ICC 17126 and ICC 17269. The flower buds of the female parents were emasculated and tagged between 3:00 p.m. and 4:00 p.m., and then pollinated with fresh pollen from wild accessions the following morning between 8:00 a.m. and 9:00 a.m. Each day for 3 consecutive days, a mixture of growth hormones (50 mg L-1 gibberellic acid + 10 mg L-1 naphthalene acetic acid + 10 mg L-1 kinetin, 1:1:1) was applied to the base of the peduncle of the pollinated buds to prevent flower drop and pod abscission. Selfed pods on the same branch were removed to encourage growth of the crossed pods. We recorded the number of pollinations and number of fully developed pods generated in each cross (Table 2).
Yellowing pods were harvested and the ovules were cultured in liquid Murashige and Skoog (MS) medium containing 3% w/v sucrose, 0.25 mg/L indole acetic acid, and 1 mg/L zeatin. Mallikarjuna (1999) [18] reported emergence of maximum number of embryos from the ovules when cultured on a medium with 0.25 mg/L indole acetic acid, and 1 mg/L zeatin. After 3 weeks, the cultured ovules were subcultured into fresh ovule culture medium until the embryos emerged from the ovules. The regenerated embryos were transferred to shoot growth medium (liquid MS containing 3% w/v sucrose, 0.25 mg/L indole acetic acid, and 1 mg/L kinetin). Well-grown shoots were cultured on root-induction medium (half-strength MS basal salts, 1.5% w/v sucrose, and 0.5 mg/L indole butyric acid). We recorded the number of ovules cultured and number of plantlets generated through ovule culture (Table 2).
|
Accession identity |
Species |
Biological status |
Country of origin |
|
ICC 4958 |
Cicer arietinum |
Advanced/Improved cultivar |
India |
|
ICCV 10 |
C. arietinum |
Cultivated variety |
India |
|
ICCV 96030 |
C. arietinum |
Cultivated variety |
India |
|
ICC 17126 |
C. pinnatifidum |
Wild species |
Turkey |
|
ICC 17276 |
C. pinnatifidum |
Wild species |
Syria |
|
ICC 17200 |
C. pinnatifidum |
Wild species |
Syria |
|
ICC 17269 |
C. pinnatifidum |
Wild species |
Turkey |
Table 1: Cultivated chickpea and Cicer pinnatifidum accessions used in this study.
|
Cross |
No. of pollinations |
No. of matured F1 seeds obtained |
No. of F1 pods harvested for ovule culture |
No. of ovules cultured |
No. of plantlets regenerated |
|
|
Female parent (Cicer arietinum) |
Pollen parent (C. pinnatifidum) |
|||||
|
ICCV 10 |
ICC 17126 |
95 |
0 |
9 |
9 |
0 |
|
ICC 17276 |
55 |
0 |
0 |
0 |
0 |
|
|
ICC 17200 |
58 |
0 |
0 |
0 |
0 |
|
|
ICC 17269 |
75 |
0 |
4 |
4 |
0 |
|
|
ICC 4958 |
ICC 17126 |
95 |
0 |
5 |
5 |
0 |
|
ICC 17276 |
55 |
0 |
0 |
0 |
0 |
|
|
ICC 17200 |
58 |
0 |
7 |
7 |
0 |
|
|
ICC 17269 |
85 |
1 |
3 |
3 |
0 |
|
|
ICCV 96030 |
ICC 17126 |
100 |
0 |
88 |
9 |
0 |
|
ICC 17269 |
100 |
0 |
68 |
7 |
1 |
|
Table 2: Crosses attempted between cultivated chickpea and Cicer pinnatifidum at ICRISAT, Patancheru, India.
Results And Discussion
One fully mature pod with a healthy F1 seed was harvested from the ICC 4958 × ICC 17269 cross. The F1 seed resembled that of the cultivated parent (ICC 4958) with respect to size, color, texture, and shape. The mature F1 seed was sown in a mixture of soil, sand, and vermiculite (3:1:1). The F1 seedling had a leaf shape similar to that of the wild C. pinnatifidum parent ICC 17269, confirming true hybridity (Figure 1). Thus, the generation of a healthy and functional F1 seed in the ICC 4958 × ICC 17269 cross was not prevented by pre-fertilization barriers such as failure of pollen germination, pollen incompatibility, arrested pollen tube growth in the stigma or style, failure of the pollen tube to penetrate the ovule, or arrested growth of the pollen tube within the ovule; or by post-fertilization barriers such as embryo abortion, or shriveled or immature F1 seeds. In contrast, earlier studies have reported strong post-zygotic barriers requiring in-ovule embryo rescue techniques for obtaining F1 hybrid plants [17-19]. This F1 seed was planted in pot and the seedling became albino (lacked chlorophyll) at 4-5 days after germination (Figure 1). This albinism is attributed to defective chloroplasts with poorly developed thylakoids and few and disorganized grana [17,22]. Our attempts to multiply this albino-type plant by regeneration through callus induction and culture of different explants (leaves, stem cuttings, and nodes) on basal MS medium containing 0.5 mg/L benzylaminopurine and 0.5 mg/L naphthalene acetic acid were unsuccessful. Thus, although geneflow in the ICC 4958 × ICC 17269 cross was not hindered by the pre- or post-fertilization barriers reported elsewhere [17,19,22,23], it was hindered by the albinism of F1 hybrid plants. It will be possible to generate more healthy F1 pods and seeds from this cross by increasing the number of pollinations and using different combinations of plant growth hormones. However, efforts are needed to address the problem of albinism in F1 seedlings.
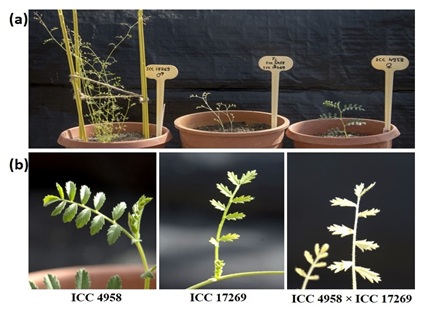 Figure 1: F1 plant generated from ICC 4958 × ICC 17269; a. Cicer pinnatifidum accession ICC 17269 (left), albino F1 plant (middle), and chickpea cultivar ICC 4958 (right); b. Leaf shape of cultivated parent ICC 4958 (left), wild parent ICC 17269 (middle), and F1 plant resembling wild parent (right).
Figure 1: F1 plant generated from ICC 4958 × ICC 17269; a. Cicer pinnatifidum accession ICC 17269 (left), albino F1 plant (middle), and chickpea cultivar ICC 4958 (right); b. Leaf shape of cultivated parent ICC 4958 (left), wild parent ICC 17269 (middle), and F1 plant resembling wild parent (right).
Unlike other crosses, interspecific crosses involving ICCV 96030 resulted in fully developed, mature pods (Table 2). However these pods lacked mature seeds. Most of the pods contained minute to small-sized colorless ovules. Thus, the pods developed normally but the ovules inside did not (Figure 2).
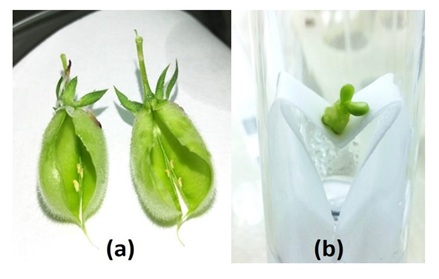 Figure 2: Ovule culture to rescue hybrid embryo from ICCV 96030 × ICC 17269; a. Well-developed pods containing ovules that failed to develop; b. Ovule cultured in liquid medium.
Figure 2: Ovule culture to rescue hybrid embryo from ICCV 96030 × ICC 17269; a. Well-developed pods containing ovules that failed to develop; b. Ovule cultured in liquid medium.
Pod development begins after fertilization. In this study, C. pinnatifidum pollen successfully fertilized ICCV 96030, leading to the differentiation of the ovary into the pod wall. However, ovules did not successfully differentiate into seeds due to some intrinsic reasons. This kind of hybrid embryo response has not been reported for other chickpea interspecific crosses. The incompatibility between cultivated chickpea ICC 96030 and all the C. pinnatifidum accessions used in this study is due to a post-zygotic barrier, specifically defective embryos that could not develop into functional seeds. Post-zygotic barriers hindering interspecific hybridization between C. arietinum and C. pinnatifidum have also been reported by [17]. Of the three cultivated chickpea cultivars, ICCV 96030 yielded the highest number of mature, fully developed pods, including a few with enlarged embryos, when pollinated with C. pinnatifidum. It will be possible to harvest mature pods with seeds from ICCV 96030 × C. pinnatifidum crosses by increasing the number of pollinations in each cross, adjusting plant growth hormone treatments to facilitate embryo/seed development, by crossing in different directions, and/or by using other C. pinnatifidum accessions, e.g., ICC 17276 and ICC 17200, as the pollen parent. In addition, immature embryos can be rescued by ovule culture.
The aborting ovules were cultured from 7-8 days after pollination. The tiny ovules did not grow upon culturing, but one larger ovule (derived from ICCV 96030 × ICC 17269) grew normally and the embryo regenerated into a seedling (Figure 2). Although the shoot was initially green in the shoot growth medium, the newly formed leaves lacked chlorophyll. The albino seedling died after 2 weeks despite sub-culturing on the ovule culture medium containing zeatin. Defective chloroplasts are the major barrier in generating interspecific hybrids between C. arietinum and C. pinnatifidum [22]. In the crosses involving ICCV 10, flower drop was the major obstacle. Most of the pollinated flower buds dropped within 1-2 days of pollination, despite the use of plant growth hormones. Although some cross combinations formed a few pods, they turned yellow within 3-4 days of pollination, and ovules from these pods did not develop further in vitro because of their small size.
Interspecific hybridization between chickpea cultivars and C. pinnatifidum produced one fully mature F1 seed (from ICC 4958 × ICC 17269). None of the other cross combinations yielded fully mature F1 seeds. Although ICCV 96030 formed the most pods, followed by ICC 4958, only one ovule from the ICCV 96030 × ICC 17269 cross regenerated into a plantlet in ovule culture. None of the three chickpea cultivars formed pods when pollinated with C. pinnatifidum ICC 17276. On the basis of the pod, ovule, and seed formation of the interspecific crosses, we concluded that the chickpea cultivars ICC 4958 and ICCV 96030 and the C. pinnatifidum accessions ICC 17269 followed by ICC 17126 and ICC 17200 exhibited good crossability.
To our knowledge, this is the first report of generating a fully mature F1 seed from an interspecific cross between cultivated chickpea and C. pinnatifidum without using embryo rescue. Our results show that the parents’ genotypes affect crossability between C. arietinum and C. pinnatifidum. The successful development of a mature healthy F1 seed from the interspecific ICC 4958 × C. pinnatifidum ICC 17269 cross confirmed the absence of pre- and post-fertilization barriers. Instead, albinism of F1 hybrids was the major obstacle hindering geneflow between C. pinnatifidum and cultivated chickpea. Embryo abortion occurred after interspecific crosses involving the chickpea cultivar ICCV 96030 and all C. pinnatifidum accessions. Using an ovule culture technique, one albino plantlet was regenerated from the ICCV 96030 × ICC 17269 cross. The interspecific crosses between chickpea cultivar ICCV 10 and C. pinnatifidum accessions were unsuccessful due to excessive flower drop and poor pod formation. These variable genotype-specific responses of pod and seed development suggest that more genotypes should be included when testing for cross-compatibility. The cultivated genotypes used in this study originate from central and southern agro-geographical areas of India. Including more genotypes preferably from other parts of India in crossability studies may be useful for identifying those that are readily crossable with C. pinnatifidum, preferably without producing albino progeny.
Overall, the results showed that it is possible to generate fully matured pods with healthy seeds in crosses between cultivated chickpea and C. pinnatifidum without embryo rescue technique and the efficiency of pod and seed formation can be improved by involving more genotypes, use of different combinations of plant growth hormones, and direction of crosses etc. However, it seems difficult to improve the geneflow between these two species due to the involvement of a strong genetic factor responsible for malformation of chloroplasts leading to albinism in the F1 hybrids. The results also show that different parental genotype combinations have different crossabilities in inter-specific crosses, indicating that some genetic factors are important for the efficient production of interspecific hybrids involving C. pinnatifidum. Further studies are, therefore, needed to identify the cross combinations which can produce healthy F1 plants without albinism.
List of Abbreviations
Not applicable
Declarations
Ethics approval and consent to participate: Not applicable
Consent for publication: Not applicable
Availability of data and materials: All the data related to this study is included in the main manuscript and additional supporting file.
Competing Interests: The authors declare that they have no competing interests.
Funding: This work was undertaken as part of the initiative “Adapting Agriculture to Climate Change: Collecting, Protecting and Preparing Crop Wild Relatives” which is supported by the Government of Norway (QZA-14/0005) and managed by the Global Crop Diversity Trust (http:// www.cwrdiversity.org/). This research was also supported by the CGIAR Research Program on Grain Legumes and Dryland Cereals (GLDC).
Author’s contribution: SS planned the study; SAL grew the materials and performed the experiments; SS, SAL, and BK analyzed the data and prepared the manuscript. All the authors read and approved the final manuscript.
Acknowledgement
Authors thank the Head-Genebank, ICRISAT for providing the seeds of four wild C. pinnatifidum accessions to carry out this study. Authors also thank the research technician, Mr. Laxmaiah and research support staff, Ms. Lakshami for their help in growing the plants and assistance in making crosses in the green house.
References
- Jukanti AK, Gaur PM, Gowda CLL, Chibbar RN (2012) Nutritional quality and health benefits of chickpea (Cicer arietinum L.): a review. British Journal Nutrition 108: 11-26.
- The Food and Agriculture Organization of the United Nations (2020) Crops and livestock products. The Food and Agriculture Organization of the United Nations. Rome, Italy.
- Stamigna C, Crino P, Saccardo F (2000) Wild relatives of chickpea: Multiple disease resistance and problems to introgression in the cultigen. Journal of Genetics and Breeding 54: 213-219.
- Collard BCY, Ades PK, Pang ECK, Brouwer JB, Taylor PW (2001) Prospecting for sources of resistance to Ascochyta blight in wild Cicer sp Australasian Plant Pathology 30: 271-276.
- Croser JS, Ahmad F, Clarke HJ, Siddique KHM (2003) Utilisation of wild Cicer in chickpea improvement—progress, constraints, and prospects. Australian Journal Agriculture Research 54: 429-444.
- Rao NK, Reddy LJ, Bramel PJ (2003) Potential of wild species for genetic enhancement of some semi-arid food crops. Genetic Resources and Crop Evolution 50: 707-721.
- Shah TM, Haq MA, Atta BM, Alam SS, Ali H (2005) Evaluation of Cicer species for resistance to Ascochyta blight. Pakistan Journal of Botany 37: 431-438.
- Pande S, Ramgopal D, Kishore GK, Mallikarjuna N, Sharma M, et al. (2006) Evaluation of wild Cicer species for resistance to Ascochyta blight and Botrytis gray mold in controlled environment at ICRISAT, Patancheru, India. Journal SAT Agricultural Research 2: 1-3.
- Stevenson PC, Haware MP (1999) Maackiain in Cicer bijugum f. associated with resistance to Botrytis grey mould. Biochemical Systematics and Ecology 27: 761-767.
- Infantino A, Puglia AP, Singh KB (1996) Screening wild Cicer species for resistance to fusarium wilt. Plant Disease 80: 42-44.
- Kashiwagi J, Krishnamurthy L, Upadhyaya HD, Krishna H, Chandra S, et al. (2005) Genetic variability of drought-avoidance root traits in the mini-core germplasm collection of chickpea (Cicer arietinum). Euphytica 146: 213-222.
- Toker C, Canci H, Yildirim T (2007) Evaluation of perennial wild Cicer species for drought resistance. Genetic Resources and Crop Evolution 54: 1781-1786.
- Toker C (2005) Preliminary screening and selection for cold tolerance in annual wild Cicer Genetic Resources and Crop Evolution 52: 1-5.
- Berger JD, Kumar S, Nayyar H, Street KA, Sandhu JS, et al. (2012) Temperature-stratified screening of chickpea (Cicer arietinum) genetic resource collections reveals very limited reproductive chilling tolerance compared to its annual wild relatives. Field Crops Research 126: 119-129.
- Canci H, Toker C (2009) Evaluation of annual wild Cicer species for drought and heat resistance under field conditions. Genetic Resources and Crop Evolution 56: 1-6.
- Sharma S, Lavale SA, Nimje C, Singh S (2021) Characterization and identification of annual wild Cicer species for seed protein and mineral concentrations for chickpea improvement. Crop Science 61: 305-319.
- Badami PS, Mallikarjuna N, Moss JP (1997) Interspecific hybridization between Cicer arietinum and pinnatifidum. Plant Breeding 116: 393-395.
- Mallikarjuna N (1999) Ovule and embryo culture to obtain hybrids from interspecific incompatible pollinations in chickpea. Euphytica 110: 1-6.
- Mallikarjuna N, Jadhav D (2008) Techniques to produce hybrid between Cicer arietinum x C. pinnatifidum Jaub. Indian Journal of Genetics and Plant Breeding 68: 398-405.
- Lulsdorf M, Mallikarjuna N, Clarke H, Taran B, Kharkwal MC (2005) Finding solutions for interspecific hybridization problems in chickpea (Cicer arietinum ) 4th International Food Legumes Research Conference, New Delhi, India.
- Sharma S, Upadhyaya HD (2019) Photoperiod response of annual wild Cicer species and cultivated chickpea on phenology, growth, and yield traits. Crop Science 59: 632-639.
- Clarke HJ, Kumari M, Khan TN, Siddique KH (2011) Poorly formed chloroplasts are barriers to successful interspecific hybridization in chickpea following in vitro embryo rescue. Plant Cell, Tissue Organ Culture 106: 465-473.
- Ahmad F, Slinkard AE (2004) The extent of embryo and endosperm growth following interspecific hybridization between Cicer arietinum and related annual wild species. Genetic Resources and Crop Evolution. 51: 765-772.
Citation: Sharma S, Lavale SA, Kilian B (2022) Fully matured F1 Seed Formed in the Cross between Cultivated Chickpea (Cicer arietinum L.) and the Tertiary Gene Pool Species, Cicer pinnatifidum without Embryo Rescue but Albinism Persists. J Genet Genomic Sci 7: 037.
Copyright: © 2022 Shivaji Ajinath Lavale, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.

